Abstract
Purpose
The purpose of this study was to review the monitoring of strontium ranelate osteoporosis therapy.
Methods
The method used in this study was comprehensive literature review with clinical perspectives.
Results
Changes in bone turnover markers (BTM) or bone mineral density (BMD) have been documented in osteoporosis clinical trials. However, neither BMD nor BTM changes fully explain the observed fracture risk reduction in treated patients. If changes in BMD or BTM on therapy would be easily discernable in individual patients, and were strongly associated with fracture risk reduction, monitoring individuals would be more useful. BMD changes in patients on strontium ranelate are of a greater magnitude and hence can be easily determined in an individual patient. In addition, there exists a better correlation between fracture risk reduction and increases in BMD.
Conclusions
The strong correlation between measured BMD increases and fracture risk reduction in patients on strontium ranelate therapy will be of clinical benefit to physicians wishing to evaluate both treatment persistence and fracture risk reduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients in clinical trials of osteoporosis therapies are selected by criteria of low bone density, prevalent fracture, or both. Such patients are at the highest risk of fracture and are optimal candidates for pharmaceutical approaches to fracture risk reduction. Clinical trials of osteoporosis therapies must demonstrate fracture risk reduction in order to achieve regulatory approval. As secondary endpoints, significant and favorable changes in biochemical markers of bone turnover or bone density have been documented in groups of patients. With different bisphosphonate dosing schedules, bone mineral density (BMD), and bone turnover markers (BTM) group changes have been acceptable proof of bioequivalence [1–3]. Such trials must be tied to prior trials demonstrating fracture risk reduction with other dosing schedules and with similar BMD and BTM changes. However, neither BMD nor BTM endpoints fully explain the observed fracture risk reduction in treated patients [4, 5]. The relevance of these measures in the estimation of treatment response and fracture risk for patients on various therapies is therefore unclear [6, 7]. In addition, the changes seen in groups of trials patients are often not discernable in individual patients seen in clinical practice. The concept of “least significant change” (LSC) has been advanced to aid in the clinical interpretation of individual’s change in BMD. For spine BMD, the LSC calculated from a precision study at a good clinical facility might be 3%. If the LSC is not exceeded then the changes in BMD can be said to be not significant. Many osteoporosis therapies would not be expected to cause changes in BMD exceeding the LSC. The WHO 10-year absolute fracture risk estimation will apply only to treatment-naïve subjects and is not intended to predict fracture risk for patients on osteoporosis therapies [8].
Evaluation of BMD by central DXA is critical for the identification of patients at sufficient risk of fracture to warrant pharmacotherapy. BMD is commonly measured at intervals in the follow-up of patients on therapy in order to monitor their response similar to what has been done in clinical trials. Strontium ranelate is an osteoporosis therapy proven effective in reducing fractures in postmenopausal women with osteoporosis. The data indicate significant vertebral, non-vertebral, and hip fracture risk reduction with good tolerability [9–11]. Distinct from other osteoporosis therapies, monitoring BMD in patients on strontium ranelate therapy may provide a better indication of fracture risk reduction than with other osteoporosis therapies.
