Abstract
Summary
Pentosidine levels were higher in diabetic patients with vertebral fractures. Trabecular bone scores were negatively associated with pentosidine levels in diabetic patients only. Our results provide further evidence that AGEs are associated with the pathogenesis of bone fragility in patients with T2DM.
Introduction
Type 2 diabetes mellitus (T2DM) is associated with fracture risk. Pentosidine, an advanced glycation end product (AGE), is associated with prevalent vertebral fractures (VFs) in patients with T2DM. Trabecular bone score (TBS) has been proposed as an index of bone microarchitecture associated with bone quality. This study evaluated the associations of urine pentosidine and TBS in T2DM and non-T2DM groups.
Methods
A total of 112 T2DM patients and 62 non-T2DM subjects were enrolled. TBS was calculated using TBS insight® software (version 2.1). Pentosidine levels were measured using high-performance liquid chromatography method. We compared the BMD, TBS, and pentosidine levels between those with and without VFs with or without adjustment for age and sex. The association with TBS, lumbar spine BMD, and pentosidine levels were also evaluated in both T2DM and non-T2DM groups.
Results
Pentosidine levels were significantly higher in T2DM patients with VFs. TBSs were significantly lower in patients with T2DM and VFs. In non-diabetic patients, there were no significant differences in TBS and pentosidine levels for those with and without VFs after adjustment for age and sex. Pentosidine levels were negatively associated with TBS only in patients with T2DM. In multivariate stepwise regression analysis, pentosidine levels were significantly associated with TBS in patients with T2DM.
Conclusions
TBS and pentosidine could be used as a method to assess bone quality to identify T2DM patients at risk of VFs. Our results also provide further evidence that AGEs are associated with the pathogenesis of bone fragility in patients with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is associated with deleterious effects on the skeleton [1]. Meta-analyses have reported that the risk of hip fracture is higher in T2DM patients compared with non-diabetic controls [2, 3]. One Japanese study reported a higher risk of vertebral fractures (VFs) in T2DM patients, even though bone mineral density (BMD) in T2DM patients was not lower than in controls [4]. Occasionally, in T2DM, the overall fracture risk cannot be predicted using BMD measurements alone. Therefore, it can be inferred that bone quality may be contributing more to the increased risk of fractures in individuals with T2DM than BMD [5]. The measurement of bone quality can be difficult. Bone microarchitecture measured using high-resolution peripheral quantitative CT (HRpQCT) can differentiate between postmenopausal women with and without fragility fractures [6, 7]; however, this technology, along with several methodologies that evaluate bone strength independent of BMD, is not readily available and currently only used as research tools [8].
Recently, the trabecular bone score (TBS) was developed, a novel method that assesses skeletal texture from lumbar spine dual-energy X-ray absorptiometry (DXA) images [9, 10]. Using the Manitoba database, one study revealed that TBS can predict the risk of major osteoporotic fracture (MOF) independent of BMD in women with diabetes and those without diabetes [11]. Based on this study, the International Society for Clinical Densitometry (ISCD) recommends that TBS can be used in postmenopausal women with T2DM to predict MOF risk [12].
Advanced glycation end products (AGEs), which are various complexes generated via a non-enzymatic reaction between reducing sugars and amine residues, influence the properties of the bone material [13]. Advanced glycation end products are one of the components of bone quality, through their accumulation in the bone collagen fibers [13]. Advanced glycation end products have been known to be closely related to the pathogenesis of fragility fracture in patients with T2DM [13, 14]. Pentosidine is one of the important surrogate markers for total AGE production because the bone content of pentosidine reflects the total amount of AGE in bone [15]. In fact, increased pentosidine levels have been reported as a risk factor for fractures in older patients with T2DM in previous studies [16, 17]. However, no study has evaluated whether pentosidine levels in patients with T2DM are associated with TBS, the novel marker for bone microarchitecture.
In this study, we compared and evaluated the association between urine pentosidine levels and TBS in patients with T2DM and non-diabetic controls with and without VFs.
