Abstract
Summary
The aim of the study was to investigate prospectively whether the levels of urinary pentosidine could predict fractures in postmenopausal women from the OFELY cohort. The results of the study suggest that urine pentosidine concentration is not an independent risk factor for fractures in postmenopausal women from a French cohort.
Introduction
Pentosidine has been described as an independent risk factor for hip and vertebral fracture in postmenopausal Japanese women. We investigated the prediction of urinary pentosidine on all fragility fracture risk in healthy untreated postmenopausal women from the OFELY cohort.
Methods
Urinary pentosidine was assessed at baseline in 396 healthy untreated postmenopausal women aged 63.3 ± 8.4 years from the OFELY cohort using high-performance liquid chromatography method. Incident clinical fractures were recorded during annual follow-up and confirmed by radiographs, and vertebral fractures were assessed on radiographs performed every 4 years. Multivariate Cox’s regression analysis was used to calculate the risk of urinary pentosidine levels after adjustment for age, prevalent fractures, and total hip bone mineral density (BMD).
Results
During a mean follow-up of 10 years, 88 of the 396 postmenopausal women have undergone incident vertebral (n = 28) and peripheral (n = 60) fractures. Fracture risk was higher in postmenopausal women with pentosidine in the highest quartile (p = 0.02), but it did not remain significant after adjustment for age, BMD, and prevalent fracture.
Conclusions
Urine pentosidine concentration is not an independent risk factor of osteoporotic fracture in healthy postmenopausal women from the OFELY cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low bone mass is a major predictor of osteoporotic fracture risk, but both bone density and bone quality affect bone strength [1]. Thus, many osteoporotic fractures occur among women with a bone mineral density (BMD) level above the diagnostic threshold of osteoporosis defined as a T-score of −2.5 SD [2, 3]. Some other determinants including clinical factors such as age, changing endocrine pattern, genetic background, lifestyle factors (dietary calcium, exercise, smoking, alcohol abuse), body weight, prevalent fractures, glucocorticoid treatment, muscle function, and propensity to fall may have significant influence on bone fragility [3–7]. The material and structural component of bone determine the bone strength independently of BMD, and their impairment results in bone fragility and an increase in fracture rate [8, 9]. Biochemical markers of bone turnover, especially those reflecting bone resorption rate such as serum type I collagen C-telopeptide (CTX) [10] and urinary type I collagen N-telopeptides (NTX) [10, 11], serum bone alkaline phosphatase (BAP) [11], undercarboxylated osteocalcin [12], IGF-1 [13], and 17βestradiol [14], have been reported to be associated with the risk of fracture independently of age and BMD.
Pentosidine, one of the most widely studied advanced glycation end products (AGEs), is a senescent nonenzymatic crosslink due to a spontaneous interaction between arginine and lysine amino acids and free sugars [15]. Pentosidine measurement is straightforward and represents the whole AGEs that are formed in vivo. Levels of AGEs are generally higher with aging and particularly in tissues characterized by a low turnover [16–18]. Pentosidine has been shown to accumulate with age in cortical bone of human femur [19]. Accumulation of AGEs in bone collagen matrix has been associated with an impairment in the mechanical properties of cortical and trabecular bone [20, 21]. Thus, Saito et al. has suggested that excessive formation of pentosidine in cortical and trabecular bone is connected to an impairment of bone quality in osteoporotic patients with hip fracture [22]. Serum and urinary pentosidine concentrations increase significantly with age [23, 24]. Menopause has no effect on serum pentosidine levels [24]. It has been shown that pentosidine levels are significantly increased in serum of osteoporotic patients—diagnosed by densitometry and without information on fracture—compared to age-matched healthy subjects [25]. In addition, Shiraki et al. has reported that a high level of urinary pentosidine was an independent risk factor for osteoporotic vertebral fractures in a 5-year prospective study in Japanese women [26].
The aim of this study was to investigate prospectively the levels of urinary pentosidine in the prediction of all types of fragility fractures in postmenopausal women in a French cohort and to determine whether pentosidine is an independent risk factor of osteoporosis.
