Abstract
Summary
This study investigated the effects of raloxifene and alendronate to follow parathyroid hormone (PTH) on bone collagen and biomechanical properties in ovariectomized rabbits. Sequential treatments of raloxifene and alendronate after hPTH(1-34) treatment improved biomechanical properties with and without bone collagen improvement, respectively.
Introduction
The standard sequential treatment to follow human parathyroid hormone (hPTH) (1-34) therapy for osteoporosis has yet to be determined. The objective of this study was to compare the effects of raloxifene and alendronate treatments to follow daily hPTH(1-34) treatment on non-enzymatic collagen cross-links, bone mass, and bone strength in ovariectomized (OVX) rabbits.
Methods
From 3 months after ovariectomy, seven month-old female New Zealand white rabbits were given either vehicle or hPTH(1-34) (8 μg/kg/day), once daily for 5 months. After hPTH(1-34) treatment, the hPTH(1-34)-treated animals were divided into two groups, and given raloxifene (10 mg/kg, daily) orally or alendronate (100 μg/kg, twice weekly) subcutaneously for 5 months. We evaluated bone mineral density (BMD), bone structural parameters, advanced glycation end product (AGE) content in collagen, and bone mechanical parameters including intrinsic parameters in the femur.
Results
Raloxifene (hPTH/RLX) and alendronate (hPTH/ALN) to follow hPTH(1-34) increased cortical thickness, maximum load, and maximum stress and decreased endocortical surface in the diaphysis, in addition to increasing total BMD in the distal metaphysis. Decreased trabecular AGE, pentosidine, and homocysteine contents and increased toughness and breaking energy were noted with hPTH/RLX treatment only. With hPTH/ALN treatment, no effects on non-enzymatic collagen cross-link AGEs were noted although increases in stiffness and elastic modulus were observed.
Conclusion
These results suggest that sequential treatments with hPTH(1-34) and antiresorptive drugs (raloxifene and alendronate) have a beneficial effect on bone mass and biomechanical properties in OVX rabbits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In theory, bone strength is derived from bone mineral density (BMD) and bone quality. Bone quality consists of structural and material properties of bone, and the determinants of the bone material properties are the degree of secondary mineralization, microdamage accumulation, and collagen cross-link formation.
Bisphosphonates such as alendronate, raloxifene (a selective estrogen-receptor modulator (SERM)), and human parathyroid hormone (hPTH) (1-34) are used to treat postmenopausal women with osteoporosis. Bisphosphonates and SERMs are used commonly as the first-line therapy for osteoporosis, and the use of hPTH(1-34) is recommended as a treatment for severe osteoporosis. Bisphosphonates are potent inhibitors of bone resorption and reduce the vertebral, non-vertebral, and hip fracture risk in patients with osteoporosis by suppressing elevated bone turnover [1]. However, long-term suppression of bone remodeling by alendronate may increase microdamage accumulation [2] and decrease heterogeneity of bone mineralization density distribution [3] in postmenopausal women. In relation to those findings, atypical femoral fracture has raised a concern for the long-term use of antiresorptives in recent years [4, 5]. Raloxifene mildly suppresses bone turnover and increases BMD, resulting in reduction in the risk of vertebral fractures but not non-vertebral fractures [6]. hPTH(1-34) is a bone-forming agent with a unique anabolic action and reduces vertebral and non-vertebral fracture risk by increasing bone turnover, BMD, and bone volume and improves both trabecular and cortical microarchitecture through its effects on bone remodeling, which are essentially the opposite of those of bisphosphonates [2, 7].
Collagen cross-links may be independent determinants of bone strength [8] and can be divided into immature and mature enzymatic cross-links and glycation or oxidation-induced non-enzymatic cross-links (advanced glycation end products (AGEs)). AGE cross-links are formed by oxidation or glycation reactions and cause deterioration of the mechanical and biological functions of bone, whereas enzymatic cross-link formation has beneficial effects for the mechanical properties of bone [9]. Pentosidine is a well-established intermolecular cross-linking AGE and is used as a surrogate marker of total AGE formation [10]. Homocysteine is also a bone quality marker reflecting the abnormalities in collagen cross-links caused by oxidative stress [11]. In addition to an increase in BMD and bone volume, hPTH(1-34) increases the total enzymatic cross-link content and decreases pentosidine coinciding with the improvement of bone strength in OVX monkeys [10]. Raloxifene ameliorates hyperhomocysteinemia-induced detrimental non-enzymatic cross-linking in OVX rabbits and is suggested to improve bone strength via the amelioration of collagen cross-links [11].