Methods
Subjects
Study participants were recruited through the Endocrinology Clinic in a medical center in South Korea from June 2015 to May 2016. For the T2DM group, inclusion criteria were adults aged ≥ 50 years who could stand alone, with the onset of diabetes at > 30 years-of-age, medical history/records consistent with T2DM, and available BMD data reviewed by trained data abstractors. Patients with a history of cancer and a serum creatinine level greater than 2.0 mg/dL were excluded. Patients who had a history of taking any drugs or hormones that affect bone metabolism, including glucocorticoids, sex steroids, warfarin, and bisphosphonates, were excluded. Any patients with other metabolic disorders and/or secondary causes of osteoporosis were also excluded. Finally, 112 subjects with T2DM were included in this study.
The control group comprised 62 eligible subjects aged ≥ 50 years with no medical history of diabetes who underwent BMD measurements. The subjects for the control group were also enrolled through the same endocrinology clinic for treatment of osteoporosis. None were receiving menopausal hormone therapy or taking corticosteroids. Any subjects with a history of cancer, a serum creatinine level greater than 2.0 mg/dL, other metabolic disorders, and/or secondary causes of osteoporosis were also not included.
All 174 enrolled participants had undergone lateral radiographs of the thoracic and lumbar spine, and VF assessments were performed to identify prevalent VFs. The time lag between the radiographs and BMD measurements was < 6 months.
The medical records of the enrolled patients were reviewed, including demographics, previous medical history (including the presence of menopausal status), alcohol intake, diabetic complications (for patients with T2DM), smoking, parental history of fractures, and medications such as glucocorticoids, estrogen, drugs for diabetes, and drugs for osteoporosis. Anthropometric parameters were measured using standard methods. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters. This study was approved by the ethical review board of this institution (AJIRB-MED-SMP-14-109) and followed the Declaration of Helsinki. All patients agreed to participate in the study and provided written informed consent.
Biochemical and BMD measurements
Fasting blood was obtained and the concentrations of fasting plasma glucose, hemoglobin A1c (HbA1c), and serum creatinine were measured by automated techniques at the laboratory of the institution. Serum 25-hydroxyvitamin D (25(OH)D) concentrations were measured using a radioimmunoassay kit (DiaSorin Inc., Stillwater, MN, USA). The densitometric examinations were performed with a Lunar iDXA apparatus (enCORE version 15.0, GE Healthcare, Madison, WI, USA). For lumbar spine BMD, when the specific vertebrae were not suitable for analysis due to a compression fracture, degenerative changes, or any other reason, BMD was calculated excluding the affected vertebrae. The coefficient of variations (CVs) for BMD were 0.339% (L1-L4), 0.679% (femur neck), and 0.794% (total hip).
Radiography and definitions of VF
Lateral radiographs of the thoracic and lumbar spine were taken in the upright position for better detection of VFs [18]. To limit exposure to radiation, a single radiograph of the thoracolumbar spine was obtained. All radiographs were taken using a tube-to-film distance of 105 cm, with the tube positioned over T11. Two musculoskeletal radiologists blinded to all data related to the patients performed the manual six-point vertebral quantitative morphometry evaluation of the radiographs. Vertebrae that were poorly visualized during VF assessments were excluded from the analysis, including degenerative changes. According to the Genant semi-quantitative approach [19], deformities were graded according to their severity using the following classification: grade 0 (< 20% deformity), grade 1, (mild deformities 20–25% deformity in Ha, Hm, or Hp), grade 2 (moderate deformities 25–40% deformity), and grade 3 (severe deformities ≥ 40% deformity). A patient was diagnosed with a VF when at least one of the three height measurements decreased by > 20% compared to the nearest uncompressed vertebra. The overall intra-observer agreement was 93% (κ = 0.81; excellent degree of agreement) when evaluating any fracture. The inter-observer agreement was 86% (κ = 0.65; good to fair agreement) when analyzing any fracture.
Measurement of TBS
Trabecular bone score was measured using TBS iNsight® software version 2.1 (Med-Imaps, Pessac, France) with anonymized spine DXA files from the database. Measurements were performed on the same vertebrae as in the BMD measurements. The investigator for TBS was blinded to all clinical parameters and outcomes. The average short-term reproducibility (CV) for TBS was 1.408%.