Materials and methods
Participants
We investigated 396 healthy untreated postmenopausal women (women were considered postmenopausal if they had not been menstruating for at least 1 year at the entry of the study) who had been enrolled in a prospective study of the determinants of bone loss (OFELY study). The whole cohort comprised 1,039 Caucasian women, 31–89 years of age, randomly selected from the regional section of a health insurance company (Mutuelle Générale de l’Education Nationale) from the Rhône district (i.e., Lyon and its surroundings in France) with an annual follow-up. Written informed consent was obtained from each woman, and the study was approved by the local ethical committee. The OFELY cohort has been described elsewhere [27, 28]. Women from this cohort were followed during a mean 10.1 ± 2.6 years. Among the 672 postmenopausal women recruited at baseline, 248 women were excluded because of the presence of treatment or disease that could influence bone metabolism (hormone replacement therapy, tamoxifen, fluoride bisphosphonates, calcitonin, thyroid hormones, corticosteroid, Paget’s disease of bone, primary hyperparathyroidism, hyperthyroidism, cirrhosis, or diabetes mellitus), 18 women because of renal impairment (creatinine clearance < 30 ml/min), and ten women because either pentosidine or creatinine level was not available, thus, leaving 396 women for this analysis.
Pentosidine measurement
For each woman, first morning void urine was collected at baseline and stored at −70°C until assayed. Pentosidine stability in frozen urine was evaluated by its measurement in five successive follow-ups. No indication of pentosidine degradation was displayed in the oldest frozen samples. Pentosidine was measured by high-performance liquid chromatography according to previously published methods with some modifications [29]. Urine samples were pretreated on SPE Chromabond® Crosslinks columns (Macherey Nagel GmbH a Co.KG, Düren, Germany) to remove interfering fluorophores. Briefly, 200 µl of urine sample were added to 1.8 ml of buffer composed of acetonitrile and acetic acid in a 8:1 ratio (v/v), respectively. Interfering fluorophores were removed by washing the column with 10 mL of a solution containing acetonitrile, acetic acid, and water (8–1–1), respectively. Pentosidine was eluted with 600 µL of 1% n-hepafluorobutyric acid (HFBA) and then separated on an Atlantis dC18, 3 µm, 4.6 × 100 mm reversed phase column protected by an Atlantis dC18, 3 µm 4.6 × 20 mm guard cartridge (Waters Corp., Milford, MA, USA) at a flow rate of 1.2 ml/ min with an isocratic elution of 12% acetonitrile in 0.12% of HFBA and quantified by fluorimetry. Effluent was monitored for fluorescence at an emission of 385 nm and an excitation of 335 nm. The amount of pentosidine was quantified using a synthetic standard previously calibrated with a calibrator of pentosidine generously gifted by Dr. Masaaki Takahashi (Hamamatsu University School of Medicine, Shizuoka, Japan). The within- and between-run imprecision were less than 1% and 5%, respectively. The pentosidine recovery rate was 93 ± 4%, and the assay was linear over the validated amount range of 0–0.5 nmol with a detection limit <0.02 pmol. Urinary pentosidine data were corrected by the urinary creatinine (Cr) concentration measured by a standard colorimetric method. Creatinine stability was checked by remeasurement of 20 urine samples already measured at the beginning of the OFELY study. Creatinine levels were not significantly changed despite urine sample long-freezing period.
Biochemistry
As previously described, bone resorption was evaluated in urine at baseline with an enzyme-linked immunosorbent assay of NTX and in serum with an enzyme-linked immunosorbent assay of CTX breakdown products [11]. The within- and between-run imprecision were less than 10% for NTX assay and 5% and 8%, respectively, for CTX assay. Serum BAP was measured with an immunoradiometric assay using two monoclonal antibodies directed against the human bone isoenzyme and bone phosphatase purified from human SAOS-2 osteosarcoma cells as a standard (OstaseTM, Hybritech, inc., San Diego, CA, USA) [11]. Serum IGF-1 was measured by radioimmunoassay after acid ethanol extraction (Diagnostic Systems Laboratories, Webster, TX, USA) [13]. The within- and between-run imprecision were less than 10% for both assay. Serum creatinine was measured by standard laboratory methods. Creatinine clearance was calculated using the Cockroft equation.