Although hPTH(1-34) has a unique mechanism of action on bone remodeling and bone collagen quality, the treatment period is limited to 24 months because of a concern for osteosarcoma [12]. From the perspective of long-term protection for fragility fracture, other drug treatments are required after the completion of the PTH(1-34) therapy to maintain bone mass gains caused by PTH(1-34), because those increases are quickly lost following simple discontinuation of PTH(1-34). The question at that point is whether patients should be treated with bisphosphonates or a drug of another class, such as raloxifene. In the present study, in order to serve as an aid to answering this question, we compared the effects of raloxifene (hPTH/RLX) and alendronate (hPTH/ALN) to follow hPTH(1-34) on bone mass, non-enzymatic collagen cross-links, and bone strength parameters including BMD and structural mechanical properties and intrinsic material properties in OVX rabbits. We used rabbits since they are the smallest species known to have Haversian bone remodeling processes similar to humans and since they had been used in a previous evaluation of raloxifene for its effects on collagen cross-links and bone strength, as mentioned above [11].
Methods
Animals
Four month-old female New Zealand white rabbits were purchased from Kitayama Labes (Nagano, Japan) on November 30, 2012 and acclimatized for 6 days. The rabbits were housed individually in wire cages and maintained at 19 ± 3 °C with a 12-h/12-h light-dark cycle and provided with ad libitum access to water and 100 g of feed (Laboratory Rabbit Diet HF #5325, PMI Nutrition International; calcium 0.9 % [min] to 1.4 % [max], phosphorus 0.44 % [min]) daily. This experiment was performed at the laboratory of Hamri Co., Ltd. (Ibaraki, Japan), which is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. The experiment protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Hamri Co., Ltd.
Phase I: OVX and sham rabbits
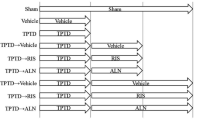
The rabbits were subjected to either bilateral OVX (n = 78) or a sham operation (n = 23) on December 10 and 11, 2012. For details of the ovariectomy procedure, refer to the previous report [13] Three months after surgery, OVX (n = 7) and sham (n = 7) rabbits were sacrificed for necropsy (Fig. 1). The rabbits were randomly selected by weight. At necropsy, 15 mL of blood samples for biochemical marker evaluations was collected from the abdominal aorta and then the rabbits were euthanized by exsanguination under anesthesia. Serum samples were prepared from blood clot samples by centrifugation and stored at −80 °C or lower. Twenty-four-hour urine samples were collected at 7 days before the end of this phase. The left and right femurs were wrapped in saline-soaked disposable paper wiper (Kimwipes; NIPPON PAPER CRECIA Co., Tokyo, Japan) in saline (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) and stored at −60 °C or lower for a three-point bending test and peripheral quantitative computed tomography (pQCT) measurement.
Phase II: hPTH(1-34) treatment in OVX rabbits
OVX rabbits were divided randomly into two groups based on weight: OVX+vehicle group (n = 29) and an hPTH(1-34) group (n = 42). The OVX rabbits were injected subcutaneously with either vehicle (5 % mannitol phosphate buffer solution, pH = 5) or hPTH(1-34) (Eli Lilly, Indianapolis, IN, USA; 8 μg/kg) dissolved in vehicle, once daily for 5 months. Sham (n = 16) rabbits were injected subcutaneously with vehicle with the same frequency and period. Since 10 μg/kg on 5 days a week (50 μg/kg/week) was the minimum dose that increased in the cortical area and cortical bone formation in rabbits [14], the dosage of hPTH(1-34) was set at 8 μg/kg/day (56 μg/kg/week) in this study. Fourteen vehicle-treated OVX rabbits, fourteen hPTH(1-34)-treated OVX rabbits, and seven sham rabbits were sacrificed for necropsy at the end of the 5-month treatment (Fig. 1). The rabbits were randomly selected by weight. The collection of biochemical samples and the necropsy procedures in this phase were the same as those described for phase I.
Phase III: sequential treatment with raloxifene or alendronate after hPTH(1-34) administration in OVX rabbits
Twenty-eight OVX rabbits which had been treated with hPTH(1-34) (8 μg/kg/day) for 5 months were divided randomly into raloxifene (n = 14) and alendronate (n = 14) groups based on weight. The hPTH(1-34)-treated OVX rabbits were orally given raloxifene (Eli Lilly, Indianapolis, IN, USA; 10 mg/kg) suspended in vehicle (10 % hydroxypropyl-β-cyclodextrin) once daily or injected subcutaneously with alendronate (Teijin Pharma, Tokyo, Japan; 100 μg/kg) dissolved in saline twice weekly, for 5 months. The doses of raloxifene and alendronate were reported in previous reports [11, 13]. The sham (n = 9) and vehicle-injected OVX (n = 15) rabbits were orally given vehicle. All rabbits were sacrificed for necropsy at the end of the 5-month treatment (Fig. 1). The collection of biochemical samples and the necropsy procedures in this phase were the same as those described for phase I.
