Abstract
The study aimed to identify factors related to bone mineral density (BMD) among older patients with heart failure (HF). A total of 70 consecutive patients with HF aged 65 years or older who were admitted to an acute hospital due to worsening condition were enrolled before discharge. BMD of the femoral neck was evaluated using the DEXA method. Physical function, as well as echocardiographic and laboratory findings including biomarker of HF severity were collected. Bivariate and multiple regression analyses were employed to determine the association between BMD and the clinical variables. Bivariate analysis determined that age, grip strength, walking speed, serum albumin, and N-terminal pro B-type natriuretic peptide (NT-proBNP) were significantly correlated with BMD (P < 0.01), whereas other clinical parameters were not. The multiple regression analysis identified NT-proBNP as an independent related factor for BMD after adjusting with confounding clinical variables. NT-proBNP was independently related to BMD among older patients with HF. Our results suggest the inclusion of bone fracture prevention strategies in disease management programs, especially for older patients with HF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis, which is characterized by compromised bone quality and strength, is a common geriatric disorder leading to increased osteoporotic fractures and disability [1]. Bone fractures, which are well-established geriatric disease due to osteoporosis [1], decreased physical function and activities of daily living, and worsening health-related quality of life.
The number of patients with heart failure (HF), especially older ones, has increased exponentially within this decade and is expected to continuously increase throughout the next decade in worldwide [2]. Despite the dramatic reduction in HF mortality rates, rehospitalization due to worsening HF remains common globally including Japan [2, 3]. Several clinical issues in older people, including mobility loss, falls and fall-related fractures, cognitive impairments, malnutrition, and mental disorders, have been considered as preventable geriatric syndromes [4]. Such geriatric syndromes should have been also considered in the HF management in accordance with the increased number of older patients with HF.
The relationship between HF and bone mineral density (BMD), which is a biomarker of osteoporosis and an established risk factor for fractures, has been pointed [5, 6]. A large-scale clinical trial reported that lower BMD was associated with higher HF risk [5]. A meta-analysis has indicated that patients with HF had significantly lower BMD compared to healthy controls [6]. Furthermore, a hazard ratio of 4.40 for hip fracture after a diagnosis of HF was reported [7]. Despite these previous studies indicating a close association between HF and BMD, most of them had enrolled patients with HF who had reduced systolic function. Considering approximately 40% of patients with HF have preserved systolic function, the study with wider range of HF severity has been required. In addition, previous studies determined HF severity through the New York Heart Association functional class, which places patients in one of four categories based on symptom severity and the amount of exertion needed to provoke symptoms [6]. In clinical settings, however, N-terminal pro B-type natriuretic peptide (NT-proBNP), a well-established biomarker for HF severity, is widely used and may be considered a more precise and appropriate index for the HF severity [8]. Considering above-mentioned limitations of the previous studies, the association between HF severity and osteoporosis among older patients with HF remains to be fully elucidated. We hypothesized that HF severity, in addition to other clinical parameters, would be related to BMD among older patients with HF.
This study was aimed to determine the relationship between BMD and HF severity among older Japanese patients with HF.
Materials and methods
Participants
A total of 70 consecutive patients with HF admitted to Fujita Health University Bantane Hospital due to worsening condition between April 2017 and March 2018 were enrolled before discharge. Patients less than 65 years, who could not walk 10 m independently, had severe dementia, had a history of femoral neck fracture or did not agree to participate were excluded. The study protocol was approved by the Research Ethics Committee of Fujita Health University (Approval No: 15-259). All study participants provided written informed consent prior to participation.
Study design and protocol
This study utilized a single-center, observational, cross-sectional design. Baseline medical examinations and physical function tests were conducted within 1 week before discharge.
Bone mineral density measurement
BMD measurements using dual-energy X-ray absorptiometry (DXA) were performed at the femoral neck, which has been reported to be significantly lower in HF patients than healthy controls [6]. DXA scans were performed using PRODIGY Fuga (GE Health Care Japan. Tokyo, Japan) with the array beam mode. Scans were analyzed blindly at our hospital using Hologic software version 7.10. This study used the mean BMD value of both femoral necks for the analyses.
Clinical assessments
Clinical characteristics were collected from patients’ medical records for age, gender, body mass index, HF etiology, and medications. Established related variables, including NT-proBNP, serum albumin, serum sodium, and estimated glomerular filtration rate (eGFR) were determined from blood tests [8]. In addition, left ventricular ejection fraction (LVEF) and E/Ea, which is the ratio of the early transmitral flow velocity to the early diastolic mitral annular velocity, were determined from an echocardiography.
Grip strengths were measured using a Jamar dynamometer. Participants were asked to sit with their wrist in a neutral position and elbow flexed at 90° [9]. Grip strength was measured two times for each hand, with the highest value being used for analysis. Walking speed was evaluated through the 10-m walk test. Participants were requested to walk at their comfortable speed for 14 m, of which the middle 10 m was timed. The test was completed twice, with the faster speed being used for the analyses.
