Abstract
The Chinese diet is low in calcium, including among adolescent girls, with an average intake around 500 mg per day. In this study, we compared the percentage change in bone mineral density and content of the spine and hip region in a 1-year follow-up study between 104 adolescent girls aged 14 to 16 years receiving 375 ml calcium-fortified soymilk supplementation and 95 girls in the control group. The mean percentage changes of bone mineral density/content (BMD/BMC) and standard deviation (SD) at 1 year for the supplementation and control groups were as follows: neck of the femur BMD 2.7±2.94%, 1.8±3.49% (P =0.08); trochanter BMD 3.3±3.27%, 1.6±2.94% (P ≤<0.001); intertrochanter BMD 3.6±3.05%, 2.32±2.95% (P =0.002); total hip BMD 3.1±2.39%, 2.05±2.22% (P =0.001); total hip BMC 3.8±3.05%, 2.6±2.96% (P =0.006). The percent difference between the percentage of bone changes in the supplementation and control groups [100× (soymilk-control)/control] ranged from 45 to 113%. We observed no differences in the spine BMD/C and no differences in changes of height and weight between the soymilk supplementation and control groups, which yielded similar results. Stepwise multivariate regression analysis including height, weight, growth stage, dietary energy, protein, calcium from usual diet and physical activity also showed that supplementation was significantly associated with a percentage increase in BMD/C at the hip. We conclude that 375 ml calcium-fortified soymilk supplementation, or an equivalent of about two glasses, is among the effective strategies for bone acquisition and the optimization of peak bone mass in adolescent girls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent studies in Hong Kong have demonstrated that the incidence of hip fracture is approaching that experienced in the Western populations [1]. Local bone mass studies have shown that according to the WHO definition of osteoporosis, the condition affects one-third to one-half of the postmenopausal and elderly women [2]. The increase of bone mass before its peak accrual at a young age and the prevention of bone loss in postmenopausal women are the two primary strategies for the prevention of osteoporotic fractures in later life [3, 4].
Soy contains protein of biological value equivalent to that of milk or egg protein, but without the cholesterol, and it is low in saturated fatty acids. Increasingly, more studies have revealed the protective role of soy foods in a wide range of health conditions including cardiovascular diseases, cancer and osteoporosis [5, 6, 7]. Recent studies have also shown the beneficial effect of soy protein supplementation on bone mass in peri- and postmenopausal women [8, 9]. Although various soy foods have traditionally been major components of the Asian diets, only limited data are available on the beneficial effects of soy in the Chinese populations. Recent studies have revealed positive associations between soy phytoestrogens and protein intake and bone mass in postmenopausal and young premenopausal women [10, 11, 12].
However, only limited data of soy protein effect on Caucasian young women [13] are available, and no study has yet reported the effect of soy protein supplementation on optimizing bone mass in the Asian adolescent or young adult populations. We conducted a 12-month controlled intervention study to investigate the effects of calcium-fortified soymilk (Calciplus) supplementation on bone mineral density and bone mineral content in Chinese adolescent girls aged 14 to 16 years.
Materials and methods
Study sample
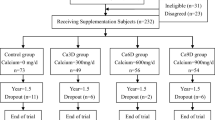
The study was conducted among 210 adolescent girls aged 14 to 16 years. The subjects were recruited from six secondary schools of comparable academic status. The participating schools were assigned either to the intervention or control group with the aim of achieving about equal numbers of girls in each group. Girls with disease conditions or medications affecting their bone mineral density and subjects with soy allergy were excluded from the study. All participants received an educational talk on “bone health in adolescents” at the beginning of the study. Ethical approval was obtained from the University Ethics Committee. Informed consent was obtained from the participants as well as from their guardians.
Baseline data collection
At baseline, all participating subjects provided demographic information, age at menarche, menstrual cycle length, medication use and history of diseases through use of a structured questionnaire administered through face-to-face interviews. Leisure-time physical activity (PA) was measured using a modification of the Minnesota Leisure Time PA Questionnaire, which had been previously validated and used in local studies on bone mass in adolescents and young women [14, 15]. The reference period used was the previous 12 months. The metabolic equivalents (MET, defined as the multiples of resting oxygen consumption) expended performing each activity were calculated based on the published and adapted values from McArdle et al. [15, 16]. The total MET was based on the reported time spent on each activity and the MET values for each activity. Physical activities were classified as either weight-bearing or non-weight-bearing in order to assess the impact of each activity on specific skeletal sites. Weight-bearing activities included PA that subjects had to perform on their feet.