BMD and strontium artifact
The greater increases in BMD are in part due to combined anti-catabolic and bone anabolic effects of strontium ranelate [12] and in part due to the higher atomic number of strontium in bone as compared to calcium. The higher atomic number of strontium (Z = 38) than calcium (Z = 20) leads to greater attenuation of X-rays and consequently an overestimation of the BMD as measured by DXA (and expressed as calcium hydroxyapatite equivalent). The greater changes in BMD are clinically useful, allowing the clinician to more easily demonstrate positive changes in BMD as an indication of patient response to therapy. With most osteoporosis therapies, BMD changes in individual patients are likely to fall within the precision error for the densitometer [13]. Although various algorithms for adjusting the bone density in patients on strontium ranelate therapy have been proposed, none are validated [14]. There are likely to be differences in strontium incorporation in bone at different skeletal sites for many reasons. Strontium is preferentially distributed in bone newly formed during strontium ranelate treatment rather than in older bone, formed before treatment initiation. [15] Strontium exchanges more readily on the surface of bone than in deeper bone. It is therefore likely that at different bone sites (trabecular and cortical bone), and with different levels of bone turnover, bone strontium content would be quite variable between individuals. Consequently, adjustment algorithms will not be clinically useful. An adjustment of the lumbar spine BMD was first proposed in the STRATOS study [16] and has been used as an attempt to approximate the increase in BMD related to the pharmacological effect of strontium ranelate. However, this algorithm for adjustment of lumbar spine BMD is complex and based on numerous assumptions. Spine bone strontium content (BSC) was estimated from iliac crest biopsies obtained from patients in the pivotal trials of strontium ranelate. Using the primate relationship between spine and iliac crest BSC, estimation of human spine BSC was calculated [17]. Patients’ plasma Sr level was correlated with iliac crest BSC (in patients undergoing bone biopsy) at each yearly time point and this relationship was used to calculate an estimated spine BSC in patients who did not have a bone biopsy [16]. Finally, the overestimation of spine BMD attributable to bone Sr was calculated by in vitro experiments comparing DXA measurements of phantoms containing different concentrations of strontium hydroxyapatite. These experiments indicated that a phantom strontium content of 1% induces a 10% overestimation of BMD expressed in calcium hydroxyapatite equivalent [18]. The mean change in adjusted-lumbar BMD in strontium ranelate-treated subjects in SOTI and TROPOS studies was approximately +7.5%, after 3 years of treatment. By the above adjustment algorithm, it is estimated that the BMD overestimation due to strontium in bone accounts for approximately 50% of the measured change in BMD after 3 years of treatment [11]. The increased BMD observed clinically in patients treated with strontium ranelate is both due to the presence of strontium in bone as well as the pharmacological antiresorptive and anabolic activity on bone cells resulting in increased bone tissue with normal bone calcium mineralization. In animal studies, measured-BMD increases correlate with improved bone strength [19] and adjustment of the BMD does not improve the strength–BMD correlation compared with the unadjusted BMD. This indicates that the measured, unadjusted BMD is optimal in predicting improved biomechanical properties in patients on strontium ranelate therapy. The easily discernable BMD increases in strontium ranelate-treated patients will assure the clinician that medication has been ingested, that strontium has been absorbed, and that anti-fracture efficacy in keeping with the results from the pivotal trials can be expected.
Changes in BMD and fracture risk reduction
Studies exploring the relationship between BMD change and fracture reduction have been conducted from clinical trials data with raloxifene [4], alendronate [20], and other agents [21]. Though post-hoc analyses and variable in their results, they suggested that increases in femoral neck BMD (in groups of patients) predicts reductions in the risk of vertebral fractures. Recently, this relationship between increases in measured femoral neck BMD and reduction in vertebral fracture risk [22] and reduction in hip fracture [23] was shown in a post-hoc analysis to be the strongest in strontium ranelate-treated patients from SOTI and TROPOS trials [22]. Groups of patients with the greatest increases in measured (unadjusted) BMD were demonstrated to have the greatest fracture risk reduction [22]. These data strongly support the monitoring of unadjusted BMD in individual strontium ranelate-treated patients where discernable increases in BMD are indicative of antifracture efficacy [22]. This is in distinction to other osteoporosis therapies where stabilization or clinically undetectable rises in BMD may often be the expected result. The change in unadjusted BMD explains about 75% of the anti-fracture efficacy of strontium ranelate [22] compared with estimates of between 4% and 28% with antiresorptive therapies [4–7, 20, 21, 24, 25] and up to 41% with PTH [26]. Furthermore, persistence with osteoporosis therapies has been poor [27] with patients frequently discontinuing therapy due to perceived lack of therapeutic benefits when BMD does not significantly increase. Since follow-up BMD is often used to assure the patient and clinician of therapeutic response and may enhance adherence to therapy, the combination of a robust BMD–fracture risk reduction relationship and greater rises in measured BMD in patients treated with strontium ranelate will prove useful.