Fracture assessment
Prevalent fractures were those that occurred after the age of 40 years and were identified at baseline by questionnaire. Incident fractures were diagnosed by a questionnaire at each annual follow-up. For women who did not come to the clinical center, a letter was sent every year to identify the occurrence of fractures. Peripheral fractures were assessed by radiographs or by surgical reports. Only low-trauma fractures (i.e., those occurring after falls from standing height or less) were taken into account. Vertebral fractures were identified on lateral X-ray films of the thoracic and lumbar spine according to the semiquantitative method of Genant [30]. The clinical vertebral fractures (radiographically confirmed) were collected in the annual questionnaire, whereas vertebral fractures that did not reach clinical attention were assessed on the X-ray films performed every 4 years.
Clinical and physical evaluation
At the initial screening women were submitted to a detailed clinical evaluation, as described previously [5]. Physical activity was assessed by a score calculated from sport or recreation, job, and home activity as described previously [5].
Bone density
BMD was measured at baseline at the lumbar spine (L1–4) and total hip by DXA with a QDR 2000 device (Hologic Inc., Waltham, MA, USA) with a short-term CV of 0.9% and 1%, respectively.
Statistical analyses
Owing to a skewed distribution, pentosidine concentrations were analyzed as quartile and as continuous, natural log-transformed values. First, comparisons of baseline characteristics according to fracture groups were made using ANOVA, for continuous variables, and Chi-squared tests for categorical variables. Then, Spearman’s correlations were calculated to determine relationships between pentosidine and potential confounders at baseline. A Cox proportional hazards model based on time to first fracture was used to evaluate fracture risk for the highest quartile compared with the three lowest quartiles (or or 1 log pentosidine increase). We have tested the proportional hazard assumption with a model including prevalent fracture, hip BMD T-score, pentosidine, and time interaction with covariables showed p = 0.32 for the proportionality test. Results were expressed as hazard ratios (HR) with 95% confidence interval. Age adjustment and multivariate analysis were done to take into account potential confounders. The multivariate analysis included age, prevalent fractures, and hip T-score. All statistical analyses were performed using the Statistical Analysis Software (SAS V9.1; SAS Institute, Cary, NC, USA).
Results
During the 10.1 ± 2.6 years follow-up, 88 women have undergone incident osteoporotic fractures. Only the first incident fracture was taken into account, including 28 vertebral fractures and 60 peripheral fractures: wrist (n = 20), hip (n = 8), humerus (n = 5), and other sites (n = 27; rib, ankle, patella, metatarsi, femur, knee, sacrum, elbow, clavicle, scapulae) within the postmenopausal cohort.
Baseline characteristics of postmenopausal women with and without incident fragility fracture are given in Table 1. Women who had fractures during the study were 4 years older, had significantly lower BMD, IGF-I levels, and creatinine clearance, and had significantly more prevalent fractures than those without incident fracture. On the other hand, body mass index (BMI), serum creatinin, and bone resorption markers levels were comparable in the two groups. In addition, women who had osteoporotic incident fractures during the follow-up had significantly higher urinary pentosidine levels than the others (p = 0.01).
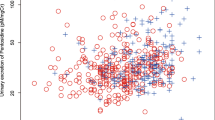
Mean pentosidine was 110.8 ± 32.4 pmol/mg Cr, and there was a significant correlation between age and natural log-transformed values of urinary pentosidine (log –Pen = 6.75–0.077 × age + 0.001 × age2, r 2 = 0.2, p < 0.0001; Fig. 1).
After adjustment for age, pentosidine levels were no longer significantly correlated with BMI, body weight, body height, and BMD (Table 2). In addition, there was no significant correlation between pentosidine levels and bone resorption markers, serum creatinine, and IGF-1 levels as well as renal creatinine clearance (Table 2).
The mean value of log-transformed pentosidine among women with prevalent fracture was significantly higher than that of women without prevalent fracture (p < 0.001), but this did remain significant after age adjustment (p = 0.29).