Measurement of pentosidine levels
Non-fasting urine samples were collected at baseline. The pentosidine detection assay followed an initial sample deproteinization method. Briefly, 200 μL of 113 acetonitrile was added to a 100-μL aliquot of urine or plasma and mixed on a vortex mixer. After vortex-mixing, the mixture was centrifuged at 12,000 rpm for 10 min, and 250 μL of the supernatant was transferred to a clean tube and evaporated under a gentle stream of nitrogen gas at 40 °C (Eyela, Tokyo, Japan). The resultant residue was reconstituted with 150 μL of 0.1 M heptafluorobutyric acid (HFBA), and 100 μL was injected onto the high-performance lipid chromatography (HPLC) system. The chromatographic equipment used was a Shimazu Prominence LC-20A HPLC system (Shimazu, Kyoto, Japan) equipped with a low-pressure gradient unit, an auto-purge, an in-line vacuum degassing auto-sampler (SIL-84 20A) with programmable temperature control, a heated column compartment, and a highly sensitive fluorescence detector (RF-20A/RF-20Axs) [20]. All components of the HPLC system were controllable through a CBM-20A system controller. Chromatographic separation of pentosidine was performed on a C18 reverse-phase column (AegisPak, 4.6 mm i.d., 25 cm, particle size 5 μm, Young Jin Biochrom, Seongnam, South Korea) after sample filtration through a 0.45-μm filter (Millipore, Billerica, MA, USA). The mobile phase used a gradient system to improve separation of pentosidine from endogenous peaks, and chromatograms were monitored by fluorescent detector set at excitation and emission wavelengths of 328 and 378 nm, respectively. The retention time for pentosidine was 24.3 min, and the lower limits of quantification (LLOQ) in human urine and plasma were 1 nM. Urine pentosidine levels were adjusted with urine creatinine levels (nmol/mg). The intra-day assay precisions (coefficients of variation) were generally low and found to be in the range of 5.19–7.49 and 4.96–8.78% for human urine and plasma, respectively. The corresponding values of the inter-day assay precisions were 9.45 and 4.27%. Accuracies (relative errors) ranged from 87.9 to 115%. All the samples were stored in a − 70 °C freezer until HPLC analysis of pentosidine.
Statistical analyses
There was a significant interaction effect of T2DM for TBS versus pentosidine (P = 0.011). Therefore, every statistical analysis was performed separately for each T2DM and control group. All data are expressed as the mean ± SD for each index considered. Student’s t tests were used to compare the continuous variables of patients with VFs and without VFs. Categorical variables were analyzed using chi-square tests. L-spine BMD, TBS, and urine pentosidine level were compared again with adjustment for age and sex using analysis of covariance (ANCOVA). The best cut-off value of urine pentosidine for the likelihood of VFs in T2DM was assessed from the receiver-operating characteristic (ROC) curve using the MedCalc version 11.5.1.0 (MedCalc Software, Ostend, Belgium). Subsequently, odd ratio (OR) according to the cut-off value for the likelihood of VFs in T2DM was estimated from logistic regression models with adjustment for age, sex, and HbA1c.
A Pearson’s correlation coefficient was used to estimate the relationships between TBS and urinary pentosidine level. A stepwise, linear, multiple regression analysis was performed using TBS as a dependent variable and pentosidine, age, sex, BMI, HbA1c (only for patients with T2DM), serum creatinine, 25(OH)D levels, and lumbar spine BMD as independent variables for each group. All multiple regression analyses were based on a single regression analysis for each predictor independent variable that allowed identification of candidate predictive variables. All analyses were conducted using SPSS version 23.0 (SPSS, Chicago, IL, USA). A P value < 0.05 was considered statistically significant.