Bone turnover markers and monitoring therapy
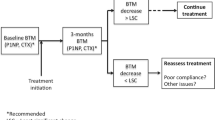
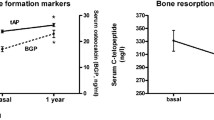
Biochemical markers of bone turnover have also been proposed as a way of monitoring treatment with some agents. Most BTMs are greatly affected by food and time of day leading to high day-to-day variability. Different therapies have various effects on BTMs. Bone anabolic therapies reduce fracture risk with increases in BTMs; antiresorptive therapies reduce fracture risk with associated decreases in BTMs. Strontium ranelate reduces fracture risk with reductions in markers of bone resorption and increases in markers of bone formation. As with some antiresorptive therapies, the changes in BTMs on strontium ranelate therapy are not of sufficient magnitude to be clinically useful in individual patients. BTMs have not routinely been advocated for either the identification of patients at risk of fracture or for monitoring of individual patients on therapy. It is possible that future BTMs will improve our ability to detect treatment response on an individual patient basis [28]. Patients being monitored with BTM’s will have to demonstrate significant changes from baseline. The best indication of this would be changes exceeding the LSC as calculated by a precision study.
Treatment discontinuation
The usefulness of either BTMs or BMD measurement to predict fracture risk in patients who have discontinued therapy is highly problematic and likely depends on the nature of the preceding treatment. Nevertheless, clinical practice may require that patients be monitored after stopping osteoporosis therapy. Unfortunately, data on patients discontinuing therapy is sparse and methodologically difficult to obtain. In the FLEX study, patients from FIT1 and FIT2 studies were treated with alendronate for a total of 5 years. They were then randomized to receive either alendronate or placebo for a further 5 years. Resolution of effect off alendronate therapy was very gradual both with regards to BMD and BTM. With continued alendronate, the further increases in BMD over 5 years were very small. Clinical vertebral fractures were significantly reduced in patients continuing on alendronate but morphometric vertebral fractures and non-vertebral fractures were not different between the two groups [29]. Such studies have inherent problems with low patient numbers, lack of placebo comparators and enrolment biases making true fracture risk evaluation problematic. Discontinuing risedronate therapy after 3 years leads to significant decreases in bone density in the fourth year off risedronate as well as increases in BTM but no increase in fracture risk in that year [30]. After 4 years of strontium ranelate therapy, patients crossed over from active therapy to placebo in the SOTI trial had an 8.9% annual incidence of vertebral fracture in the year off therapy as compared to patients maintained on treatment (6.9%; p = NS). No effect of treatment discontinuation was observed on the proportion of patients with new non-vertebral fractures in the fourth to the fifth year (4.0% in the year off therapy vs. maintained treatment 4.5%; p = NS) [11]. During this fourth year off strontium ranelate therapy, L2–L4 BMD decreased 3.4% ± 5.8; patients continuing therapy experienced further increases in BMD of 1.5% ± 6.0% (p < 0.001). Spine BMD after 4 years of strontium ranelate followed by 1 year of placebo remained 13% higher (0.860 ± 0.147 g/cm²) than at initial trial randomization (0.733 ± 0.119 g/cm²) [31].
The resolution of bone metabolic effects induced by strontium ranelate can also be demonstrated by following BTM after treatment discontinuation. In the SOTI trial, patients on strontium ranelate therapy experienced a rise in BSAP. After 4 years of therapy, treatment was stopped. Three months later, significant decreases in BSAP and increases in sCTX were observed. This may indicate rapid resolution of anabolic and antiresorptive effects after treatment discontinuation.
Primate studies confirm that after 1 year of strontium ranelate therapy (at doses giving three times the plasma strontium level seen in humans at therapeutic dose), bone strontium content falls rapidly on treatment discontinuation. After 10 weeks off therapy, iliac crest bone strontium content by necropsy decreased by 50% [32]. A slow phase of strontium excretion likely follows this rapid mobilization of strontium from the skeleton and is likely attributable to delayed mobilization of buried strontium not readily available for calcium exchange [32]. Although patient bone biopsy data is difficult to obtain following treatment discontinuation, models based upon the specific non-invasive determination of bone strontium content [33] may permit better modeling of strontium resolution of effect at the bone tissue level after treatment discontinuation.