The mean concentrations in urinary pentosidine were 80.8 ± 6.9, 97.1 ± 4.3, 114.8 ± 5.9, and 157.2 ± 33.7 pmol/mg Cr from the lowest (Q1) to the highest quartile (Q4). There were 13 fractures (four vertebral and nine peripheral), 23 (five vertebral and 18 peripheral), 23 (ten vertebral and 13 peripheral), and 29 (nine vertebral and 20 peripheral), respectively, from the lowest to the highest quartile. Figure 2 shows the incidence of vertebral and peripheral fractures and time of follow-up according to quartiles of pentosidine in urine. The fracture rate was significantly (p = 0.02) higher in the highest quartile (Q4) than in the lowest quartile of urinary pentosidine (Q3 to Q1). The incident fracture rate was the lowest in Q1. Rates were comparable in Q2 and Q3. In addition, age increased significantly across those quartiles from 59.8 ± 6.1 in the lowest to 68.4 ± 9.8 in the highest quartile (p < 0.0001).
The HR for 1 log pentosidine increment was 2.65 (95% CI, 1.23–5.76, p = 0.01). However, after adjustment for age, prevalent fracture, and BMD, high levels of urinary pentosidine were no longer associated with an increased fracture risk (p = 0.62; Table 3). After 10 years of follow-up, low levels of serum IGF-I (1 log decrease) at baseline were associated with an increased fracture risk after age adjustment with a HR of 0.45 (95% CI, 0.2–0.997), p = 0.049, whereas high values of serum BAP only tended to be associated with fracture risk with a HR of 1.63 (95% CI, 0.96–2.80), p = 0.07. High value of Serum CTX and urinary NTX were not significant predictors of fracture risk.
When our analysis was restricted to 5 years follow-up, 46 women had sustained incident osteoporotic fractures. Among them, there was no significant association between pentosidine levels and fracture risk with a HR of 1.7 (95%CI 0.23–12.8), p = 0.61. In addition, we found that the highest quartile of serum BAP was a significant predictor of fracture risk with a HR of 2.9 (95% CI, 1.5–5.5), p = 0.02. In the present analysis, at 5 years of follow-up, high levels of urinary NTX and serum CTX were not significantly associated with risk of fracture.
Discussion
In the present study, we found that increased levels of urinary pentosidine were not significantly associated with increased risk of all fragility fracture after adjusting for age, BMD, and prevalent fracture in a large prospective cohort of healthy untreated postmenopausal women from 50 to 89 years.
Pentosidine is one of the well-characterized AGEs in humans. Its assessment in tissues and biological fluids has been greatly facilitated by its acid stability and its natural fluorescent properties. Pentosidine accumulates with age and diabetes, predominantly in long-lived molecules such as collagen, in a variety of normal and diabetic human tissues including skin, tracheal cartilage, bone, aorta, cardiac muscle, lung, liver, and kidney [31]. In blood, pentosidine is mainly associated with plasma proteins and is markedly elevated with kidney failure [32]. Pentosidine levels in urine and serum has been also regarded as a potential biomarker in the assessment of chronic age-related diseases such as diabetes mellitus, rheumatic disease, atherosclerosis, and renal failure [33–36].
Shiraki et al. have recently shown in a 5-year prospective study among Japanese postmenopausal women from the Nagano cohort that urine pentosidine levels were positively associated with the presence of vertebral fractures independently of others fractures risks such as prevalent fracture, BMD, or age [26]. They have reported a significant HR for incident fracture of 1.33 (95%CI, 1.01–1.76, p = 0.04) comparing the highest quartile of urinary pentosidine versus the three others. In the Shiraki study, it should be noticed that 16.7% of postmenopausal women underwent incident vertebral fractures after 5 years of follow-up when 8.6% of postmenopausal women had pre-existing vertebral fractures. In comparison, the proportion of incident vertebral fractures was only of 4.3 after 5 years of follow-up in our study when 7.4% of postmenopausal women had prevalent vertebral fractures.