Dietary intake was measured at baseline and at 12 months using 3-day food records that were completed by each girl after receiving 30 min of training at school about food amounts, portion estimation and utensil sizes. The girls were also given food portion photo booklets to help them estimate and record the amounts of the foods and beverages taken. The dietary data were later analyzed for nutrients using Nutritionist Pro Version 2.1 nutrient composition software (First DataBank, Inc., Indianapolis).
Bone mineral density (BMD) was measured at baseline and at 12 months with a Delphi QDR series, Hologic dual-energy X-ray densitometre (Hologic, Inc., Bedfold, Mass.) at the spine (L1-L4) and hip (femoral neck, trochanter and intertrochanter and total hip). Calibration was performed daily on a lumbar spine phantom. The coefficient of variation (CV) of measurements with the spine phantom was 0.372. The in-vivo CV% based on 27 subjects was 0.879 for spine BMD, 1.044 for hip BMD, 1.74 for femoral neck BMD, 1.32 for trochanter BMD and 1.66 for intertrochanter BMD. Height (to the nearest mm) and body weight (to the nearest 0.1 kg) were measured with an upright balance scale (Health-O-Meter, Inc. Bridgeview, Ill.).
Urine samples were obtained at baseline, at 6 and 12 months. Urinary N-telopeptide (NTx), a bone resorption marker, was measured by a commercially available enzyme-linked immunosorbent assay (ELISA) that uses a specific monoclonal antibody directed against the N-telopeptide intermolecular cross-linking domain of type I collagen of bone (Ostepmark, Ostex International, Inc., Seattle, Wash.). Intra-assay variability was assessed using 61 duplicate urine specimens. The CV% was 7.2%. Total assay precision was evaluated by testing the level I urine control and the level II urine control. The inter-assay variability was 5.52% (level I) and 9.13% (level II) at a mean value of 374.9 nM BCE and 1,324 nM BCE, respectively. The intra-assay variability was 4.70% (level I) and 9.24% (level II).
Intervention
The intervention began after baseline 3-day food records had been obtained, PA questionnaires had been administered and physical measurements of bone mineral density had been completed. Girls in the intervention group were supplied daily with 375 ml of calcium-fortified soymilk (Calciplus) beverage containing 142.5 kcal, 6.75 g protein, 4.1 g fat, 600 mg Ca and 54 mg isoflavones. The control group girls did not take a placebo. None of the subjects in either group received instructions regarding diet or physical activities. The subjects were unaware of the specific study hypothesis or of the participation of another group.
Follow-up data collection
A repeat of baseline measurements was performed among all girls in both groups after 12 months. The growth stage of each girl was ascertained by the gain in height over the follow-up period. Classification of growth stage was adopted from that used by Bollen [17], with no gain in height classified as non-growth stage: 2–4 cm/year was classified as descending growth stage, and <2 cm/year as ‘end-of-growth stage.’
Statistical analysis
One hundred ninety-nine subjects completed the study. Five girls from the control group and six from the intervention group refused to participate in the follow-up study. Reasons for drop-outs included change of school, overseas study and bulky feeling after drinking soymilk. An intention-to-treat analysis was performed using all available data from the 199 subjects. Comparisons of the baseline characteristics of the participants, including the dietary intake, physical activity, bone mineral density (BMD) and content (BMC) and reproductive history, were made between the intervention and control subjects. T -tests or chi-square tests were used to compare the baseline and follow-up characteristic between the two groups.
Analyses included comparisons of the two study groups in their baseline characteristics. Percentage changes (defined as follow-up BMD/C – baseline BMD/C divided by baseline BMD/C) of BMD/C at the spine and hip regions were compared between the intervention and control groups by two-sample t -tests, as well as by analysis of covariance, taking into account the covariates (height, weight, baseline and follow-up dietary calcium intake, total energy intake, weight bearing and total PA as well as growth stage). Stepwise multivariate analysis was used controlling for the covariates (with group forced in the model; F to enter =0.05, F to remove =0.01).
Results
The baseline characteristics of the participants were comparable in the two groups. The mean follow-up time was 11.62 (SD=0.76) months. The mean age of the participants was 14.5 (0.6) years. The mean height and weight were similar, and mean body mass index was 19.4. The mean dietary calcium intake was around 510 mg/day. Except for soy protein, which was slightly higher in the intervention group, the energy intake and other dietary variables were similar between the two groups. None of the girls smoked. Only one claimed to have an alcoholic drink at least once per week. A few girls in the intervention group had a history of fracture, while none was reported by the control group. More girls in the control group reported a history of fracture among their paternal grandmothers, but no statistically significant difference was found.
