Abstract
Introduction
Heart failure and osteoporosis are common conditions in older, frail individuals. It is important to investigate interactions of the common problems in the aging population to devise relevant interventions.
Methods
Sixty individuals (43 men, mean age 77±9 years, and 17 women, mean age 78±12 years) with heart failure (HF) and 23 age- and gender-matched non-HF controls (15 men, eight women; mean age 77±9 years) underwent hip and bone mineral density (BMD) assessments; frailty assessment; physical performance assessment including 6-min walk, grip strength, and self-reported physical activity; and biochemical assessment including calcium, parathyroid hormone (PTH), 25-hydroxy vitamin D (25-OHD), estradiol, creatinine (Cr), and blood urea nitrogen levels (BUN).
Results
Significant differences between HF and control groups were found for BMD Z-scores of the femoral neck, total femur, and trochanteric region at the femur (p<.05). Further differences between groups included frailty score (p=.02), 6-min walking distance (p<.001), and self-reported physical activity (p=.001). In addition, several differences between groups were present for calcium (p=.054), PTH (p<.001), 25-OHD (p=.01), Cr (p=.04), and BUN (p=.01). In regression analysis, HF (defined as case, by ejection fraction, or by New York Heart Association class), frailty status, and vitamin D were significant predictors of lower bone mass at the femur.
Conclusions
Individuals with HF have lower BMD, in part related to lower vitamin D status and higher frailty rates. Interventions to optimize vitamin D and physical activity should be explored to prevent bone loss in individuals with heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has recently been a dramatic increase in the aging of the population, and with aging comes an increase in medical complexity due to multiple comorbidities. The relationship between multiple diseases and disease treatment is becoming increasingly important. For the older adult, heart failure (HF) and osteoporosis are two common chronic conditions that are gaining importance for healthcare. Both HF and osteoporosis are more common in frail individuals [1–3]. It is important to investigate interactions of the common problems in the aging population to devise relevant interventions.
Heart failure is a major public health problem and the most common cause of morbidity and mortality in older adults [4]. The lifetime risk for HF is 1 in 5 for both men and women [5]. The aging of the population and improved therapy for HF is leading to an increase in the number of older adults who will live with this disabling condition. A recent epidemiologic study revealed that the incidence of HF has remained unchanged in the last 20 years, but 5-year survival with the disease has improved from 43% in 1979–1984 to 52% in 1996–2000 (p<.001) [6]. Recognition of the chronic physiologic changes that affect a variety of systems in the aging HF patient is important to slow time to disability. The increased survival and the chronicity of HF will result in a greater impact of comorbidities.
Osteoporosis is a bone-thinning disease that results in fractures that occur with minimal trauma. The direct healthcare costs related to osteoporosis are estimated to be $14 billion per year, comparable to costs of HF [7]. Osteoporosis is a common complication in individuals with severe HF who receive cardiac transplant [8–11]. Shane et al. [8] evaluated 101 patients undergoing evaluation for cardiac transplant and found that BMD values, controlled for weight and height, were similar to those of population norms in the men but were lower in the women. There was also a high prevalence of vitamin D insufficiency. Analysis of the relationship of osteoporosis and HF and the interaction with frailty in the progression to dependency and disability is important to tailor interventions. If HF is a major factor in the pathogenesis of osteoporosis and fracture, development of early interventions to slow bone loss would be warranted in frail HF patients.
We evaluated BMD in 60 older individuals with HF not requiring cardiac transplantation and compared them with 23 community-based controls of similar age to explore the role of HF in bone mass.
Methods
The study was reviewed and approved by the Institutional Review Board at the University of Connecticut. HF participants were recruited from the University of Connecticut Heart Failure Center. Patients age ≥60 years with HF and an ejection fraction (EF) of ≤40% as measured by echocardiogram, cine angiography, or radionuclide angiography within the preceding year were offered participation in the study. Patients were excluded if they had metastatic, active, or advanced cancer; were undergoing chemotherapy, radiation treatment, or hormonal therapy; had systemic rheumatologic or connective tissue disorders; consumed more than three alcoholic drinks per day; had used androgen, estrogen, dehydroepiandrosterone (DHEA), or hormone receptor antagonists in the preceding year; had advanced liver disease or renal disease requiring dialysis; had Parkinson’s disease; were unable to ambulate; or had had a myocardial infarction within 3 months prior to the study. Control participants from the greater Hartford, Connecticut, area were recruited by newspaper advertisements, physician referrals, and contacts at senior citizen centers. Exclusion criteria were the same, and controls had no history of HF.