Thus, in the present study, we have decided to analyze the predictive value for all types of bone fragility fractures including vertebral and other peripheral sites of fractures during a longer follow-up, and we have found that high levels of urinary pentosidine were not significantly associated with the incident fracture rate after adjusting for age, BMD, and prevalent fracture. When we have limited our analyses to 5 years of follow-up, results were unchanged. As in the Shiraki study, urinary pentosidine levels were higher in postmenopausal women with prevalent fractures than women without fracture, but this was no longer significant after age adjustment. In our study, after 5 and 10 years of follow-up, low serum IGF-1 predicts the risk of fracture independently of other risk factors confirming data previously published for postmenopausal women from OFELY cohort followed during 5 years [14]. Our data show that high serum BAP level predicts the risk of fracture after 5 years of follow-up as previously published but is no longer significant after 10 years of follow-up [11]. In addition, we could not find a significant association between serum CTX and the risk of fracture after 5 and 10 years of follow-up as previously reported for postmenopausal women from OFELY cohort followed during 5 years [11]. At 5 years of follow-up, we find a HR in the highest quartile of 1.7 (95% CI 0.83–3.6), p = 0.14. The discrepancy between the two studies could be explained by a smaller number of women with and without incident fracture in the present analysis (46 and 350, respectively) compared to the prior published analysis (55 and 380, respectively), as the point estimate is similar to that previously reported but with wider confidence interval. Garnero et al. have shown that most postmenopausal women with high bone turnover at baseline were similarly classified 4 years later [37], suggesting that the absence of prediction by bone markers after 10 years of follow-up does not depend on a change in bone turnover rate. In addition, BMD is still a major predictor of fracture risk after 10 years of follow up, but the effect is diminished during the course of long-term follow-up probably diluted by other risk factors. Taken together, those data indicate that the long-term prediction of fracture using biological variables is less strong than over the first years [38–40].
Among the baseline characteristics of the two populations, age was similar, OFELY postmenopausal women were slightly taller and bigger, and their lumbar spine BMD and urinary NTX levels were slightly lower than that of the Nagano postmenopausal women. However, urinary levels of pentosidine were 2.6 times higher in the OFELY than in the NAGANO postmenopausal women (110.8 + 32.4 versus 42.6 + 17.7, respectively).
Different factors could explain these discrepancies. Differences between pentosidine concentrations obtained in the two cohorts could stem from two HPLC methods using their own calibrators which have not been standardized. In addition, pentosidine is not specific of bone tissue, and its urinary excretion reflects only in part bone turnover, so it lacks specificity as a bone marker. Consequently, the influence of others tissues and nutrition habits among the two populations should be taken into consideration. Indeed, it has been shown that caloric restriction decreases age-dependent accumulation of pentosidine in tissues [41, 42] and that serum and urinary pentosidine excretion is significantly influenced by food dietary intake [43]. In addition, studies have suggested that the kidney plays a critical role in pentosidine disposal. In plasma, its half life is directly related to renal function and pentosidine accumulates in renal failure [44]. In rats, it has been shown that pentosidine is filtered through the glomeruli and reabsorbed in the proximal tubules where it is modified or degraded to be eventually cleared in the urine, and intact pentosidine represents only 20–30% of the urine metabolites [45]. We have excluded from our study women with renal failure (creatinine clearance < 30 ml/min). In our population, after age adjustment, creatinine clearance was not associated with pentosidine levels. Unfortunately, we could not compare the renal function between the two cohorts as these data were not reported in Shiraki’s study.
Our study has some limitations. Despite a long follow-up, we had relatively few vertebral and peripheral fractures as the OFELY women are healthy and relatively young. Pentosidine concentrations were measured at baseline in urine samples which were kept frozen since the beginning of the study. The effect of nutrition on pentosidine content in tissues and its urinary excretion levels has not been taken into account when the study was set up. Indeed, our results are based on a single measurement of urinary pentosidine which may not accurately reflect the bone turnover status because of the presence of pentosidine in the other tissues and the possible variability in its measurement due to dietary intake of glycation compounds and to pentosidine renal catabolism.
In conclusion, we could not confirm that the urinary pentosidine level is an independent risk factor of fragility fractures in healthy postmenopausal women over the age of 50 years. Pentosidine appears to be a marker of frailty reflecting health, nutritional state, and renal impairment which is significantly associated to aging. Its measurement could provide some information concerning mechanisms leading to bone fragility but may be of limited clinical interest in the assessment of fracture risk.