Results
Table 1 summarizes the baseline characteristics of the patients with T2DM. Among the 112 patients, 32 had VFs (28.6%). The mean age of the patients with VFs (65.2 ± 8.5 years) was significantly higher than of patients without VFs (60.6 ± 6.8 years, P = 0.010). Femur neck BMD, total hip BMD, and L-Spine BMD were significantly lower in the patients with VFs (P = 0.002, P = 0.001, and P = 0.005, respectively). The percentage of diabetic nephropathy, insulin use, osteoporosis drug use, and vitamin D supplement use were higher in patients with VFs in T2DM (P = 0.032, P = 0.034, P = 0.002, and P = 0.004, respectively). There were significant differences in TBS and pentosidine (P = 0.001 and P = 0.018, respectively). These significant differences were still remained even after adjustment for age and sex (P = 0.039 in TBS and P = 0.044 in pentosidine) (Fig. 1). No significant differences were found between patients with T2DM with VF and patients with T2DM without VF in terms of the other parameters.
Table 2 summarizes the baseline characteristics of the non-diabetic controls. Among the 62 patients, 21 had VFs (33.9%). The mean age of the patients with VFs (70.3 ± 6.6 years) was significantly higher than that of those without VFs (59.5 ± 7.1 years, P < 0.001). The mean height of patients with VFs (152.2 ± 8.3 cm) was significantly lower than that of those without VFs (157.4 ± 6.6 cm, P = 0.009). Femur neck BMD, total hip BMD, and L-Spine BMD were significantly lower in the patients with VFs (P < 0.001, P = 0.004, and P < 0.001, respectively). Serum 25(OH)D level was significantly higher in patients with VFs (P = 0.020). The percentage of osteoporosis drug use, and vitamin D supplement use, was higher in patients with VFs in controls (P = 0.001 and P = 0.030, respectively). TBS was also significantly lower in the patients with VFs (P = 0.019). There was no significant difference between two groups in pentosidine (P = 0.612). After adjustment for age and sex, there were no significant differences between two groups in L-spine BMD and TBS (P = 0.062 and P = 0.918) (Fig. 1). No significant differences were found between controls with VF and controls without VF in terms of the other parameters. The best cut-off value of urine pentosidine for the likelihood of VFs in T2DM was also assessed from the ROC curve (Supplementary Figure). The best cut-off that maximized sensitivity and specificity was 1.08 nmol/mg. Subsequently, the OR according to the cut-off value for the likelihood of VFs in T2DM was estimated from logistic regression models with adjustment for age, sex, and HbA1c. The OR for the likelihood of fracture was 2.723 (95% CI 1.008–7.355, P = 0.048).
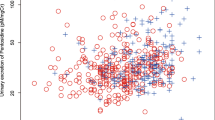
Figure 2 shows the correlations between TBS and urinary pentosidine level in controls and patients with T2DM. Pentosidine was negatively correlated with the TBS in patients with T2DM (r = − 0.325, P < 0.001). However, pentosidine was not significantly correlated with L-spine BMD in patients with T2DM (r = − 0.169, P = 0.075). There were no significant correlations between pentosidine and TBS, or pentosidine and L-spine BMD in controls.
Stepwise linear multiple regression analysis for TBS as a dependent variable was performed on the non-diabetic patients and patients with T2DM. For patients with T2DM, this analysis generated three different models, confirming the significant role of pentosidine as a predictor of TBS (Table 3). Other predictors were age and 25(OH)D. On the other hand, pentosidine was ruled out as a significant predictor of TBS in non-diabetic patients. L-spine BMD and age were the only predictors of TBS in non-diabetic patients (Table 4).
Discussion
Pentosidine levels were higher in T2DM patients with VFs compared to those without VFs. TBS was significantly lower in T2DM patients with VFs. However, there were no significant differences in TBS and pentosidine between non-diabetic patients with and without VFs after adjustment for age and sex. Pentosidine levels were negatively associated with TBS only in T2DM patients even after adjustment for confounding factors.
