Abstract
Vitamin D (25(OH)D) increases the efficiency of intestinal calcium absorption. Low levels of serum calcium stimulate the secretion of parathyroid hormone (PTH), which maintains serum calcium levels at the expense of increased bone turnover, bone loss and increased risk of fractures. We studied the association between 25(OH)D and PTH levels, and their associations with bone mineral density (BMD), bone loss, and prevalence of hip fractures in 615 community-dwelling postmenopausal aged 50–97 years. Mean level of 25(OH)D and PTH were 102.0 nmol/l±35.0 nmol/l and 49.4 ng/l±23.2 nmol/l, respectively; 49% of women were current hormone therapy users. The overall prevalence of vitamin D insufficiency (25(OH)D<50 nmol/l) was 2%, and prevalence of high PTH levels (>65 ng/l) was 17.4%. In multiple linear regression analyses hip BMD was negatively and independently associated with PTH levels ( p =0.04), and positively and independently associated with 25(OH)D levels ( p =0.03). There were only 23 women (3.7%) who experienced a hip fracture. In age-adjusted analyses there were no significant differences of 25(OH)D and PTH levels by hip fracture status. Across the entire range of values, the overall correlation between 25(OH)D and PTH was moderate ( r =−0.20). However, after the threshold vitamin D level of 120 nmol/l, all PTH values were below 65 ng/l. Further studies are necessary to identify the optimal vitamin D levels necessary to prevent secondary hyperparathyroidism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D contributes to calcium homeostasis by increasing the efficiency of intestinal calcium and phosphorus absorption [1]. Low levels of calcium in serum result in negative calcium balance, which stimulates the secretion of parathyroid hormone (PTH) [2]. This compensatory secondary hyperparathyroidism maintains serum calcium levels at the expense of increased bone turnover, bone loss, and an increased risk of hip and other nonvertebral fractures [3, 4, 5, 6]. Approximately 90% of plasma vitamin D is produced endogenously [7] after adequate ultraviolet exposure (10–15 min/day in the summer months). Dietary sources of vitamin D in the USA include fatty saltwater fish, eggs, and fortified milk. Combined vitamin D and calcium supplementation reduces the risk of hip and other nonvertebral fractures among older men and women [8, 9], but clinical trials of treatment with vitamin D alone have had mixed results [10, 11, 12, 13].
Vitamin D insufficiency, often defined as 25-hydroxyvitamin D (25(OH)D) ≤50 nmol/l [14–16], affects over 40% of individuals aged 70 and older [17, 18]; even higher prevalence is found during winter and at high latitudes [19, 20]. The main causes of low 25-hydroxyvitamin D levels in older persons include inadequate sun exposure, age-related decline in the capacity of the skin to produce vitamin D [21], and possibly reduced dietary intake of vitamin D as a consequence of reduced dairy product use. In southern California, which has a sunny temperate climate year-round, vitamin D deficiency is thought to be uncommon in community-dwelling ambulatory adults and may not be considered a risk factor for osteoporosis. However, this assumption of vitamin D sufficiency has not been rigorously examined. In this paper we report the association between 25(OH)D and parathyroid hormone (PTH) levels, and their association with bone mineral density (BMD) and prevalence of hip fracture in older community-dwelling women.
Methods
Between 1997 and 2000 all surviving participants from the Rancho Bernardo Study cohort in southern California were invited to participate in a study of osteoporosis. A total of 676 women, approximately 60% of the surviving cohort, participated. Main reasons for non-participation included having moved away, becoming too sick or too busy, or being institutionalized. Twenty of the women were unable to lie prone for the BMD measurement, and another 22 did not have adequate blood samples for measurement of 25(OH)D and PTH levels. We also excluded 19 women with creatinine clearance values <30 ml/min. This analysis includes the remaining 615 postmenopausal women aged 50–97 years. All were ambulatory and gave written, informed consent. The study was approved by the Institutional Review Board of the University of California, San Diego.
Participants completed a self-administered questionnaire about intake of dairy products, current smoking, alcohol use, regular physical activity, and fracture history. Current medication use, including calcium and vitamin supplementation, was ascertained by questionnaire and was verified by examination of pills and prescriptions brought to the clinic. Postmenopausal hormone therapy (HT) was defined as current estrogen or estrogen plus progestin use at the time of the visit. For measurements of height and weight, women wore light clothing and no shoes. Body mass index (BMI) was calculated as body weight (in kilograms) divided by height (in meters) squared. Bone mineral density was measured at the total hip by dual energy X-ray absorptiometry (Hologic QDR model 1000; Hologic, Bedford, MA, USA). Bone densitometers were calibrated daily using a calibration standard, with measurements maintained within the manufacturers’ precision standards of ≤1.5%. Non-vehicular accident hip fractures occurring after age 45 were classified as osteoporotic. Ninety-five percent of self-reported hip fractures were confirmed by examination of radiology reports.