After a patient gave informed consent, his or her medical history was reviewed, including the cause of HF, comorbidities, hospitalization history, and the need for intravenous medications in the Heart Failure Center in the 6 months prior to the study visit. A physical exam was performed to assess HF stability and New York Heart Association (NYHA) class. The frailty phenotype was determined, and a 6-min walk test was performed. The frailty phenotype evaluation based on that described by Fried et al. [12] included self-reported weight loss of ≥10 pounds in the preceding year, grip strength as measured by handheld Jamar dynamometer, sense of exhaustion as evaluated by two questions from the Center of Epidemiologic Studies Depression Scale [13], walking speed as measured by an 8-foot walk, and level of physical activity reported in kcal/week from the Physical Activity Scale in the Elderly (PASE) [14]. Individuals were reported as frail if they met criteria in three or more of the five characteristics. The 6-min walk was performed according to the protocol of Guyatt et al. [15].
Bone density measurement
A dual-energy x-ray absorptiometry machine (DXA-IQ, Lunar, Madison, WI, USA; software version 4.6f) was used to measure BMD (g/cm2). One technician scanned and analyzed all measures of BMD of the left femur (femoral neck, trochanter, total femur). The coefficient of variation of BMD measurement at the proximal femur was <1%. The difference between an individual’s BMD and the mean BMD for a reference population (National Health and Nutrition Examination Survey data) can be expressed in standard deviation units. When an individual’s BMD is compared with the mean BMD score in a young, healthy population, this standard deviation is referred to as the T-score, and when compared with a similar gender-, age-, weight-, and height-matched population, it is referred to as a Z-score.
Biochemical measurements
Serum was divided into 0.5-ml aliquots and stored at −70°C. Participants underwent measurement of fasting serum calcium (Ca), blood urea nitrogen (BUN), estradiol, creatinine, parathyroid hormone (PTH), and 25-hydroxy vitamin D levels (25-OHD); 25-OHD measurements were measured by enzyme immunoassay (Immunodiagnostic Systems, Fountain Hills, AZ, USA), with an intraassay coefficient of variation of <6.6%. Intact PTH was measured by ELISA (Diagnostic Systems Labs, Webster, TX, USA) with an average intraassay variability of 5%.
Statistical analysis
Distributions of baseline characteristics and clinical assessments in the study samples were summarized with counts and percentages or with means and standard deviations as appropriate. A contrast of the distributions for each measurement between the HF and control samples was performed using either the χ2 test, Student’s t-test, Welch’s corrected t-test, or Wilcoxon’s rank sum test as warranted by the scale, normality, and variance properties of the particular measure. Means and standard deviations were also determined to characterize distributions of bone density measures between the two samples. The t-tests and rank sum test were again used to determine the statistical significance of differences in the centers of those distributions. The BMD measurements were used to classify subjects as osteoporotic, osteopenic, or normal; frequencies of these categories were compared between the HF and control samples using Fisher’s exact test.
Multivariate linear regression analysis was applied to evaluate the dependence of BMD Z-scores on a number of potentially predictive factors (HF, vitamin D, PTH level, frailty score, PASE score, 6-min walking distance, creatinine clearance, and diuretic use). These independent variables were chosen because of their known effects on bone metabolism and/or their clinical importance in HF. Model building started with a backwards, stepwise, variable selection strategy. After the backwards strategy was applied, a forward stepwise selection strategy was applied, and the sources of differences in results between the two approaches were identified and resolved. The model-building process was applied to each dependent variable three times: once using HF as an independent variable, a second time using EF (control vs. HF with EF <20% vs. HF with EF ≥20%) in place of HF, and a third time using NYHA class (no HF vs. HF with NYHA classes I and II vs. HF with NYHA classes III and IV) in place of HF. The second and third applications of the model-building process were undertaken to determine whether refinement of the HF classification would lead to different conclusions regarding predictors of BMD. Regression diagnostics were applied to the final models for each BMD measure (femoral total, neck, and trochanter Z-scores) to rule out effects of outliers, nonnormality, heteroscedasticity of variance, and collinearity on modeling results. A 5% significance level was used in all statistical tests and in applying the variable selection strategies.