Although not all studies have reported a higher risk of VF in individuals with T2DM [21, 22], most studies have reported more VFs in patients with T2DM compared to non-diabetic patients [23,24,25]. Advanced glycation end products, especially pentosidine, have been suggested important contributors to the decreased material properties of bone in patients with T2DM [13]. Previous studies have reported a relationship between pentosidine and VF. Yamamoto et al. [17] reported an increased risk of VF in women with T2DM with higher urine pentosidine levels, but the same was not seen in men. On the contrary, Schwarz et al. [26] also found a significant association between prevalent VF and pentosidine in both genders. In our study, pentosidine was also significantly higher in patients with T2DM with VFs, which was consistent with these previous studies. Shiraki et al. [27] reported an increased risk of VF with higher pentosidine levels, even in elderly women without T2DM. However, Schwarz et al. [26] could not find a significant association between pentosidine and risk of VFs in patients without T2DM. We could not find any correlation between pentosidine and the prevalence of VFs in patients without T2DM.
In our study, TBS was significantly decreased in patients with T2DM and VFs compared to those without VFs. Patients with T2DM have been reported to have an increased osteoporotic fracture risk, even when they have higher BMDs than non-diabetic individuals [2, 3]. Therefore, it is possible that bone quality may be contributing more to the high risk of fractures in individuals with T2DM than BMD. It has been suggested that TBS can provide indirect information on bone quality in secondary osteoporosis, such as in T2DM [11, 28, 29]. Recently, we reported that TBS could be a potential supplementary tool for the discrimination of VFs in postmenopausal women with T2DM [30]. This study has confirmed these previous findings.
TBS was significantly lower in patients with VFs in the control group. However, this pattern was not found after adjustment for age and sex. It has been known that TBS was also significantly lower in the subjects with fractures even in general population [31]. Assessment by one-tailed test (α = 0.05) showed that 25 subjects were needed for each group with or without VFs in T2DM to reach sufficient statistical power (1-β = 0.8) to reproduce the similar difference in TBS between the VFs and non-VFs group according to our previous study (1.31 vs 1.23) [30]. Therefore, 32 subjects for VF group in T2DM fulfilled this requirement. However, 32 subjects were needed for each group with or without VFs in controls (α = 0.05 and 1-β = 0.8) to reproduce the similar difference in TBS between VFs and non-VFs group for general population according to the previous Japanese study (1.20 vs 1.13) [31]. With only 21 subjects with VFs in our study, only about 65% of statistical power was calculated. That might contribute to the loss of significance in the comparison of TBS in controls after adjustment for age and sex. However, the main purpose of our study was to evaluate the associations of urine pentosidine and TBS in T2DM and non-T2DM groups. We could find the prominent difference in the association of TBS and pentosidine between T2DM and non-DM controls with a relatively small number of subjects.
AGEs significantly influence the properties of the bone material [13]. AGEs, which are rich in bone material especially in T2DM, have been known to be closely related to the pathogenesis of fragility fracture in patients with T2DM [13, 14]. Recently, TBS has been reported to be closely related to bone quality and fracture risks in T2DM patients [11, 12, 30]. If TBS is a good surrogate marker of bone quality in T2DM and pentosidine, one of the surrogate markers for total AGEs, is closely related to bone quality, it makes sense that TBS and pentosidine are significantly associated. However, to our knowledge, there have been no studies on the association between TBS and pentosidine in patients with T2DM. Although there have been some studies on the association between pentosidine and bone quality, they have been undertaken using other measurement tools, and not TBS. In a study using micro-CT, a positive correlation was found between pentosidine content and trabecular number and connectivity density, and a negative correlation was found between pentosidine content and trabecular separation in human cadaveric bones [32]. In a recent study, non-enzymatic glycation and degree of mineralization increased, especially in trabecular bone, in patients with type 1 diabetes mellitus (T1DM) and fractures. In one study, invasive iliac crest bone biopsies from five patients with type 1 diabetes mellitus (T1DM) and fractures, five patients with T1DM patients and no fractures, and five healthy subjects were performed, and the degree of mineralization of bone was measured by micro-CT [33]. Even though micro-CT could be one of the most accurate tools for the measurement of bone quality, an invasive bone biopsy is required and is not appropriate for studies with large populations. Moreover, there have only been animal studies on the association between pentosidine and bone quality in T2DM [34, 35]. In our study, we found that pentosidine could be related to TBS, a possible surrogate marker of bone quality, in patients with T2DM even after correction for confounding factors. It was also meaningful that pentosidine still showed significant association with TBS even after adjustment for strong confounding factors such as age and L-spine BMD. Therefore, this could be further evidence that pentosidine may reflect bone quality and the susceptibility of fragility fractures in patients with T2DM.