Blood was collected in tubes that were protected from sunlight, and serum was stored at −70°C within 30 min of processing. Serum 25(OH)D [25(OH)D2+25(OH)D3] levels were analyzed by a competitive protein binding assay (Vitamin D Research Laboratory, Dr. Michael Holick, Boston University), as described by Chen et al. [22]. The rat serum vitamin D-binding protein used in the assay has high affinity for 25(OH)D. The intra-assay and inter-assay variations were 8% and 10%, respectively. The limit of detection was 12.5 nmol/l and reference range was 25–130 nmol/l. Intact PTH values were determined in the same laboratory using a chemiluminescence assay kit (Nichols Institute Diagnostics, San Juan Capistrano, CA). This assay has both intra-assay and inter-assay coefficients of variation of 6% and a reference range of 10–65 ng/l. Serum creatinine levels were measured by Smith Kline Beecham clinical laboratories. Creatinine clearance was calculated by the modified Cockcroft-Gault formula [140 minus age (in years)] multiplied by weight (in kilograms) divided by [72 × serum creatinine (mg/dl)] and multiplied by 0.85 (correction factor for females) [23].
Data analyses
SPSS (SPSS, SPSS Base 11 for Windows User’s Guide) and SAS (SAS Institute SAS User’s Guide, Version 8.2) were used for analysis. Vitamin D insufficiency was defined as 25(OH)D level ≤50 nmol/l [14], and vitamin D deficiency was defined as 25(OH)D level <30 nmol/l [24]. Results were expressed as mean ±95% confidence interval (CI) or percentages, and were compared using the Student t -test, one-way analyses of variance (ANOVA), or chi-square tests, as appropriate. Multivariate linear regression models were carried out to assess the independent associations between 25(OH)D and PTH levels with total hip BMD, as well as BMD at the femoral neck and trochanter, using both forward and backward approaches. Risk factors previously associated with BMD in this cohort (age, BMI, use of thiazide [yes/no], thyroid medication [yes/no], alcohol intake [≥3/week], exercise [≥3/week], current smoking [yes/no], calcium and vitamin D supplementation, and current hormone therapy use) were included one at a time and in combinations, until an optimal regression model was achieved. Because both 25(OH)D and hormone therapy enhance calcium absorption from the gut, an interaction term between HT and 25(OH)D was initially included in the multivariate models. The interaction term was not significant ( p value >0.1), and further analyses were performed without it. All statistical tests were two-tailed, and statistical significance was defined as p <0.05.
Results
On average, the 615 postmenopausal women in this study were aged 74.6±10.0 (range 50–97) years, (Table 1). Their mean levels of 25(OH)D and PTH were 102.0 nmol/l ±35.0 nmol/l (range 10.0–337.0) and 49.4 ng/l ±23.2 ng/l (range 6.0–288.0), respectively. Approximately half of all women were current HT users (mean years of use was 19.8, range 5–52 years); one-fifth reported thyroid hormone use, and 16% reported use of thiazide medication. Mean creatinine clearance was 71.8 ml/min ±26.0 ml/min (range 30.1–223.8). Creatinine clearance was correlated with total hip BMD ( r =0.40, p <0.001), but there was no association between creatinine clearance and levels of 25(OH)D ( r =0.02, p =0.63) or PTH ( r =0.03, p =0.47). Use of vitamin D supplements or calcium supplements was reported by 29.3% and 55.4%, respectively. Women who used vitamin D supplements had higher levels of 25(OH)D (111.3 nmol/l vs 98.0 nmol/l, p <0.001) than non-users. Most women (171 of 180) who took vitamin D supplements also took calcium supplements.
Age-adjusted 25(OH)D and PTH levels varied with the season in which they were sampled. Winter (December 21–March 20) was characterized by lower levels of 25(OH)D, while higher levels were observed in fall (September 21–December 20) (95.6 nmol/l vs 114.3 nmol/l, p =0.001). Lower levels of PTH were found in fall, and higher levels were found in spring (March 21–June 20) (46.4 ng/l versus 52.3 ng/l, p =0.04). The associations between season and vitamin D and PTH levels were independent of HT.