Results
One hundred sixty-nine patients (68 women and 101 men) with HF were recruited for the study. Eighty-four were eligible, and 58 (41 men, mean age 76±9, and 17 women, mean age 78±12) agreed to participate. Twenty-three non-HF controls (15 men, eight women) with a mean age of 77±9 years (77±9 for men, 78±11 for women) were enrolled in the study. HF was defined by an EF ≤40%. Among the cases, 90% (52) had EFs ≥20%, and 10% (six) had EFs <20%. The frequencies of the NYHA classes among the HF cases were 2% (one) in class I, 57% (33) in class II, 36% (21) in class III, and 5% (three) in class IV.
Descriptive characteristics for both samples are presented in Table 1. Significant differences existed between the HF and the control groups in measures of physical performance, including composite score of the frailty model (p=.02), self-reported physical activity (p=.001), usual walking speed (p=.002), and 6-min walking distance (p<.001), but not hand grip strength (p=.26). Twenty-seven percent of HF subjects met the criteria for frailty, whereas none of the control subjects did (p=.02).
As reported in Table 1, significant differences between groups were also noted in biochemical parameters. There was significantly lower 25-OHD in the HF group (p=.01). Total calcium was also lower in the HF cases, but the difference did not reach statistical significance (p=.054). PTH was significantly higher in HF patients (p<.001). Eighty-three percent of HF patients and 43% of controls were above the normal range for PTH (upper limit of normal 65 pg/ml). No differences in estradiol levels were detected between groups (p=.67). Measures of renal function were different, including BUN (p=.01) and creatinine (p=.04), although no difference was noted in creatinine clearance (p=.43). This discrepancy may indicate that the differences in BUN and creatinine were due to prerenal azotemia or could reflect variability in measuring creatinine clearance.
Comparisons of BMD of the total femur, femoral neck, and trochanteric region of the femoral neck are shown in Table 2. Significantly lower differences between BMD Z-scores, which were corrected for age, gender, height, and weight, were found in the femoral neck, total femur, and trochanteric region of the femur for those with and without HF (p<.05 for all). BMD T-score values were also consistently lower in HF patients than in control subjects, but p-values for these differences generally failed to fall below the 5% threshold for statistical significance. BMD at the femoral neck revealed that 19% (11) of the HF patients had osteoporosis compared with 5% (one) of the controls, 49% (28) of HF patients had osteopenia compared with 57% (12) of controls, and 32% (18) of HF patients compared with 38% (eight) of controls were normal. An application of Fisher’s exact test established that the differences in frequencies of osteoporosis and osteopenia between patients and controls were not statistically significant (p=0.35). (Note that three subjects—one HF case and two controls—did not have BMD assessed and are not included in these tabulations.) In the multivariate regressions (Table 3), HF and its reexpression in terms of EF level and NYHA class were always significant predictors of the Z-scores for total femur and the femoral trochanter. HF cases tended to have lower Z-scores than controls on BMD assessments at these two sites. Furthermore, among the HF cases, those with low EFs or with high NYHA classes tended to have lower Z-scores than cases with high EFs or low NYHA classes, respectively.