In our study, we could not find any correlation between pentosidine and TBS in non-diabetic patients. If pentosidine does have a deleterious effect on TBS, then the correlation between pentosidine and TBS should have also been found in non-diabetic patients. For non-diabetic patients, we recruited patients who visited our hospital for the treatment of osteoporosis. Therefore, a higher proportion of patients had VFs and used anti-osteoporosis medications. A reduction in bone turnover induced by anti-resorptives may result in retardation of collagen turnover, potentially leading to the accumulation of non-enzymatic glycation or oxidation-induced cross-links of collagen [36]. Therefore, anti-resorptive use could influence the association between pentosidine and TBS. However, we could not find any differences in urinary pentosidine level between anti-resorptive users and non-users (P = 0.713). Therefore, we concluded that anti-resorptive use was not a factor. The number of patients with high urinary pentosidine levels was smaller in the non-diabetic patients, and this may have made it difficult to find any association between pentosidine and TBS in these patients. More studies are needed to ascertain any association between pentosidine and TBS in non-diabetic patients.
This study has some limitations. First, the study was cross-sectional. Thus, it can only demonstrate associations between pentosidine and TBS, and not the causations. Second, we measured pentosidine levels in urine and did not directly measure the AGE levels in bone. However, AGE levels in serum and urine have been reported as a surrogate marker for levels in bone collagen. Odetti et al. [37] reported that plasma pentosidine levels were closely correlated with levels in cortical bone from the femur removed during surgery. Moreover, direct measurements of AGE levels in the collagen of bone require invasive procedures, and these are not available in most studies [26]. Third, a bone turnover marker was not available in our patients. Fourth, the existence of back pain was not investigated. Therefore, we could not discriminate VFs between symptomatic and asymptomatic VFs. Fifth, a relatively small number of patients with VFs were enrolled for the control group. More subjects should have been enrolled to confirm the significant difference in TBS between VFs and non-VFs group in the controls.
However, the pentosidine levels were measured in all patients with T2DM and non-diabetic patients using the accurate HPLC method. Each TBS and pentosidine has been known to be associated with fracture risks in T2DM patients, separately. No study has reported the association of TBS and pentosidine in patients with T2DM. To our knowledge, this study showed the significant association of TBS and pentosidine in patients with T2DM at first. In this regard, the present study has strength.
In conclusion, our results provide further evidence that AGEs are associated with the pathogenesis of bone fragility in patients with T2DM. Pentosidine levels may reflect fracture susceptibility in patients with T2DM. In the clinical setting, TBS and pentosidine can be used as a non-invasive method to assess bone quality to identify T2DM patients at risk of VFs.