Biochemical parameters
Procollagen type-I N-terminal propeptide (P1NP) (UniQ PINP RIA; Orion Diagnostica, Espoo, Finland), bone alkaline phosphatase (BAP) (Access Ostase; Beckman Coulter Inc., Tokyo, Japan), N-telopeptide of type I collagen cross-links (NTX) (Osteomark; Alere Inc. Waltham, MA, USA), and pentosidine (FSK Pentosidine; Fushimi Pharmaceutical Co., Ltd., Kagawa, Japan) were measured using a commercial kit. Homocysteine was labeled with 4-fluoro-7-sulfamoylbenzofurazan and measured by using a high-performance liquid chromatography (YMC-UltraHT Pro C18; YMC Co.,Ltd., Kyoto, Japan) with fluorescence detection at 375 nm excitation and 505 nm emission.
Bone mineral density
The diaphysis and distal metaphysis of the right femur was scanned by pQCT using an XCT Research SA+ (Stratec Medizintechnik, Pforzheim, Germany) system with a pixel size of 0.12 × 0.12 mm and a slice thickness of 0.46 mm. The scan area of the diaphysis was positioned at the midpoint of the femur. The scan area of distal metaphysis was positioned at 4 mm proximal to the epiphyseal plate of the femur. The position of the epiphyseal plate was confirmed by the difference in X-ray permeability on a scan image. The total and cortical BMD, including periosteal and endocortical surface, were determined from the scanned single slice (0.46 mm) using the pQCT software, version 6.20, at the diaphysis and distal metaphysis. The cortical area was determined with a threshold value of 630 (395 mg/cm3). Strength strain index was determined with a threshold value of 700 (464 mg/cm3).
Bone mechanical test
The bone strength of the left femur was measured with a three-point bending method using a mechanical testing machine (MZ-500S, Maruto, Tokyo, Japan). The support span was 80 mm and the loading bar was positioned at the midpoint of the femur. The bending load was applied to the posterior surface up of the midshaft with a crosshead speed of 10 mm/min until fracture. Maximum load, stiffness, and breaking energy were calculated from the load-displacement curve using the analysis software (CTRwin Ver. 1.05; System Supply, Nagano, Japan). Maximum stress, elastic modulus, and toughness were calculated by normalizing the structural parameters.
Bone powder preparation for collagen and collagen cross-links contents
Bone powder was prepared from the diaphysis and distal metaphysis of the left femur after bone mechanical test. To obtain cortical bone samples, a 2-mm-thick ring-shaped cortical bone was collected from just distal to diaphysis of the fracture point using a hand saw (K-100, HOZAN Co., Osaka, Japan) and cut up using bone scissors. To obtain trabecular bone samples, the trabecular bone was scraped out from the longitudinally split distal metaphysis of the femur using a sharp curette and bone scissors (B-20; Natsume Seisakusho, Tokyo, Japan). The bone marrow and soft tissue in the excised trabecular bone was carefully removed using a disposable paper wiper (Kimwipes, NIPPON PAPER CRECIA Co., Tokyo, Japan). The cortical and trabecular bone samples were frozen in liquid nitrogen and crushed with pestle and mortar in order to prepare the bone powder. Finally, the weights of the collected bone powder from the cortical and trabecular bone per specimen were approximately 200 and 50 mg, respectively.
Collagen content in the bone
Collagen content in the femoral cortical and trabecular bone was calculated from the hydroxyproline (Hyp) content, which is expressed as a percentage of tissue lyophilized dry weight. An aliquot of bone powder was weighed by 3 mg (dry weight) and hydrolyzed in 6 M hydrochloric acid at 110 °C for 24 h. The amount of collagen in the bone was determined from the Hyp content measured by a high-performance liquid chromatography (HPLC) method [15]. It was assumed that collagen weighed 7.5 times the measured Hyp weight, with a molecular weight of 300,000. The resulting data were used to calculate the cross-link content as moles per mole of collagen.
Measurement of collagen cross-links
The reduction of collagen in the bone with sodium borohydride (NaBH4) (Sigma-Aldrich, St. Louis, MO, USA) and the measurement of cross-links were carried out with a previously reported method [15]. Briefly, each 3-mg (dry weight) bone powder aliquot was demineralized twice with 0.5 M EDTA in 50 mM Tris buffer, pH 7.4, at 4 °C for 96 h. Demineralized bone residues were then suspended in potassium phosphate buffer, pH 7.6 (0.15 ionic strength) and reduced at 37 °C with NaBH4. The reduced specimens were hydrolyzed in 6 N hydrochloric acid at 110 °C for 24 h. Hydrolysates were then analyzed for hydroxyproline with an HPLC system (LC9, Shimadzu, Shizuoka, Japan) fitted with a cation exchange column (0.9 × 10 cm, Aa pack-Na, JASCO, Tokyo, Japan) linked to an online fluorescence flow monitor (RF10AXL, Shimadzu, Shizuoka, Japan). The total content of AGEs was determined by the method of Saito et al. [16]. Briefly, AGE content was determined by using a fluorescence reader at 370 nm excitation and 440 nm emission (JASCO FP6200, JASCO) and normalized to a quinine sulfate standard. Total fluorescent AGE content is expressed as moles per mole of collagen.