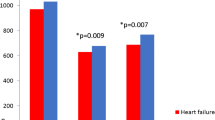
In the models of total femur and femoral trochanter Z-scores, the only significant predictor other than HF status was the frailty score. In general, higher frailty scores were associated with lower Z-scores, and the trends in mean Z-scores across the three ordinal frailty score categories appeared to be linear (Fig. 1). However, the frailty score was not a significant predictor of the femoral trochanter Z-score in the presence of NYHA class. In that circumstance, the frailty score was excluded from the final model because the p-value for its slope estimate (.08), though near the 5% threshold for inclusion, failed to reach or cross that threshold. Although the frailty score was always a significant predictor of the total Z-score, in the model with NYHA class the magnitude of its slope (−0.38) was much smaller than in the models with HF and EF level (−0.49 and −0.53, respectively). This suggests a correlation between the frailty score and NYHA class. Correlation analysis established that the relationship between frailty score and NYHA class (R=+0.47) was statistically significant (p<.0001).
The final models for total femur and femoral trochanter Z-scores explained between 14.5% and 22.3% of variance in the dependent variables. In fact, all models that included the frailty score as a predictor variable had R 2 values at or above 18.5%.
In the models for femoral neck Z-score, vitamin D was the only consistent predictor. NYHA class was also a significant predictor (p=.03) in conjunction with vitamin D, but HF status (p=.08) and EF level (p=.06) missed the 5% cutoff for inclusion in the corresponding models.
The frailty score was also excluded from the final models for femoral neck Z-score. A comparison of the p-values for frailty score between models (p=.08 in the model with vitamin D alone and p=.29 in the model with both vitamin D and NYHA class) again suggests an overlap in the information conveyed by the frailty score and NYHA class in predicting the femoral neck Z-score. That overlap was confirmed by results of the correlation analysis reported above. Further, a significant correlation between vitamin D (after square root transformation) and frailty exists; the correlation is −0.32, and the p-value is 0.003.
Vitamin D explained 12.1% of the variation in femoral neck Z-score. In combination with NYHA class, it explained 17.3% of variation in the dependent variable. Figure 2 illustrates the relationship between vitamin D levels and the femoral neck Z-scores both with and without concurrent adjustment for the effects of NYHA class. To account for skewness, the independent variable representing vitamin D levels was transformed to a square root scale. Inversion of that transformation after model fitting resulted in a curvilinear relationship between vitamin D and the femoral neck Z-score.
Discussion
We evaluated 58 older individuals with EF ≤40 who had predominantly NYHA class II-III HF. Significant differences in femoral Z-scores were found between individuals with HF and community-dwelling controls. One cause of the difference could be increased weight from edema, which would falsely decrease Z-score, but that is unlikely in this group of individuals with well-controlled HF and whose weights did not differ from those of the controls. HF was a significant predictor of lower BMD as defined by our inclusion criteria of EF ≤40 or by further differentiation of EF or NYHA class. The BMD was also affected by frailty status and vitamin D levels. Individuals with HF were significantly more likely to have poor physical performance, a higher frailty composite score, and lower 25-OHD levels than the community-dwelling age- and gender-matched controls. Thus, our study suggests that the association between HF and lower bone density may be due to fact that HF patients are more likely to be frail, have lower endurance, and have poor nutrition and/or decreased exposure to sunlight.
Although frailty has not been directly measured previously, in another study assessing BMD in HF patients, lower total body BMD was seen in cachectic HF patients compared with the noncachectic HF patients or controls, but hip or spine BMD values were not reported [16]. Weight loss is a component of the frailty definition, and these results suggest that frailty and poor nutrition or elevated cytokines in conjunction with HF can increase bone loss. Shane et al. evaluated the bone health of NYHA class III-IV pretransplant HF patients (mean age for men, 54 years; for women, 37 years) and found normal Z-scores but an increased prevalence (26%) of low 1,25-dihydroxy vitamin D levels associated with impaired renal function [10]. The patients in this study were young and may not have had time to develop significant bone loss related to HF.
The association between fracture and frailty is clear, but the association between osteoporosis, defined as low bone density, and frailty is not established. Components of the definition of frailty used in this study such as poor strength, low levels of physical activity, and weight loss associated with malnutrition are risk factors for osteoporotic fracture [17–19]. Others have reported that individuals with HF are at increased risk of frailty [2], and we found that 27% of individuals with HF met the criteria for frailty, compared with the usual 7% in community-dwelling adults over age 65 years [12] and none in our control group. One study of older women found no association between level of frailty and BMD; frailty was defined from a nurse’s or physiotherapist’s subjective impression of an individual’s level of frailty on a scale of 1 (not frail) to 100 (frail) after a brief meeting with the subject [20]. In our study of 392 older community-dwelling men, the frailty model was not associated with BMD, although walking speed and hand grip strength were [21].