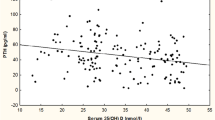
The overall prevalence of vitamin D insufficiency, defined as 25(OH)D levels equal or less than 50 nmol/l was 2%. Only six women (1%) had levels of 25(OH)D indicative of vitamin D deficiency according to the classical criterion of less than 30 nmol/l. Prevalence of PTH levels greater than 65 ng/l was 17.4%. Vitamin D and PTH levels were inversely associated ( r =−0.20, age-adjusted linear regression β=−0.31, p <0.001). As shown in a scatter plot (Fig. 1), no women with 25(OH)D levels greater than 120 nmol/l had PTH levels above 65 ng/l. Analyses adjusted for creatinine clearance and current HT use showed that serum 25(OH)D levels were lower and PTH levels were higher at older ages ( p for trend <0.001 and 0.05, respectively) (Fig. 2).
Table 2 shows the association of 25(OH)D, PTH, and other risk factors with total hip BMD. Age and BMI each made a substantial contribution to the variance in hip BMD ( R 2 of 0.08 and 0.12, respectively). For every year of age there was a decline of 0.005 g/cm2 in hip BMD ( p <0.001), and for every unit increase of BMI there was an increase of 0.013 g/cm2 in BMD ( p <0.001). Women who used HT had total hip BMD 0.06 g/cm2 higher than women not using HT ( p <0.001). PTH levels ( p =0.04 ) and alcohol intake ( p =0.01) were each negatively and independently associated with BMD, while 25(OH)D levels ( p =0.03) and use of thiazides ( p =0.02) were each positively and independently associated with BMD. Neither dietary calcium intake nor calcium supplementation were independently associated with BMD in the multivariate model, but use of calcium plus vitamin D supplements was positively, although marginally, associated with hip BMD ( p =0.05). The season when blood samples were collected, regular exercise, creatinine clearance, and current smoking were not associated with hip BMD. Results were similar in analyses using BMD at femoral neck or trochanter as the outcome (data not shown).
There were only 23 women (3.7%) who experienced a hip fracture. In age-adjusted analyses, there were no significant differences of 25(OH)D and PTH levels by hip fracture status.
Discussion
Consistent with other reports, we found an inverse correlation between 25(OH)D and PTH [20, 25, 26], and 25(OH)D levels declined and PHT increased with age [27, 28, 29]. Although only 2% of these older women had vitamin D insufficiency according to the classic definition, serum levels of PTH indicative of hyperparathyroidism were present in about 18%. This suggests that the existing criterion for vitamin D insufficiency may be too low.
In these ambulatory community-dwelling women, we found that 25(OH)D levels were independently and positively associated with hip BMD, similar to results of other studies of Caucasian postmenopausal women [30–32]. The negative and independent association of PTH levels with BMD is also in accord with other studies [33–35]. PTH and 25(OH)D levels did not account for a large portion of the variation on BMD in this cohort. However, we believe that 25(OH)D and PTH levels are clinically relevant, because secondary hyperparathyroidism can be prevented, unlike intractable risk factors for bone loss, such as increasing age.
In a recent meta analyses of 25 randomized clinical trials, vitamin D treatment was found to reduce the incidence of vertebral fractures ( RR =0.63, 95% CI 0.45–0.88, p <0.01) and showed a trend toward reduced incidence of nonvertebral fractures ( RR =0.77, 95% CI 0.57–1.04, p =0.09). However, the authors noted that most of the studies had methodological weaknesses, the results were often inconsistent, and secure inferences from the available clinical trials were limited [10].
There is a growing consensus that serum 25(OH)D concentrations of at least 80 nmol/l are needed to achieve the maximal efficiency of vitamin D-induced intestinal calcium transport and for optimal bone health [1, 36, 37]. While in our study the overall correlation between 25(OH)D and PTH was moderate, we observed a threshold effect, with PTH levels below 65 ng/l in every woman who had 25(OH)D levels equal or greater than 120 nmol/l. In accord with Heaney [38], the findings of this study suggest that secondary hyperparathyroidism could be prevented in most or all individuals by increasing serum 25(OH)D to at least 120 nmol/l year-round. Such an increase can be achieved with 1,000 IU/day supplementation with vitamin D3, which is comfortably below the level of 2,000 IU/day, the maximum intake where no adverse effects would be expected [38, 39].