References
Epstein S, Leroith D (2008) Diabetes and fragility fractures: a burgeoning epidemic? Bone 43(1):3–6. https://doi.org/10.1016/j.bone.2008.03.017
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes: a meta-analysis. Osteoporos Int 18(4):427–444. https://doi.org/10.1007/s00198-006-0253-4
Janghorbani M, Van Dam RM, Willett WC, Hu FB (2007) Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 166(5):495–505. https://doi.org/10.1093/aje/kwm106
Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T (2009) Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res 24(4):702–709. https://doi.org/10.1359/jbmr.081207
Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, Yu Q, Zillikens MC, Gao X, Rivadeneira F (2012) Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol 27(5):319–332. https://doi.org/10.1007/s10654-012-9674-x
Stein EM, Liu XS, Nickolas TL, Cohen A, McMahon DJ, Zhou B, Zhang C, Kamanda-Kosseh M, Cosman F, Nieves J, Guo XE, Shane E (2012) Microarchitectural abnormalities are more severe in postmenopausal women with vertebral compared to nonvertebral fractures. J Clin Endocrinol Metab 97(10):E1918–E1926. https://doi.org/10.1210/jc.2012-1968
Nishiyama KK, Macdonald HM, Hanley DA, Boyd SK (2013) Women with previous fragility fractures can be classified based on bone microarchitecture and finite element analysis measured with HR-pQCT. Osteoporos Int 24(5):1733–1740. https://doi.org/10.1007/s00198-012-2160-1
Silva BC, Leslie WD (2017) Trabecular bone score: a new DXA-derived measurement for fracture risk assessment. Endocrinol Metab Clin N Am 46(1):153–180. https://doi.org/10.1016/j.ecl.2016.09.005
Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA (2011) Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom 14(3):302–312. https://doi.org/10.1016/j.jocd.2011.05.005
Pothuaud L, Carceller P, Hans D (2008) Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the studyof human trabecular bone microarchitecture. Bone 42(4):775–787. https://doi.org/10.1016/j.bone.2007.11.018
Leslie WD, Aubry-Rozier B, Lamy O, Hans D, Manitoba Bone Density Program (2013) TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab 98(2):602–609. https://doi.org/10.1210/jc.2012-3118
Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD (2015) Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD official positions part 2: trabecular bone score. J Clin Densitom 18(3):309–330. https://doi.org/10.1016/j.jocd.2015.06.008
Yamamoto M, Sugimoto T (2016) Advanced glycation end products, diabetes, and bone strength. Curr Osteoporos Rep 14(6):320–326. https://doi.org/10.1007/s11914-016-0332-1
Saito M, Marumo K (2010) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int 21(2):195–214. https://doi.org/10.1007/s00198-009-1066-z
Dong XN, Qin A, Xu J, Wang X (2011) In situ accumulation of advanced glycation end products (AGEs) in bone matrix and its correlation with osteoclastic bone resorption. Bone 49(2):174–183. https://doi.org/10.1016/j.bone.2011.04.009
Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, Resnick HE, Tylavsky FA, Black DM, Cummings SR, Harris TB, Bauer DC, Health, Aging, and Body Composition Study (2009) Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab 94(7):2380–2386
Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T (2008) Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 93(3):1013–1019. https://doi.org/10.1210/jc.2007-1270
Mehta JS, Reed MR, McVie JL, Sanderson PL (2004) Weight-bearing radiographs in thoracolumbar fractures: do they influence management? Spine (Phila Pa 1976) 29(5):564–567. https://doi.org/10.1097/01.BRS.0000113873.07484.5D
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8(9):1137–1148. https://doi.org/10.1002/jbmr.5650080915
Du ES, Moon HS, Lim SJ, Chang SY, Kim SH (2016) High performance liquid chromatographic determination of YJC-10592, a new chemokine receptor 2 (CCR-2) antagonist, in biological samples. J Pharm Investig 46(5):495–504
Hanley DA, Brown JP, Tenenhouse A, Olszynski WP, Ioannidis G, Berger C, Prior JC, Pickard L, Murray TM, Anastassiades T, Kirkland S, Joyce C, Joseph L, Papaioannou A, Jackson SA, Poliquin S, Adachi JD, Canadian Multicentre Osteoporosis Study Research Group (2003) Associations among disease conditions, bone mineral density, and prevalent vertebral deformities in men and women 50 years of age and older: cross sectional results from the Canadian multicentre osteoporosis study. J Bone Miner Res 18(4):784–790. https://doi.org/10.1359/jbmr.2003.18.4.784