Physical activity is a known contributor to bone health [22–26]. Our data suggest that the poor physical performance of older frail adults affects bone health and that HF patients are more likely to perform poorly. Exercise programs have successfully been used in HF patients to improve exercise tolerance and duration [27]. Exercise is the focus of a large ongoing multicenter trial in HF patients to evaluate the effect on mortality, hospitalizations, and quality of life [28]; it would be appropriate to add bone measurements or fractures to the clinical outcomes.
Vitamin D levels were found to be low and PTH increased in individuals with HF compared with controls. Shane et al. also found decreased 25-OHD levels in hospitalized HF patients compared with nonhospitalized HF patients. They speculate that the decreased 25-OHD levels were due to decreased sun exposure. Other causes for the diminished 25-OHD levels in HF patients may be due to malabsorption secondary to intestinal congestion or decreased synthesis of 25-OHD due to hepatic congestion [29]. While mean 25-OHD levels were in the normal range for most of the subjects in the study, the elevated PTH level in 81% of HF subjects and 39% of controls indicates that levels were suboptimal and that these patients require vitamin D supplementation.
There is evidence that vitamin D is as important for muscle as it is for bone [30–33]. In this study, 25-OHD levels correlated with 6-min walking performance. Low serum 25-OHD has been associated with diminished exercise tolerance in HF patients [8]. Therefore, in addition to the direct effects of vitamin D on bone, vitamin D’s effects on muscle and physical functioning may indirectly contribute to low BMD.
The importance of low BMD in this population is that it is likely to increase fracture risk. This will be compounded by poor physical performance, which will increase the risk of falls. A fracture is likely to have even more devastating consequences in a patient with HF. Hence, analysis of the risk factors and appropriate interventions are critical for this population.
Conclusions
The aging of the population and improved therapy for HF will lead to an increase in the prevalence of older adults with this chronic disabling condition. Recognition of the physiologic changes that affect a variety of systems in the aging HF patient is important to slow time to disability. Osteoporosis with or without fracture is a well-recognized geriatric condition and an area of intense research. Our study suggests that individuals with HF are at increased risk of bone loss perhaps due, in part, to effects of HF on vitamin D status and physical performance. Interventions to optimize vitamin D levels and physical activity may prevent bone loss and reduce fracture risk.
References
Newman AB, Gottdiener JS, McBernie MA, Hirsch CH, Kop WJ, Tracy RP, Walston JD, Fried LP (2001) Associations of subclinical cardiovascular disease with frailty. J Gerontol 56A(3):M158–M166
Walston JD, McBernie MA, Newman AB, Tracy RP, Kop WJ, Hirsch CH, Gottdiener JS, Fried LP (2002) Frailty and activation of the inflammation and coagulation systems with and without co-morbidities. Arch Intern Med 162:2333–2341
Kenny AM, Waynik IV, Smith J, Fortinsky R, Kleppinger A, McGee D. Association between osteoporosis and frailty in older community dwelling men. (Unpublished work)
Hunt SA, Baker DW, Chin MH, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure); International Society for Heart and Lung Transplantation; Heart Failure Society of America (2001) ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. Circulation 104(24):2996–3007
Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB et al (2002) Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 106(24):3068–3072
Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ (2004) Trends in heart failure incidence and survival in a community-based population. JAMA 292(3):344–350
Ray NF, Chann JK, Thamer M, Melton LJ (1997) Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res 12:24–35
Shane E, Mancini D, Aaronson K et al (1997) Bone mass, vitamin D deficiency, and hyperparathyroidism in congestive heart failure. Am J Med 103(3):197–207