Statistical analyses
Data are presented as mean and standard deviation for continuous variables and number and percentages for categorical data. In bivariate analyses, Pearson’s or Spearman’s correlation coefficients were used to determine the association between BMD scores and clinical variables. Given the right-skewed distribution of NT-proBNP data, logarithmic conversion was performed. Variables with a p value of less than 0.1 upon bivariate analyses were entered into a multiple regression model with a forced entry method to determine independent factors for BMD. All analyses were performed using SPSS 24.0 software package (SPSS Inc., Tokyo, Japan), with a p value of less than 0.05 being considered statistically significant.
Results
Clinical characteristics are summarized in Table 1. Among the 70 patients included (mean age: 81.3 ± 8.5 years), 39 were men. The mean LVEF was 52.4%, while 23 patients (32.8%) had LVEF less than 40%. A total of 57 patients (81.4%) received loop diuretics, while no patient underwent pharmacotherapy for osteoporosis.
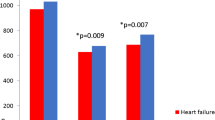
Correlations between BMD and clinical variables are presented in Table 2. Age, gender, BMI, serum albumin, HbA1c, log-transformed NT-proBNP, grip strength, and gait speed were significantly correlated with BMD, whereas LVEF and eGFR were not. Prior to multiple regression analysis, we excluded gender and grip strength from independent variables because of higher multicollinearity between age and gender (r = −0.769, p < 0.001), and grip strength (r= −0.806, p < 0.001). The multiple regression analysis identified that log-transformed NT-proBNP was selected as an independent correlated factor for BMD after adjustment for other several clinical variables. (Table 3).
Discussion
The current study showed that NT-proBNP, a well-establish biomarker of HF severity, was closely correlated with BMD among older patients with HF. To the best of our knowledge, this has been the first study to demonstrate that HF severity was an independent related factor for BMD among older patients with HF.
Although lower BMD [6] and higher risk of hip fracture [7] were observed in patients with HF, no study has addressed the association of BMD and HF severity. After adjusting for several clinical variables including age, medication, and physical function, our results demonstrated that log-transformed NT-proBNP was an independent correlated factor for BMD. The finding could be attributed to hormonal abnormalities and inflammatory activation among patients with HF. The patients with HF frequently develop significant bone loss over time related to testosterone depletion [10]. Healthy controls with high CRP and tumor necrosis factor-α group had lower BMD values and higher risk for hip fracture as compared to low group [11], while inflammatory cytokines have been well known and relevant markers of HF severity and prognosis [12]. These pathophysiological changes related to HF support the close association between HF severity and BMD among older patients with HF.
Age and walking speed were independent correlated factors for BMD in the study. Clinical guidelines have indicated that increasing age, female sex, low body weight, rheumatoid arthritis, current smoking, alcohol intake, vitamin D deficiency, low calcium intake, physical inactivity, and polypharmacy or long-term use of certain medications, such as glucocorticoids, anticoagulants, and diuretics, are all risk factors for osteoporosis [1]. Our data support previous findings suggesting aging and physical function as risk factors for osteoporosis. It is well known that physical function decreases with heart failure severity [8]. In our result, we could find independent association between BMD and NT-proBNP after adjustment for grip strength and walking speed. Thus, our data also showed that older patients with HF were more likely to develop osteoporosis due to not only aging or physical inactivity but also HF severity.
There was no relationship between BMD and pharmacotherapy. The current study included 16 patients on oral anticoagulants (six receiving a vitamin K antagonist and 10 receiving a non-vitamin K antagonist oral anticoagulant). Previous reports have shown that non-vitamin K antagonists promote lower risk for osteoporosis than vitamin K antagonists [13]. Moreover, most of our patients (approximately 80%) had been placed on loop diuretics, a first-line medication for patients with HF. Considering our patients’ pharmacological background, the much lower rate of anticoagulant use and much higher rate of loop diuretic use could perhaps explain why we detected no relationship between BMD and pharmacotherapy.
Previous guidelines and scientific statements have provided several disease management strategies for older patients with HF. Some lifestyle-related factors, such as salt intake, physical activity, and medication [14], could help manage HF conditions and prevent future rehospitalization due to HF deterioration. However, no information has been available regarding non-HF-related rehospitalizations, including those due to bone fracture or other diseases. Given that HF is a risk factor for bone fractures, as previously discussed, preventing all-cause rehospitalizations is important for improving health-related quality of life. Our results suggest that increased bone loss in conjunction with HF could likely increase fracture risk, especially among older patients with HF. Thus, strategies for optimal HF treatment and for optimizing physical activity or physical function among older patients with HF who have double the disease burden are critical.