Clinicians are aware of the need to initiate therapy in patients who are at risk of fracture and to continue therapy as long as required to maintain fracture risk reduction. Although tempting, treating to BMD targets or for specified arbitrary periods of time is not well supported by evidence. There is no proof of persistence of fracture risk reduction off therapy. Other risk factors such as prevalent fracture, age, fall risk, and steroid therapy must be considered prior to treatment discontinuation and patients at high fracture risk would be best advised to continue proven osteoporosis therapy.
As with antiresorptive therapies, the BMD decline after stopping treatment with strontium ranelate has not been associated with an increase in short-term fracture risk. More clinical trials documenting resolution of effect after osteoporosis therapies over the long-term are essential.
With strontium ranelate therapy, persistence of antifracture efficacy as well as safety over 5 years (vs. placebo) has been demonstrated. In SOTI and TROPOS studies 5 year sustained vertebral, nonvertebral, and hip antifracture efficacy has been demonstrated [34].
Conclusions
Absolute fracture risk determined by BMD and other risk factors is essential in determining candidates for osteoporosis pharmacotherapy in treatment naïve patients. The role of follow-up BMD on therapeutic decision making is less clear. Clinicians must be aware of the different fracture risk reduction relationships associated with BMD changes on various therapies. Though this relationship is again different with strontium ranelate compared with other osteoporosis therapies, the usefulness of BMD as a marker of therapeutic efficacy is likely greater than with other therapies. Since BMD changes can be easily determined on an individual patient basis and there exists a better correlation with fracture risk reduction and increases in BMD, strontium ranelate BMD monitoring will prove more useful than with other therapies [22]. This may in turn enhance patient adherence to therapy leading to greater fracture risk reduction.
Osteoporosis is a chronic disease requiring long-term therapy. Treatment discontinuation might be considered because of inefficacy or safety concerns. In this case, further treatment decisions will be based on the interpretation of a BMD measurement, which could have been influenced by the previous treatment. Fracture risk in such a patient changing therapies is not easily evaluated and cannot be considered equivalent to that of a treatment-naïve patient. It is possible that a patient would switch therapy due to a lack of increase in BMD on strontium ranelate. In such a patient, the lack of BMD increase likely indicates absence of significant strontium in the skeleton and likely a lack of strontium biological response. This could be due to non-adherence or due to lack of absorption of strontium ranelate. In such cases, subsequent BMD monitoring on another osteoporosis therapy would not be greatly influenced by the potential concerns of strontium BMD artefact. However, should strontium ranelate-treated patients with significant increases in BMD change to another therapy, at least 1 year should pass before a new BMD baseline be determined. Longitudinal BMD changes on the new therapy can then likely be determined from this new baseline. Intolerance to strontium ranelate such as diarrhea or nausea is unlikey but if problematic would likely emerge early in the course of therapy when little bone strontium uptake has occurred. At this early stage, strontium artefact on subsequent BMD estimations would likely be minimal and of no clinical concern.
Strontium ranelate therapy is associated with a significant decrease in fracture risk associated with robust increases in measured BMD. The unadjusted, measured-BMD may lead to overestimation of the BMD changes on treatment which will be useful in overcoming the precision error of the bone density instruments. The strong correlation between measured BMD increases and fracture risk reduction in patients on strontium ranelate therapy will be of clinical benefit to physicians wishing to evaluate both treatment persistence and the associated reduced fracture risk. Osteoporosis requires long-term therapy in order to reduce fracture risk. It is important that patients be encouraged to continue their treatment for many years. This confirmation of treatment effect may be achieved through measurable increases in BMD resulting in better adherence to therapy.