Shane E, Rivas M, McMahon DJ, Staron RB, Silverberg SJ, Seibel MJ, Mancini D, Michler RE, Aaronson K, Addesso V, Lo SH (1997) Bone loss and turnover after cardiac transplantation. J Clin Endocrinol Metab 82(5):1497–1506
Muchmore JS, Cooper DK, Ye Y, Schlegel V, Pribil A, Zuhdi N (1992) Prevention of loss of vertebral bone density in heart transplant patients. J Heart Lung Transplant 11(5):959–963
Lee AH, Mull RL, Keenan GF, et al (1994) Osteoporosis and bone morbidity in cardiac transplant recipients. Am J Med 96(1):35–41
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA (2001) Frailty in older adults: evidence for a phenotype. J Gerontol 56A(3):M146–M156
Orme JG, Reis J, Herz EJ (1986) Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol 42(1):28–33
Washburn RA, Smith KW, Jette AM, Janney CA (1993) The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46(2):153–162
Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB (1985) The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 132(8):919–923
Anker SD, Clark AL, Teixeira MM, Hellewell PG, Coats AJ (1999) Loss of bone mineral in patients with cachexia due to chronic heart failure. Am J Cardiol 83(4):612–615, A10
World Health Organization (1994) WHO technical report series #843: assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organization, Geneva
Orwoll E (2000) Assessing bone density in men. J Bone Miner Res 15(10):1867–1870
Amin S, Feldson DT (2001) Osteoporosis in men. Rheum Dis Clin North Am 27:19–47
Gerdhem P, Ringsberg KA, Magnusson H, Obrant KJ, Akesson K (2003) Bone mass cannot be predicted by estimations of frailty in elderly ambulatory women. Gerontology 3:168–172
Kenny AM, Waynik IY, Smith JA, Fortinksy R, Kleppinger A, McGee D. The association between level of frailty and bone mineral density in community-dwelling men. (Submitted for publication)
Gleeson PB, Protas EJ, LeBlanc AD, Schneider VS, Evans HJ (1990) Effects of weight lifting on bone mineral density in premenopausal women. J Bone Miner Res 5(2):153–158
Snow-Harter C, Bouxsein ML, Lewis BT, Carter DR, Marcus R (1992) Effects of resistance and endurance exercise on bone mineral status of young women: a randomized exercise intervention trial. J Bone Miner Res 7(7):761–769
Nelson ME, Fiatarone MA, Morganti CM, Trice I, Greenberg RA, Evans WJ (1994) Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. A randomized controlled trial. JAMA 272(24):1909–1914
Dalsky GP, Stocke KS, Ehsani AA, Slatopolsky E, Lee WC, Birge SJ Jr (1988) Weight-bearing exercise training and lumbar bone mineral content in postmenopausal women. Ann Intern Med 108(6):824–828
Judge JO, Kleppinger A, Kenny A, Smith JA, Biskup B, Marcella G (2005) Home-based resistance training improves femoral bone mineral density in women on hormone therapy. Osteoporos Int 16(9):1096–1108
Piña IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ (2003) Exercise and heart failure: a statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention. Circulation 107:1210–1225
HF-Action: http://www.clinicaltrials.gov/ct/show/NCT00047437
Reichel H, Koeffler HP, Norman AW (1989) The role of the vitamin D endocrine system in health and disease. New Eng J Med 320:980–991
Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C (2000) Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res 15:1113–1118
Bischoff HA, Staehelin HB, Dick W, Akos R, Theiler R, Pfeiffer M, Begerow B, Lew RA, Conzelmann M (2001) Fall prevention by vitamin D and calcium supplementation. J Bone Min Res 16(supp1):S163
Young A, Brenton DP, Edwards R (1978) Analysis of muscle weakness in osteomalacia. Clin Sci Mol Med 54(2):31
Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, Dawson-Hughes B (2004) Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged >or =60 y. Am J Clin Nutr 80(3):752–758
Acknowledgements
This work was supported by the General Clinical Research Center (MO1-RR06192) and R01 AG18887. We wish to thank Linda Gregory, Alison Kleppinger, and MaryBeth Barry for their help in completing this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kenny, A.M., Boxer, R., Walsh, S. et al. Femoral bone mineral density in patients with heart failure. Osteoporos Int 17, 1420–1427 (2006). https://doi.org/10.1007/s00198-006-0148-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0148-4