Statistics
All statistical analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC, USA). The differences between OVX and sham at each phase or OVX and hPTH(1-34) at phase II were analyzed by t test. Differences among all groups except sham at phase III were analyzed by one-way ANOVA followed by Tukey’s honestly significant difference. Differences were considered significant for values of p < 0.05 (two-sided). All data are presented as means ± SEM.
Results
Confirmation of successful ovariectomy
The success of ovariectomy was confirmed in all OVX rabbits by clear atrophy of the uterine horns at necropsy.
Bone markers
There were no significant differences in P1NP, bALP, urinary NTX (uNTX), pentosidine, or homocysteine between the sham and OVX groups at any phase (Table 1). P1NP and bALP levels were significantly higher in the hPTH(1-34) group than in the OVX group (+50 and +19 %, respectively). On the other hand, the hPTH/RLX and hPTH/ALN groups showed no significant differences in P1NP, bALP, and uNTX levels compared with the OVX group. Pentosidine and homocysteine levels in the hPTH/RLX group were significantly lower than in the OVX group (−19 and −21 %, respectively) (Table 1).
BMD and bone structure in the distal metaphysis and diaphysis of the femur
In the femoral distal metaphysis, the sham and OVX groups showed significant difference in the total BMD at phases I and II. Total BMD was significantly high in the hPTH/RLX and hPTH/ALN groups as well as in the hPTH(1-34) group, when compared with the OVX group (Table 2). In the femoral diaphysis, the sham and OVX groups showed no difference in cortical BMD, area, thickness, periosteal surface, or endocortical surface at any phase, except cortical BMD at phase II. The cortical area in the hPTH(1-34) group was significantly larger than that in the OVX group, whereas the cortical areas in the hPTH/RLX and hPTH/ALN groups did not show any significant difference from that in the OVX group. Cortical thickness was significantly high in the hPTH/RLX and hPTH/ALN groups as well as in the hPTH(1-34) group, when compared with the OVX group. Furthermore, endocortical surface, but not periosteal surface, was significantly lower in the hPTH/RLX and hPTH/ALN groups than in the OVX group (Table 2).
Total AGE contents in collagen
In the present study, we measured the total AGEs in cortical and trabecular bone of the femur because there was more total AGEs in trabecular bone than in the cortical bone [17]. Except for the trabecular bone at phase II and the cortical bone at phase I, the total AGE contents of the trabecular and cortical bones were significantly increased in the OVX group compared with the sham group (Fig. 2). The hPTH(1-34) group showed significantly decreased trabecular and cortical total AGE contents compared with the OVX group. The trabecular total AGE contents in the hPTH/RLX group showed significantly low values when compared with both the OVX and hPTH/ALN groups. On the other hand, the cortical total AGE contents in the hPTH/RLX group showed no significant difference in comparison with both the OVX and hPTH/ALN groups.
Bone mechanical properties
There were no significant differences in the maximum load or stiffness between the sham and OVX groups at any phase, although the breaking energy in the OVX group was significantly higher than that in the sham group at phase III. The hPTH(1-34) group showed significant improvement of the maximum load compared with the OVX group. The hPTH/RLX and hPTH/ALN groups showed significantly increased maximum load compared with the OVX group. The hPTH/RLX group showed significantly increased breaking energy compared with both the OVX group and the hPTH/ALN group; on the other hand, there was no significant difference in the breaking energy between the OVX group and the hPTH/ALN group. Stiffness was significantly higher in the hPTH/ALN group than in the OVX group (Fig. 3).
Intrinsic material properties were further evaluated. Although no significant differences in maximum stress, elastic modulus, or toughness were observed between the sham group and the OVX group at phase I or II; significant differences in elastic modulus and toughness between the sham group and the OVX group were shown at phase III. The hPTH(1-34) group showed significant improvement of the maximum stress compared with the OVX group. The hPTH/RLX and hPTH/ALN group significantly improved the maximum stress compared with the OVX group. In addition, the hPTH/RLX group showed a greater increase in toughness than the OVX group and the hPTH/ALN group. Elastic modulus was significantly higher in the hPTH/ALN group than in the OVX group (Fig. 4).
Discussion
In the present study, raloxifene and alendronate were each given to OVX rabbits for 5 months after a 5-month daily hPTH(1-34) treatment, and their effects on non-enzymatic collagen cross-links, bone mass, and bone strength were directly compared.