References
Rizzoli R, Greenspan SL, Bone G, Schnitzer TJ, Watts NB, Adami S, Foldes AJ, Roux C, Levine MA, Uebelhart B, Santora AC 2nd, Kaur A, Peverly CA, Orloff JJ (2002) Alendronate Once-Weekly Study Group. Two-year results of once-weekly administration of alendronate 70 mg for the treatment of postmenopausal osteoporosis. J Bone Miner Res 17(11):1988–96
Brown JP, Kendler DL, McClung MR, Emkey RD, Adachi JD, Bolognese MA, Li Z, Balske A, Lindsay R (2002) The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int 71(2):103–11
Miller PD, McClung MR, Macovei L, Stakkestad JA, Luckey M, Bonvoisin B, Reginster JY, Recker RR, Hughes C, Lewiecki EM, Felsenberg D, Delmas PD, Kendler DL, Bolognese MA, Mairon N, Cooper C (2005) Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 20(8):1315–22
Sarkar S, Mitlak BH, Wong M, Stock JL, Black DM, Harper KD (2002) Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res 17(1):1–10
Cummings SR, Karpf DB, Harris F et al (2002) Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med 112(4):281–289
Miller PD (2005) Bone density and markers of bone turnover in predicting fracture risk and how changes in these measures predict fracture risk reduction. Curr Osteoporos Rep 3:103–10
Wasnich RD, Miller PD (2000) Anti-fracture efficacy of anti-resorptive agents are related to changes in bone density. J Clin Endoc Metab 85(1):231–236
Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ 3rd, O’Neill T, Pols H, Reeve J, Silman A, Tenenhouse A (2005) Predictive value of BMD for hip and other fractures. J Bone Miner Res 20(7):1185–94
Kendler D (2006) Strontium ranelate—data on vertebral and non vertebral fracture efficacy and safety: mechanism of action. Current Osteoporosis Reports 4:34–39
Reginster JY, Seeman E, De Vernejoul MC, Adami S, Compston J, Phenekos C, Devogelaer JP, Diaz-Curiel M, Sawicki A, Goemaere S, Sorensen OH, Felsenberg D, Meunier PJ (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: TROPOS Study. JCEM 90(5):2816–2822
Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, Cannata J, Balogh A, Lemmel E-M, Pors-Nielsen S, Rizzoli R, Genant HK, Reginster J-Y (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350:459–468
Blake GM, Lewiecki EM, Kendler DL, Fogelman I (2007) A review of strontium ranelate and its effect on DXA scans. J Clin Densitom 10(2):113–9
Blake G, Fogelman I (2005) Bone densitometry: an update. editorial Lancet 366(9503):2068–2070
Blake GM, Fogelman I (2005) Long-term effect of strontium ranelate treatment on BMD. J Bone Miner Res 20(11):1901–4
Boivin G, Khebbab MT, Jaurand X, Farlay D, Meunier PJ, Delmas PD (2007) Localization of strontium, by X-ray microanalysis cartography, in bone biopsies of postmenopausal osteoporotic women treated for 3 years with strontium ranelate. Calcif Tissue Int 80:S118
Meunier PJ, Slosman DO, Delmas PD (2002) Strontium ranelate: dose-dependent effects in established postmenopausal vertebral osteoporosis—a 2-year randomized placebo controlled trial. J Clin Endocrinol Metab 87:2060–2066
Dahl SG, Allain P, Marie PJ (2001) Incorporation and distribution of strontium in bone. Bone 28:446–453
Pors Nielsen S, Slosman D, Sorensen OH, Basse-Cathelinat B, De Casin P, Roux C, Meunier PJ (1999) Influence of strontium on bone mineral density and bone mineral content measurements by dual x-ray absorptiometry. J Clin Densitom 2:371–379
Ammann P, Shen V, Robin B, Mauras Y, Bonjour JP, Rizzoli R (2004) Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female rats. J Bone Miner Res 19:2012–2020
Hochberg MC, Ross PD, Black D, Cummings SR, Genant HK, Nevitt MC et al (1999) Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis Fracture Intervention Trial Research Group. Arthritis Rheum 42:1246–54