References
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM (2001) Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 286:2815–2822
Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JP, Pols HA (2004) Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 34:195–202
Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD (2005) Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res 20:1813–1819
Albrand G, Munoz F, Sornay-Rendu E, DuBoeuf F, Delmas PD (2003) Independent predictors of all osteoporosis-related fractures in healthy postmenopausal women: the OFELY study. Bone 32:78–85
Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM (1995) Risk factors for hip fracture in white women. Study of osteoporotic fractures research group. N Engl J Med 332:767–773
Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35:375–382
Seeman E, Delmas PD (2006) Bone quality—the material and structural basis of bone strength and fragility. N Eng J Med 354:2250–2261
Chavassieux P, Seeman E, Delmas PD (2007) Insights into material and structural basis of bone fragility from diseases associated with fractures: how determinants of the biomechanical properties of bone are compromised by disease. Endocrine Rev 28:151–164
Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, Cormier C, Breart G, Meunier PJ, Delmas PD (1996) Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res 11:1531–1538
Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD (2000) Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 15:1526–1536
Vergnaud P, Garnero P, Meunier PJ, Bréart G, Kamihagi K, Delmas PD (1997) Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: the EPIDOS Study. J Clin Endocrinol Metab 82:719–724
Garnero P, Sornay-Rendu E, Delmas PD (2000) Low serum IGF-1 and occurrence of osteoporotic fractures in postmenopausal women. Lancet 355:898–899
Chapurlat RD, Garnero P, Bréart G, Meunier PJ, Delmas PD (2000) Serum estradiol and sex hormone-binding globulin and the risk of hip fracture in elderly women: the EPIDOS study. J Bone Miner Res 15:1835–1841
Sell DR, Monnier VM (1989) Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem 264:21597–21602
Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR, Baynes JW (1993) Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest 91:2463–2469
Verzijl N, DeGroot J, Oldehinkel E, Bank RA, Thorpe SR, Baynes JW, Bayliss MT, Bijlsma JW, Lafeber FP, Tekoppele JM (2000) Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J 350:381–387
Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, Federici A (2005) Advanced glycation end products and bone loss during aging. Ann NY Acad Sci 1043:710–717
Saito M, Marumo K, Fujii K, Ishioka N (1997) Single-column high-performance liquid chromatographic-fluorescence detection of immature, mature, and senescent cross-links of collagen. Anal Biochem 253:26–32
Garnero P, Borel O, Gineyts E, Duboeuf F, Solberg H, Bouxsein ML, Christiansen C, Delmas PD (2006) Extracellular posttranslational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone 38:300–309
Viguet-Carrin S, Roux JP, Arlot ME, Merabet Z, Leeming DJ, Byrjalsen I, Delmas PD, Bouxsein ML (2006) Contribution of the advanced glycation endproduct pentosidine and of maturation of type I collagen to compressive biomechanica properties of human lumbar vertebrae. Bone 39:1073–1079
Saito M, Fujii K, Soshi S, Tanaka T (2006) Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int 17:986–995
Yoshihara K, Nakamura K, Kanai M, Nagayama Y, Takahashi S, Saito N, Nagata M (1998) Determination of urinary and serum pentosidine and its application to elder patients. Biol Pharm Bull 21:1005–1008
Takahashi M, Oikawa M, Nagano A (2000) Effect of age and menopause on serum concentrations of pentosidine, an advanced glycation end product. J Gerontol A Biol Sci Med Sci 55:137–140
Hein G, Wiegand R, Lehmann G, Stein G, Franke S (2003) Advanced glycation end-products pentosidine and Nε-carboxymethyllysine are elevated in serum of patients with osteoporosis. Rheumatology 42:1242–1246
Shiraki M, Kuroda T, Tanaka S, Saito M, Fukunaga M, Nakamura T (2008) Nonenzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures. J Bone Miner Metab 26:93–100
Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD (1996) Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 11:337–349