When the OVX group was compared with the sham group, no clear evidence showing OVX-induced bone fragility was noted although the trabecular and/or cortical total AGE contents were increased in each phase and elastic modulus was decreased at phase III in the OVX group. In the results consistent with its anabolic action, hPTH(1-34) treatment increased PINP and bALP and bone formation markers and improved bone geometry and mechanical properties, as evidenced by increases in the cortical area, cortical thickness, maximum load, and maximum stress. In addition, decreased trabecular and cortical AGE contents were noted. Saito et al. [10] reported that 18-month hPTH(1-34) treatment decreased pentosidine and increased the total enzymatic cross-link content and trabecular thickness and improved structure mechanical properties in OVX monkeys. The findings obtained in the present study using rabbits were consistent with those reported in OVX monkeys.
With sequential raloxifene treatment after hPTH(1-34) treatment, increased cortical thickness, maximum load, and maximum stress were noted compared with the OVX group although increased bone formation markers were not seen. The cortical area was not increased, but the endocortical surface was decreased, and the trabecular AGE content was decreased in the hPTH/RLX group. These results indicate that bone structure and collagen quality were improved by the sequential treatment with raloxifene after hPTH(1-34). In addition to the findings seen during hPTH(1-34) treatment, decreased pentosidine and homocysteine contents and increased toughness and breaking energy were newly observed in the hPTH/RLX group. The decreased trabecular AGE content and increased toughness and breaking energy in the hPTH/RLX group were significantly different from the corresponding values in the hPTH/ALN group as well as those in the OVX group.
With sequential alendronate treatment after hPTH(1-34) treatment, increased cortical thickness, maximum load, and maximum stress and decreased endocortical surface were noted, similar to the results in the hPTH/RLX group. Moreover, increased stiffness and elastic modulus, which were not noted in the hPTH/RLX or hPTH(1-34) group, were observed in the hPTH/ALN group. In contrast to the hPTH/RLX group, no effects on non-enzymatic collagen cross-link AGE content were noted.
As the abovementioned results show, the sequential treatments with raloxifene or alendronate after hPTH(1-34) could improve overall bone geometry and biomechanical properties. However, a difference between the hPTH/RLX and hPTH/ALN groups was noted in effect on collagen cross-link content in the trabecular bone. Bone strength is defined by BMD and bone quality, and the former contributes 70 % of the bone strength in theory [18]. Collagen cross-links have a crucial role in stabilizing collagen fibers by covalently binding adjacent collagen molecules and have been reported to have a critical effect on bone quality in diabetes mellitus [8, 19] and chronic kidney disease (CKD) [20]. There are two types of collagen cross-links, enzymatic collagen cross-links and non-enzymatic collagen cross-links. Enzymatic collagen cross-links, which contain immature cross-links (dehydrodihydroxylysinonorleucine, dehydrohydroxylysinonorleucine, and dehydrolysinonorleucine) and mature cross-links (pyridinoline and deoxypyridinoline), are formed by lysyl hydroxylase (LOX) and lysyl oxidase. These physiological cross-links contribute to the strength of the collagen fibers [21] and the process of matrix calcification [8]. On the other hand, non-enzymatic collagen cross-links which contain AGEs, such as pentosidine and vesperlysine, are formed by oxidative stress and glycation. Increases in non-enzymatic collagen cross-links have been observed in people with aging [22, 23] and patients with CKD [24].
In this study, the hPTH/RLX group decreased the total AGE content in the femoral trabecular bone and decreased the pentosidine and homocysteine contents. Reductions in AGEs in collagen (e.g., pentosidine) have been observed with the administration of a number of drugs used to treat osteoporosis, including hPTH(1-34) [10] and eldecalcitol [16] in OVX monkeys, supporting the suggestion that non-enzymatic collagen cross-links have an impact on bone quality. In addition, in this study, the hPTH/RLX group increased bone toughness. This increase in bone toughness was consistent with findings in rabbits showing that raloxifene ameliorated the reduction in bone toughness as well as the decrease in enzymatic cross-links and increase in non-enzymatic AGE cross-links caused by hyperhomocysteinemia [11]. Therefore, we considered that reduction in AGEs may contribute to the amelioration of bone mechanical properties. Nojiri et al. [25] reported that superoxide dismutase (SOD) deficiency-induced oxidative stress led to the accumulation of AGEs in bones. Several reports have described the relationship between estrogen and oxidative stress. Estradiol treatment has been reported to restore SOD activity after the reduction due to ovariectomy-induced estrogen deficiency [26]. Raloxifene ameliorated hyperhomocysteinemia, which is known to increase oxidative stress marker levels (malondialdehyde and protein carbonyl) and to accelerate accumulation of AGEs [27]. Raloxifene also suppressed vascular reactive oxygen species in rats [28]. Thus, raloxifene may decrease AGE accumulation in collagen through the reduction of oxidative stress. Saito et al. [11] reported that raloxifene increased enzymatic cross-links in bones. Accordingly, we considered that an increase in enzymatic cross-links due to raloxifene treatment may have been additionally involved in improved biomechanical properties in this study. In addition, raloxifene-induced improvements of collagen spacing [29] and bone hydration [30] might also contribute to an improvement of intrinsic biomechanical properties.