Winter is mild in southern California, with mean temperatures in January of 57.4°F and with an average of 12 days of clear sky every month; nevertheless, there was substantial seasonal variation in 25(OH)D levels. Fall marked the highest 25(OH)D level, consistent with the accumulation of 25(OH)D during the summer and the 3-week half life of vitamin D [40, 41]. There was little seasonal variation for PTH, probably reflecting the overall adequate values of 25(OH)D. However, the associations between 25(OH)D and PTH with BMD observed here were independent of season of sampling.
Both vitamin D and HT increase intestinal calcium absorption [42]. Recent findings from the Women’s Health Initiative indicate that HT may not be safe for long-term therapy, and women may be discontinuing HT as a result. Those who discontinue estrogen use may experience secondary hyperparathyroidism due to decreased calcium absorption.
It is important to redefine the amount of vitamin D intake necessary to maintain the desirable level of PTH, and minimize the possibility of further bone loss. Carefully planned clinical trials would be helpful to identify the appropriate dosage of vitamin D for prevention of secondary hyperparathyroidism and its long-term adverse effects on bone.
References
Heaney RP, Dowell MS, Hale CA, Bendich A (2003) Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 22:142–146
Fukugawa M, Kurokawa K (2002) Calcium homeostasis and imbalance. Nephron 92 [Suppl 1]:41–45
Lips P (2001) Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22:477–501
Sahota O, Masud T, San P, Hosking DJ (1999) Vitamin D insufficiency increases bone turnover markers and enhances bone loss at the hip in patients with established vertebral osteoporosis. Clin Endocrinol (Oxf) 51:217–221
Ooms ME, Roos JC, Bezemer PD, van der Vijgh WJ, Bouter LM, Lips P (1995) Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double-blind trial. J Clin Endocrinol Metab 80:1052–1058
Scharla SH, Scheidt-Nave C, Leidig G, Woitge H, Wuster C, Seibel MJ, Ziegler R (1996) Lower serum 25-hydroxyvitamin D is associated with increased bone resorption markers and lower bone density at the proximal femur in normal females: a population-based study. Exp Clin Endocrinol Diabetes 104:289–292
Norris JM (2001) Can the sunshine vitamin shed light on type 1 diabetes? Lancet 358:1476–1478
Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ (1992) Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 327:1637–1642
Dawson-Hughes B, Harris SS, Krall EA, Dallal GE (1997) Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 337:670–676
Papadimitropoulos E, Wells G, Shea B, Gillespie W, Weaver B, Zytaruk N, Cranney A, Adachi J, Tugwell P, Josse R, Greenwood C, Guyatt G (2002) Meta-analyses of therapies for postmenopausal osteoporosis. VIII: Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr Rev 23:560–569
Gillespie WJ, Avenell A, Henry DA, O’Connell DL, Robertson J (2001) Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev CD000227
Trivedi DP, Doll R, Khaw KT (2003) Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 326:469
Meyer HE, Smedshaug GB, Kvaavik E, Falch JA, Tverdal A, Pedersen JI (2002) Can vitamin D supplementation reduce the risk of fracture in the elderly? A randomized controlled trial. J Bone Miner Res 17:709–715
Malabanan A, Veronikis IE, Holick MF (1998) Redefining vitamin D insufficiency. Lancet 351:805–806
Oliveri B, Plantalech L, Bagur A, Wittich AC, Rovai G, Pusiol E, Lopez Giovanelli J, Ponce G, Nieva A, Chaperon A, Ladizesky M, Somoza J, Casco C, Zeni S, Parisi MS, Mautalen CA (2004) High prevalence of vitamin D insufficiency in healthy elderly people living at home in Argentina. Eur J Clin Nutr 58:337–342
Standing Committee on the Scientific Evaluation of Dietary Reference Intakes IoM (1997) Dietary reference intakes: calcium, phosphorus, magnesium, vitamin D, and fluoride. National Academy Press, Washington, DC
van der Wielen RP, Lowik MR, van den Berg H, de Groot LC, Haller J, Moreiras O, van Staveren WA (1995) Serum vitamin D concentrations among elderly people in Europe. Lancet 346:207–210
Peacock M, Liu G, Carey M, McClintock R, Ambrosius W, Hui S, Johnston CC (2000) Effect of calcium or 25OH vitamin D3 dietary supplementation on bone loss at the hip in men and women over the age of 60. J Clin Endocrinol Metab 85:3011–3019