Koh WP, Wang R, Ang LW, Heng D, Yuan JM, Yu MC (2010) Diabetes and risk of hip fracture in the Singapore Chinese health study. Diabetes Care 33(8):1766–1770. https://doi.org/10.2337/dc10-0067
Melton LJ III, Leibson CL, Achenbach SJ, Therneau TM, Khosla S (2008) Fracture risk in type 2 diabetes: update of a population-based study. J Bone Miner Res 23(8):1334–1342
Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, Johnson KC, Margolis KL (2006) Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational study. J Clin Endocrinol Metab 91(9):3404–3410. https://doi.org/10.1210/jc.2006-0614
Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T (2009) Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res 24(4):702–709. https://doi.org/10.1359/jbmr.081207
Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, Resnick HE, Tylavsky FA, Black DM, Cummings SR, Harris TB, Bauer DC, Health, Aging, and Body Composition Study (2009) Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab 94(7):2380–2386
Shiraki M, Kuroda T, Tanaka S, Saito M, Fukunaga M, Nakamura T (2008) Nonenzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures. J Bone Miner Metab 26(1):93–100. https://doi.org/10.1007/s00774-007-0784-6
Cohen A, Dempster DW, Müller R, Guo XE, Nickolas TL, Liu XS, Zhang XH, Wirth AJ, van Lenthe GH, Kohler T, McMahon DJ, Zhou H, Rubin MR, Bilezikian JP, Lappe JM, Recker RR, Shane E (2015) Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporos Int 21(2):263–273
Choi YJ, Chung YS (2016) Type 2 diabetes mellitus and bone fragility: special focus on bone imaging. Osteoporos Sarcopenia 2(1):20–24. https://doi.org/10.1016/j.afos.2016.02.001
Choi YJ, Ock SY, Chung YS (2016) Trabecular bone score (TBS) and TBS-adjusted fracture risk assessment tool are potential supplementary tools for the discrimination of morphometric vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Densitom 19(4):507–514. https://doi.org/10.1016/j.jocd.2016.04.001
Iki M, Tamaki J, Kadowaki E, Sato Y, Dongmei N, Winzenrieth R, Kagamimori S, Kagawa Y, Yoneshima H (2014) Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: the Japanese population-based osteoporosis (JPOS) cohort study. J Bone Miner Res 29(2):399–407. https://doi.org/10.1002/jbmr.2048
Viguet-Carrin S, Follet H, Gineyts E, Roux JP, Munoz F, Chapurlat R, Delmas PD, Bouxsein ML (2010) Association between collagen cross-links and trabecular microarchitecture properties of human vertebral bone. Bone 46(2):342–347. https://doi.org/10.1016/j.bone.2009.10.001
Farlay D, Armas LA, Gineyts E, Akhter MP, Recker RR, Boivin G (2016) Nonenzymatic glycation and degree of mineralization are higher in bone from fractured patients with type 1 diabetes mellitus. J Bone Miner Res 31(1):190–195. https://doi.org/10.1002/jbmr.2607
Devlin MJ, Van Vliet M, Motyl K, Karim L, Brooks DJ, Louis L, Conlon C, Rosen CJ, Bouxsein ML (2014) Early-onset type 2 diabetes impairs skeletal acquisition in the male TALLYHO/JngJ mouse. Endocrinology 155(10):3806–3816. https://doi.org/10.1210/en.2014-1041
Saito M, Fujii K, Mori Y, Marumo K (2006) Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int 17(10):1514–1523. https://doi.org/10.1007/s00198-006-0155-5
Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D (2009) Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int 20(6):887–894. https://doi.org/10.1007/s00198-008-0754-4
Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, Federici A (2005) Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci 1043(1):710–717. https://doi.org/10.1196/annals.1333.082
Acknowledgements
This research was partly supported by the Korea Health Technology R&D Project (HI16C0992), through the Korea Health Industry Development Institute (KHIDI), which is funded by the Ministry of Health and Welfare, Republic of Korea (So Hee Kim). The authors are grateful to the technologist, Byung Joo Lee, for his assistance with the analyses of trabecular bone score. The authors are also grateful to professor Yunhwan Lee for his advice on the statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Electronic supplementary material
ESM 1
Receiver operating characteristic curve analysis of pentosidine with VFs. (GIF 14 kb)
Rights and permissions
About this article
Cite this article
Choi, Y.J., Ock, S.Y., Jin, Y. et al. Urinary Pentosidine levels negatively associates with trabecular bone scores in patients with type 2 diabetes mellitus. Osteoporos Int 29, 907–915 (2018). https://doi.org/10.1007/s00198-017-4359-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4359-7