On the other hand, in this study, since the hPTH(1-34)/ALN group showed a similar level of total AGE content in collagen to the OVX group, it was considered that the sequential treatment with alendronate after hPTH(1-34) might not improve AGE content. Bisphosphonates such as alendronate and risedronate clearly increased the pentosidine content in collagen at the clinical dose in dogs [31], whereas other reports indicated a five times higher than clinical doses, but not clinical doses, of alendronate and risedronate increased AGEs content in collagen [32]. From these data, we cannot exclude the possibility that the dose of alendronate in this study (100 μg/kg/twice a week) may be excessive compared with the clinical dose.
However, there are four limitations to the interpretation of the results of the present study. Firstly, we used the OVX rabbits since they are the smallest species known to have Haversian bone remodeling processes similar to humans and since they had been used for an evaluation of the effects of raloxifene on collagen cross-links and bone strength, as mentioned in the “Introduction” section [11]. Castañeda et al. [33] reported that OVX alone may induce osteopenia in rabbits. Consistent with that report, OVX did not induce clear femoral cortical bone fragility, including a decrease in cortical BMD, in the present study. Breaking energy and toughness in the OVX groups showed higher values than in the sham group at 13 months after ovariectomy. It is not clear why ovariectomy could increase breaking energy and toughness because this is the first report that observed the long-term effect of ovariectomy on bone strengths in rabbits. However, since the value of P1NP and bALP in the OVX groups was 20 % higher than in the sham group without an increase in uNTX at phase III, the acceleration of bone formation might provide benefit for bone strength. In addition, water in the bone is known as one of the important factors in bone strength. Dehydration increased stiffness but decreased energy absorbed to failure without significant change in maximum stress in human femoral cortical bone [34, 35]. Furthermore, a recent report indicated not only a positive correlation between the amount of collagen-bound water and bone toughness but also a negative correlation between the amount of mineral-bound water and bone modulus in bovine femoral cortical bone [36]. Since the OVX group at phase III showed a decrease in elastic modulus but an increase in toughness in femoral cortical bone, it is speculated that the OVX group might have more mineral- and collagen-bound water in the cortical bone compared with the sham group at phase III. Further studies will be needed to elucidate the mechanism of this phenomenon. Moreover, BMD in the diaphysis of the femur was not affected by daily treatment with hPTH(1-34) in contrast to BMD in the distal metaphysis of the femur in this study. Thus, OVX rabbits may have some limitations as a disease model for osteoporosis, especially with regard to detection of effects on cortical BMD and bone strength. We also examine the influence of time points and/or estrogen status on bone-related parameters such as AGEs, bone strength, and QCT parameters. From the result of the two-factor ANOVA (Supplemental Data 1), time points in those parameters were more critical than estrogen status. Since Brown et al. reported that they confirmed spontaneous annual variations in endocrine gland weights in rabbits [37], there might also be the variations in bone-related parameters. These data suggested that the influence of age and/or season on the bone-related parameters should be carefully considered when using rabbits. Secondly, we set a vehicle-injected OVX group which did not receive hPTH(1-34) for comparisons with the hPTH/RLX and hPTH/ALN groups; we did not set a group to receive vehicle after the 5-month hPTH(1-34) treatment. In the present study, increases in thickness, maximum load, and maximum stress were noted with the hPTH/RLX and hPTH/ALN; and furthermore, a decrease in trabecular AGE content was noted with the hPTH/RLX as well as the hPTH(1-34) group, but it is difficult to mention that raloxifene or alendronate maintained, improved, or worsened those improvements by hPTH(1-34) therapy. Thirdly, the dose of ALN (100 μg/kg/twice a week) was threefold lower than that in the previous report which set ALN group as a positive control [13]. In the previous report, does-finding study of ALN was performed in OVX rabbits and the result showed that both of the 100 and 300 μg/kg/twice a week doses had same efficacy on lumbar spine BMD [13], but it is unknown whether the both doses indicate the same efficacy on the long bone BMD, bone markers, and so on. Thus, the effect of ALN (100 μg/kg/twice a week) on bone-related parameters may not reach the full efficacy in this study. Fourthly, we did not confirm demineralization of bone powder in this study. The method of demineralization in the trabecular and cortical bone was reported in our previous studies [15, 16, 38, 39]. In those studies, the degree of the mineralization (calcium and phosphorus content) was measured using atomic emission spectroscopy (ICP-AES, Nippon Jarrell-Ash Co., Ltd., Kyoto, Japan) and then it was confirmed that the bone specimens were completely demineralized. Since we adopted the same method in this study, we did not measure the content of calcium and phosphorus content in each bone powder.