Ooms ME, Lips P, Roos JC, van der Vijgh WJ, Popp-Snijders C, Bezemer PD, Bouter LM (1995) Vitamin D status and sex hormone binding globulin: determinants of bone turnover and bone mineral density in elderly women. J Bone Miner Res 10:1177–1184
Melin A, Wilske J, Ringertz H, Saaf M (2001) Seasonal variations in serum levels of 25-hydroxyvitamin D and parathyroid hormone but no detectable change in femoral neck bone density in an older population with regular outdoor exposure. J Am Geriatr Soc 49:1190–1196
Holick MF, Matsuoka LY, Wortsman J (1989) Age, vitamin D, and solar ultraviolet. Lancet 2:1104–1105
Chen TC, Turner AK, Holick MF (1990) Methods for the determination of the circulating concentration of 25-hydroxyvitamin D. J Nutr Biochem 1:315–319
Papaioannou A, Ray JG, Ferko NC, Clarke JA, Campbell G, Adachi JD (2001) Estimation of creatinine clearance in elderly persons in long-term care facilities. Am J Med 111:569–573
Lips P, Wiersinga A, van Ginkel FC, Jongen MJ, Netelenbos JC, Hackeng WH, Delmas PD, van der Vijgh WJ (1988) The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab 67:644–650
Khosla S, Atkinson EJ, Melton LJ 3rd, Riggs BL (1997) Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: a population-based study. J Clin Endocrinol Metab 82:1522–1527
LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J (1999) Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA 281:1505–1511
Jacques PF, Felson DT, Tucker KL, Mahnken B, Wilson PW, Rosenberg IH, Rush D (1997) Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr 66:929–936
Need AG, Horowitz M, Morris HA, Nordin BC (2000) Vitamin D status: effects on parathyroid hormone and 1, 25-dihydroxyvitamin D in postmenopausal women. Am J Clin Nutr 71:1577–1581
Mezquita-Raya P, Munoz-Torres M, Luna JD, Luna V, Lopez-Rodriguez F, Torres-Vela E, Escobar-Jimenez F (2001) Relation between vitamin D insufficiency, bone density, and bone metabolism in healthy postmenopausal women. J Bone Miner Res 16:1408–1415
Collins D, Jasani C, Fogelman I, Swaminathan R (1998) Vitamin D and bone mineral density. Osteoporos Int 8:110–114
Fradinger EE, Zanchetta JR (2001) Vitamin D and bone mineral density in ambulatory women living in Buenos Aires, Argentina. Osteoporos Int 12:24–27
Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B (2004) Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med 116:634–639
Roe SD, Porter CJ, Godber IM, Hosking DJ, Cassidy MJ (2004) Reduced bone mineral density in male renal transplant recipients: evidence for persisting hyperparathyroidism. Osteoporos Int
Pluijm SM, Visser M, Smit JH, Popp-Snijders C, Roos JC, Lips P (2001) Determinants of bone mineral density in older men and women: body composition as mediator. J Bone Miner Res 16:2142–2151
Sigurdsson G, Franzson L, Steingrimsdottir L, Sigvaldason H (2000) The association between parathyroid hormone, vitamin D and bone mineral density in 70-year-old Icelandic women. Osteoporos Int 11:1031–1035
Dawson-Hughes B (2004) Racial/ethnic considerations in making recommendations for vitamin D for adult and elderly men and women. Am J Clin Nutr 80:1763S–1766S
Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ (1997) Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7:439–443
Heaney RP (2000) Vitamin D: How much do we need, and how much is too much? Osteoporos Int 11:553–555
Vieth R, Chan PC, MacFarlane GD (2001) Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr 73:288–294
Haddad JG Jr, Rojanasathit S (1976) Acute administration of 25-hydroxycholecalciferol in man. J Clin Endocrinol Metab 42:284–290
Holick MF (1990) The use and interpretation of assays for vitamin D and its metabolites. J Nutr 120 [Suppl 11]:1464–1469
Gennari C, Agnusdei D, Nardi P, Civitelli R (1990) Estrogen preserves a normal intestinal responsiveness to 1,25-dihydroxyvitamin D3 in oophorectomized women. J Clin Endocrinol Metab 71:1288–1293
Acknowledgments
Supported by the National Institute of Diabetes and Digestive and Kidney Diseases, grant DK31801, and the National Institute on Aging, grant AG07181
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
von Mühlen, D.G., Greendale, G.A., Garland, C.F. et al. Vitamin D, parathyroid hormone levels and bone mineral density in community-dwelling older women: The Rancho Bernardo Study. Osteoporos Int 16, 1721–1726 (2005). https://doi.org/10.1007/s00198-005-1910-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-005-1910-8