Arlot ME, Sornay-Rendu E, Garnero P, Vey-Marty B, Delmas PD (1997) Apparent pre- and postmenopausal bone loss evaluated by DXA at different skeletal sites in women: the OFELY cohort. J Bone Miner Res 12:683–690
Viguet-Carrin S, Gineyts E, Bertholon C, Delmas PD (2009) Simple and sensitive method for quantification of fluorescent enzymatic mature and senescent crosslinks of collagen in bone hydrolysate using single-column high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 877:1–7
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Sell DR, Nagaraj RH, Grandhee SK, Odetti P, Lapolla A, Fogarty J, Monnier VM (1991) Pentosidine: a molecular marker for the cumulative damage to proteins in diabetes, aging, and uremia. Diabetes Metab Rev 7:239–251
Odetti P, Fogarty J, Sell DR, Monnier VM (1992) Chromatographic quantitation of plasma and erythrocyte pentosidine in diabetic and uremic subjects. Diabetes 41:153–159
Miyata T, Ishiguro N, Yasuda Y, Ito T, Nangaku M, Iwata H, Kurokawa K (1998) Increased pentosidine, an advanced glycation end product, in plasma and synovial fluid from patients with rheumatoid arthritis and its relation with inflammatory markers. Biochem Biophys Res Commun 244:45–49
Sugiyama S, Miyata T, Ueda Y, Tanaka H, Maeda K, Kawashima S, Van Ypersele de Strihou C, Kurokawa K (1998) Plasma levels of pentosidine in diabetic patients: an advanced glycation end product. J Am Soc Nephrol 9:1681–1688
Kitauchi T, Yoshida K, Yoneda T, Saka T, Yoshikawa M, Ozono S, Hirao Y (2004) Association between pentosidine and arteriosclerosis in patients receiving hemodialysis. Clin Exp Nephrol 8:48–53
Sanaka T, Funaki T, Tanaka T, Hoshi S, Niwayama J, Taitoh T, Nishimura H, Higuchi C (2002) Plasma pentosidine levels measured by a newly developed method using ELISA in patients with chronic renal failure. Nephron 91:64–73
Garnero P, Mulleman D, Munoz F, Sornay-Rendu E, Delmas PD (2003) Long-term variability of markers of bone turnover in postmenopausal women and implications for their clinical use: the OFELY study. J Bone Miner Res 18:1789–1794
Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR (2003) BMD at multiple sites and risk of fracture of multiple types: long-term results from the study of osteoporotic fractures. J Bone Miner Res 18:1947–1154
Taylor BC, Schreiner PJ, Stone KL, Fink HA, Cummings SR, Nevitt MC, Bowman PJ, Ensrud KE (2004) Long-term prediction of incident hip fracture risk in elderly white women: study of osteoporotic fractures. J Am Geriatr Soc 52:1479–1486
Hillier TA, Stone KL, Bauer DC, Rizzo JH, Pedula KL, Cauley JA, Ensrud KE, Hochberg MC, Cummings SR (2007) Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women: the study of osteoporotic fractures. Arch Intern Med. 167:155–160
Cefalu WT, Bell-Farrow AD, Wang ZQ, Sonntag WE, Fu MX, Baynes JW, Thorpe SR (1995) Caloric restriction decreases age-dependent accumulation of the glycoxidation products, N epsilon-(carboxymethyl) lysine and pentosidine, in rat skin collagen. J Gerontol A Biol Sci Med Sci 50:B337–B341
Sell DR, Monnier VM (1997) Age-related association of tail tendon break time with tissue pentosidine in DBA/2 vs C57BL/6 mice: the effect of dietary restriction. J Gerontol A Biol Sci Med Sci 52:B277–B284
Förster A, Kühne Y, Henle T (2005) Studies on absorption and elimination of dietary maillard reaction products. Ann N Y Acad Sci 1043:474–481
Miyata T, Ueda Y, Shinzato T, Iida Y, Tanaka S, Kurokawa K, Ypersele de Strihou C van, Maeda K (1996) Accumulation of albumin-linked and free-form pentosidine in the circulation of uremic patients with end-stage renal failure: renal implications in the pathophysiology of pentosidine. J Am Soc Nephrol 7:1198–1206
Miyata T, Ueda Y, Horie K, Nangaku M, Tanaka S, Ypersele de Strihou C van, Kurokawa K (1998) Renal catabolism of advanced glycation end products: the fate of pentosidine. Kidney Int 53:416–422
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gineyts, E., Munoz, F., Bertholon, C. et al. Urinary levels of pentosidine and the risk of fracture in postmenopausal women: the OFELY study. Osteoporos Int 21, 243–250 (2010). https://doi.org/10.1007/s00198-009-0939-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-0939-5