In conclusion, sequential treatment with raloxifene and alendronate after daily hPTH(1-34) treatment improved the overall bone geometry and bone strength parameters including biomechanical properties. Furthermore, the sequential treatment with raloxifene improved bone quality markers such as AGE content in collagen and serum levels of homocysteine and pentosidine. These results suggest that sequential treatments with hPTH(1-34) and antiresorptive drugs (raloxifene and alendronate) have a beneficial effect on biomechanical properties in OVX rabbits.
References
Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD (2003) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 18:1051–1056
Dobnig H, Stepan JJ, Burr DB, Li J, Michalská D, Sipos A, Petto H, Fahrleitner-Pammer A, Pavo I (2009) Teriparatide reduces bone microdamage accumulation in postmenopausal women previously treated with alendronate. J Bone Miner Res 24:1998–2006
Misof BM, Paschalis EP, Blouin S, Fratzl-Zelman N, Klaushofer K, Roschger P (2010) Effects of 1 year of daily teriparatide treatment on iliacal bone mineralization density distribution (BMDD) in postmenopausal osteoporotic women previously treated with alendronate or risedronate. J Bone Miner Res 25:2297–2303
Shane E, Burr D, Ebeling PR et al (2010) Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 25:2267–2294
Papapoulos S, Lippuner K, Roux C, Lin CJ, Kendler DL, Lewiecki EM, Brandi ML, Czerwiński E, Franek E, Lakatos P, Mautalen C, Minisola S, Reginster JY, Jensen S, Daizadeh NS, Wang A, Gavin M, Libanati C, Wagman RB, Bone HG (2015) The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM extension study. Osteoporos Int 26:2773–2783
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Glüer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators JAMA 282:637–645
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Saito M, Marumo K (2010) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int 21:195–214
Saito M, Fujii K, Mori Y, Marumo K (2006) Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int 17:1514–1523
Saito M, Marumo K, Kida Y, Ushiku C, Kato S, Takao-Kawabata R, Kuroda T (2011) Changes in the contents of enzymatic immature, mature, and non-enzymatic senescent cross-links of collagen after once-weekly treatment with human parathyroid hormone (1-34) for 18 months contribute to improvement of bone strength in ovariectomized monkeys. Osteoporos Int 22:2373–2383
Saito M, Marumo K, Soshi S, Kida Y, Ushiku C, Shinohara A (2010) Raloxifene ameliorates detrimental enzymatic and nonenzymatic collagen cross-links and bone strength in rabbits with hyperhomocysteinemia. Osteoporos Int 21:655–666
Tashjian AH Jr, Chabner BA (2002) Commentary on clinical safety of recombinant human parathyroid hormone 1-34 in the treatment of osteoporosis in men and postmenopausal women. J Bone Miner Res 17:1151–1161
Pennypacker BL, Duong LT, Cusick TE, Masarachia PJ, Gentile MA, Gauthier JY, Black WC, Scott BB, Samadfam R, Smith SY, Kimmel DB (2011) Cathepsin K inhibitors prevent bone loss in estrogen-deficient rabbits. J Bone Miner Res 26:252–262
Hirano T, Burr DB, Turner CH, Sato M, Cain RL, Hock JM (1999) Anabolic effects of human biosynthetic parathyroid hormone fragment (1-34), LY333334, on remodeling and mechanical properties of cortical bone in rabbits. J Bone Miner Res 14:536–545
Saito M, Marumo K, Fujii K, Ishioka N (1997) Single-column high-performance liquid chromatographic-fluorescence detection of immature, mature, and senescent cross-links of collagen. Anal Biochem 253:26–32
Saito M, Grynpas MD, Burr DB, Allen MR, Smith SY, Doyle N, Amizuka N, Hasegawa T, Kida Y, Marumo K, Saito H (2015) Treatment with eldecalcitol positively affects mineralization, microdamage, and collagen crosslinks in primate bone. Bone 73:8–15
Karim L, Tang SY, Sroga GE, Vashishth D (2013) Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone. Osteoporos Int 24:2441–2447
Osteoporosis Prevention, Diagnosis, and Therapy (2000) NIH consensus statement 17:1–45
Tomasek JJ, Meyers SW, Basinger JB, Green DT, Shew RL (1994) Diabetic and age-related enhancement of collagen-linked fluorescence in cortical bones of rats. Life Sci 55:855–861
Iwasaki Y, Kazama JJ, Yamato H, Fukagawa M (2011) Changes in chemical composition of cortical bone associated with bone fragility in rat model with chronic kidney disease. Bone 48:1260–1267
Depalle B, Qin Z, Shefelbine SJ, Buehler MJ (2015) Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J Mech Behav Biomed Mater 52:1–13