Some potential limitations of the present study are worth noting. First, considering that this study was performed on patients with HF from a single institution, generalization of the results should be done with caution. Second, the cross-sectional design of our research does not allow for determining causal relationships. Then, longitudinal studies are needed to prove that strategies aimed at preventing bone fractures should be an integral component of disease management programs. Finally, we could not determine hormonal, inflammatory, and nutritional status. Thus, this study may serve as only preliminary research regarding the impact of HF severity on BMD among older patients with HF. Nevertheless, the results presented herein provide novel information about the relation between HF severity and BMD, and also suggested the optimal management of older patients with HF to prevent not only HF-related rehospitalizations but also all-cause hospitalizations.
In conclusion, the present study showed that NT-proBNP was an independent related factor for femoral neck BMD among older patients with HF. The results of this study suggest that strategies aimed at preventing bone fractures could be an integral component of disease management programs especially for older patients with HF.
References
Qaseem A, Forciea MA, McLean RM, Denberg TD, Clinical Guidelines Committee of the American College of Physicians (2017) Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med 166:818–839
Okura Y, Ramadan MM, Ohno Y, Mitsuma W, Tanaka K, Ito M, Suzuki K, Tanabe N, Kodama M, Aizawa Y (2008) Impending epidemic: future projection of heart failure in Japan to the year 2055. Circ J 72:489–491
Shah RU, Tsai V, Klein L, Heidenreich PA (2011) Characteristics and outcomes of very elderly patients after first hospitalization for heart failure. Circ Heart Fail 4:301–307
Integrated care for older people. Guidelines on community-level interventions to manage declines in intrinsic capacity (2017). https://apps.who.int/iris/bitstream/handle/10665/258981/9789241550109-eng.pdf;jsessionid=141EC7D80E377F103C2381F604CD8FA3?sequence=1. Accessed 10 Mar 2020
Fohtung RB, Brown DL, Koh WJ, Bartz TM, Carbone LD, Civitelli R, Stein PK, Chaves PH, Kestenbaum BR, Kizer JR (2017) Bone mineral density and risk of heart failure in older adults: the Cardiovascular Health Study. J Am Heart Assoc. https://doi.org/10.1161/JAHA.116.004344
Xing W, Lv X, Gao W, Wang J, Yang Z, Wang S, Zhang J, Yan J (2018) Bone mineral density in patients with chronic heart failure: a meta-analysis. Clin Interv Aging 13:343–353
Sennerby U, Melhus H, Gedeborg R, Byberg L, Garmo H, Ahlbom A, Pedersen NL, Michaëlsson K (2009) Cardiovascular diseases and risk of hip fracture. JAMA 302:1666–1673
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group (2016) Eur Heart J 37:2129–2200
Fess EE, Moran C (1981) Clinical assessment recommendations. American Society of Hand Therapists, Indianapolis, pp 6–8
Jankowska EA, Jakubaszko J, Cwynar A, Majda J, Ponikowska B, Kustrzycka-Kratochwil D, ReczuchvK B-N, Banasiak W, Poole-Wilson PA, Ponikowski P (2009) Bone mineral status and bone loss over time in men with chronic systolic heart failure and their clinical and hormonal determinants. Eur J Heart Fail 11:28–38
Lim HS, Park YH, Kim SK (2016) Relationship between serum inflammatory marker and bone mineral density in healthy adultsJ. J Bone Metab 23:27–33
Braunwald E (2008) Biomarkers in heart failure. N Engl J Med 358:2148–2159
Gu ZC, Zhou LY, Shen L, Zhang C, Pu J, Lin HW, Liu XY (2018) Non-vitamin K antagonist oral anticoagulants vs warfarin at risk of fractures: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol 9:348
Izawa H, Yoshida T, Ikegame T, Izawa KP, Ito Y, Okamura H, Osada N, Kinugawa S, Kubozono T, Kono Y, Kobayashi K, Nishigaki K, Higo T, Hirashiki A, Miyazawa Y, Morio Y, Yanase M, Yamada S, Ikeda H, Momomura S, Kihara Y, Yamamoto K, Goto Y, Makita S (2019) Japanese Association of Cardiac Rehabilitation Standard Cardiac Rehabilitation Program Planning Committee. Standard Cardiac Rehabilitation Program for Heart Failure. Circ J 83:2394–2398
Funding
This work was supported by JSPS KAKENHI grant no. 16K09460. None of the work in this article has been presented at a conference or in any other publications.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hideo Izawa has received grant support through his institution from Takeda, Shionogi, Dainippon-Sumitomo, Otsuka, Pfizer, and Daiichi-Sankyo and honoraria for lectures from Otsuka and Daiichi-Sankyo.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study has not been presented at any conference or published in any journal in whole or in part.
Rights and permissions
About this article
Cite this article
Kono, Y., Izawa, H., Aoyagi, Y. et al. Impact of heart failure severity on bone mineral density among older patients with heart failure. Heart Vessels 36, 1856–1860 (2021). https://doi.org/10.1007/s00380-021-01884-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-021-01884-1