Delmas PD, Li Z, Cooper C (2004) Relationship between changes in bone mineral density and fracture risk reduction with antiresorptive drugs: some issues with metaanalyses. J Bone Miner Res 19:330–7
Bruyere O, Roux C, Detilleux J, Slosman DO, Spector TD, Fardellone P, Brixen K, Devogelaer JP, Diaz-Curiel M, Albanese C, Kaufman JM, Pors-Nielsen S, Reginster JY (2007) Relation between bone mineral density changes and fracture risk reduction in patients treated with strontium ranelate. J Clin Endocrinol Metab 92:3076–3081
Bruyere O, Roux C, Badurski J, Isaia G, De Vernejoul MC, Cannata J, Ortolani S, Slosman D, Detilleux J, Reginster J-Y (2007) Relationship between change in femoral neck bone mineral density and hip fracture incidence during treatment with strontium ranelate. Curr Med Res Opin 23(12):3041–5
Watts NB, Cooper C, Lindsay R, Eastell R, Manhart MD, Barton IP, van Staa TP, Adachi JD (2004) Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom 7:255–61
Chapurlat RD, Palermo L, Ramsay P, Cummings SR (2005) Risk of fracture among women who lose bone density during treatment with alendronate The Fracture Intervention Trial. Osteoporos Int 16:842–8
Chen P, Miller PD, Delmas PD, Misurski DA, Krege JH (2006) Change in lumbar spine BMD and vertebral fracture risk reduction in teriparatide-treated postmenopausal women with osteoporosis. J Bone Miner Res 21:1785–90
Cramer JA, Amonkar MM, Hebborn A, Altman R (2005) Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 21(9):1453–60
Bouxsein ML, Delmas PD (2008) Considerations for development of surrogate endpoints for antifracture efficacy of new treatments in osteoporosis: a perspective. J Bone Miner Res 23(8):1155–67
Black DM, Schwartz AV, Ensrud KE et al (2006) Effects of continuing or stopping alendronate after 5 years of treatment—The fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 396:2927–2938
Watts NB, Chines A, Olszynski WP, McKeever CD, McLung MR, Zhou X, Grauer A (2008) Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int 2007. Osteoporos Int 19(3):365–72
Ortolani S, Diaz-Curiel M (2007) Strontium ranelate: changes in BMD one year after treatment discontinuation. Osteoporos Int 18:S25
Farlay D, Boivin G, Panczer G, Lalande A, Meunier PJ (2005) Long-term strontium ranelate administration in monkeys preserves characteristics of bone mineral crystals and degree of mineralization of bone. J Bone Miner Res 20(9):1569–1578
Pors Nielsen S, Barenholdt O, Barenholdt-Schioler C, Mauras Y, Allain P (2004) Noninvasive measurement of bone strontium. J Clinical Densitometry 7:262–268
Reginster JY, Felsenberg D, Boonen S, Diez-Perez A, Rizzoli R, Brandi ML, Spector TD, Brixen K, Goemaere S, Cormier C, Balogh A, Delmas PD, Meunier PJ, Reginster JY, Brixen K, Cormier C, Cannata J (2008) Effects of long-term strontium ranelate treatment on the risk of nonvertebral and vertebral fractures in postmenopausal osteoporosis: results of a five-year, randomized, placebo-controlled trial. Arthritis Rheum 58(6):1687–95
Conflicts of interest
Robert G. Josse, financial disclosure: speaking honoraria, member of advisory board of Novartis, P&G/Sanofi-Aventis, Servier. David Kendler, financial disclosure: consultancies, honoraria, research funding from Novartis, Merck, Procter & Gamble, Nycomed, Eli Lilly, Pfizer, Amgen, Wyeth, GSK, Zelos and Servier. Jonathan D. Adachi, financial disclosure/conflicts of interest: consultant, speaker for or conducted clinical trials with the following companies: Amgen, AstraZeneca, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, Procter & Gamble, Roche, sanofi-aventis, Servier, and Wyeth
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kendler, D.L., Adachi, J.D., Josse, R.G. et al. Monitoring strontium ranelate therapy in patients with osteoporosis. Osteoporos Int 20, 1101–1106 (2009). https://doi.org/10.1007/s00198-009-0886-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-0886-1