Wang X, Shen X, Li X, Agrawal CM (2002) Age-related changes in the collagen network and toughness of bone. Bone 31:1–7
Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, Federici A (2005) Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci 1043:710–717
Mitome J, Yamamoto H, Saito M, Yokoyama K, Marumo K, Hosoya T (2011) Nonenzymatic cross-linking pentosidine increase in bone collagen and are associated with disorders of bone mineralization in dialysis patients. Calcif Tissue Int 88:521–529
Nojiri H, Saita Y, Morikawa D, Kobayashi K, Tsuda C, Miyazaki T, Saito M, Marumo K, Yonezawa I, Kaneko K, Shirasawa T, Shimizu T (2011) Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J Bone Miner Res 26:2682–2694
Guerra RC, Zuñiga-Muñoz A, Guarner Lans V, Díaz-Díaz E, Tena Betancourt CA, Pérez-Torres I (2014) Modulation of the activities of catalase, cu-zn, mn superoxide dismutase, and glutathione peroxidase in adipocyte from ovariectomised female rats with metabolic syndrome. Int J Endocrinol 2014:175080
Mori H, Okada Y, Kishikawa H, Inokuchi N, Sugimoto H, Tanaka Y (2013) Effects of raloxifene on lipid and bone metabolism in postmenopausal women with type 2 diabetes. J Bone Miner Metab 31:89–95
Wassmann S, Laufs U, Stamenkovic D, Linz W, Stasch JP, Ahlbory K, Rösen R, Böhm M, Nickenig G (2002) Raloxifene improves endothelial dysfunction in hypertension by reduced oxidative stress and enhanced nitric oxide production. Circulation 105:2083–2091
Newman CL, Creecy A, Granke M, Nyman JS, Tian N, Hammond MA, Wallace JM, Brown DM, Chen N, Moe SM, Allen MR (2016) Raloxifene improves skeletal properties in an animal model of cystic chronic kidney disease. Kidney Int 89:95–104
Gallant MA, Brown DM, Hammond M, Wallace JM, Du J, Deymier-Black AC, Almer JD, Stock SR, Allen MR (2014) Burr DB (2014) bone cell-independent benefits of raloxifene on the skeleton: a novel mechanism for improving bone material properties. Bone 61:191–200
Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD (2008) Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int 19:329–337
Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D (2009) Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int 20:887–894
Castañeda S, Calvo E, Largo R, González-González R, de la Piedra C, Díaz-Curiel M, Herrero-Beaumont G (2008) Characterization of a new experimental model of osteoporosis in rabbits. J Bone Miner Metab 26:53–59
Evans FG, Lebow M (1951) Regional differences in some of the physical properties of the human femur. J Appl Physiol 3:563–572
Sedlin ED, Hirsch C (1966) Factors affecting the determination of the physical properties of femoral cortical bone. Acta Orthop Scand 37:29–48
Unal M, Akkus O (2015) Raman spectral classification of mineral- and collagen-bound water’s associations to elastic and post-yield mechanical properties of cortical bone. Bone 81:315–326
Brown WH, Pearce L, Van Allen CM (1926) The occurrence and trend of spontaneous variations in organ weights of normal rabbits. J Exp Med 44:653–666
Saito M, Fujii K, Soshi S, Tanaka T (2006) Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int 17:986–995
Saito M, Fujii K, Marumo K (2006) Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcif Tissue Int 79:160–168
Acknowledgments
The authors acknowledge Shin Nippon Biomedical Laboratories, Ltd., for the assistance with the preparation of the manuscript and Soshi Nagaoka (Eli Lilly Japan K.K.) for performing the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Eli Lilly Japan K.K.
Conflicts of interest
Shuichi Kimura and Yoshitaka Isaka are employees of Eli Lilly Japan K.K. Mitsuru Saito received research grants and/or consulting or speaking fees from Pfizer Inc., Eli Lilly, Chugai, Dai-ichi Sankyo, Asahikasei Pharma, Astellas Pharma, Taisho Toyama Pharma, Teijin Pharma, and Ono Pharma. Yoshikuni Kida, Azusa Seki, and Keishi Marumo declare that they have no conflict of interest.
Electronic supplementary material
Supplemental Data 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Kimura, S., Saito, M., Kida, Y. et al. Effects of raloxifene and alendronate on non-enzymatic collagen cross-links and bone strength in ovariectomized rabbits in sequential treatments after daily human parathyroid hormone (1-34) administration. Osteoporos Int 28, 1109–1119 (2017). https://doi.org/10.1007/s00198-016-3812-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3812-3