The mean age of menarche was about 12.1 (1.07) years in both groups. The amounts of time spent in weight-bearing and non-weight-bearing activities were also similar. One-third of the girls considered they were less active compared with girls of similar age. Tables 1 and 2 show the follow-up measurements. Both groups had an overall similar increase in height and weight, and over 90% of the girls in both groups had already approached the end stage of their growth. The dietary nutrient intakes were similar except for lower Ca and phosphorus intakes in the intervention group before taking into account the soy milk supplementation.
The BMDs and total BMC at the spine and hip sites were also similar at baseline (Table 3). An increase in bone acquisition was observed in both groups (Fig. 1). The percentage increases in the spine BMD and BMC were similar. However, the intervention group had a statistically significantly higher percentage increase of BMC for the total hip as well as BMDs at the trochanteric, intertrochanteric and total hip. A reduction of urinary excretion of type I collagen cross-linked N-telopeptides (NTx) was noted in both groups over the follow-up period at 6 and 12 months.
The associations between soy supplementation and bone acquisition at the various bone sites were similar when individually adjusted for growth stage, body size, mean physical activity or mean dietary intake variables of baseline and follow-up values. The stepwise multivariate regression analyses simultaneously controlling for these covariates also confirmed that soy supplementation had a statistically significant effect on percentage increase in BMC and BMD at the hip sites (Table 4). Growth stage was an important predictor of bone acquisition and was retained all the final models.
Discussion
We found that a 1-year calcium-fortified soy milk supplementation of 375 ml per day had a positive effect on bone accretion at the hip sites in adolescent girls. Girls in the iintervention group were consuming about 54 mg isoflavones per day. Previous intervention trials with soy protein containing this amount of isoflavones in Caucasians have been ineffective in improving bone health in postmenopausal women [8, 9]. However, observational studies among Chinese women, including those of cross-sectional [11] and longitudinal design [10], have generally shown positive associations between habitual soy intake and bone health, particularly at the hip sites. A daily consumption of 54 mg soy isoflavones would be at the high intake range of these population-based studies. No study has yet specifically investigated the effect of soy isoflavone supplementation on adolescent bone health.
Previous local studies have shown higher habitual soy food intake or soy isoflavone supplementation of 80 mg per day was associated with better hip bone mass in postmenopausal Chinese women [11, 12]. A study by Lydeking-Olsen [18] also showed that calcium-fortified soymilk was well tolerated by postmenopausal women and was associated with better bone health. Very few studies have investigated soy intake in young women or adolescents. A longitudinal study in young premenopausal Chinese women noted an association between higher habitual soy intake and better maintenance of peak bone mass [10]. However, a study by Anderson et al. [13] observed no effect of soy protein supplementation on bone mass in young women, but the study sample size was small.
The mechanisms of the effect of soy on bone health are still unclear. Researchers have proposed several mechanisms by which soy might benefit bone health. Increased isoflavone intake could alter the serum estrogen concentrations, but the limited available data so far have shown inconsistent findings [19, 21]. Soy isoflavones could also exert a direct effect on estrogen-receptors in bone cells, and might inhibit bone resorption or stimulate bone formation [22, 23]. Other mechanisms could be related to the effect of soy in the enhancement of calcium absorption and the reduction of urinary calcium excretion [24, 25]. However, the calcium-conserving effect of soy protein or isoflavones are still inconclusive.
Another postulated mechanism could be related to the promotion of insulin-like growth factor-I (IGF-I). Arjmandi et al. [23] have reported that soy or its isoflavones promote IGF-I production in rats and in humans [25]. As IFG-I is known to increase osteoblastic activities in humans [26, 27], the anabolic effect may result in appositional growth resulting in an increase in BMC as observed in this study. In an 18-month controlled trial, Cadogan et al. [28] reported an increased serum IGF-I in the milk supplementation group of adolescent girls, and a predominantly enhanced bone acquisition in the lower body segment. Our observation is consistent with these findings with a higher percentage of bone gain at the sites of the hip rather than the spine. Previous observation and clinical trial studies in older Chinese women have also reported stronger soy protein or isoflavone effects on the hip BMD or BMC than that of the spine [11, 12]. Other explanations for the differential effects of calicum-fortified soymilk supplementation with significant effect on the hip, but not on the spine, might be due to the largely completed vertebral growth among these girls who were approaching the end stage of growth. The physical activities have also been comparable among the intervention and control groups. However, the exact mechanism for the inconsistent effects on the spine and hip remain uncertain.
The soymilk supplement was fortified with 600 mg calcium. Although there is no universal agreement on how much calcium is needed by adolescents for optimal bone mineral accretion, a customary calcium intake at a mean intake of 500 to 550 mg/day is inadequate according to the recommended intake of 1,200 mg for Chinese adolescents aged 13–15 years and 1,000 mg for Chinese adolescents aged 16–17 years [29]. Thus, the 600 mg supplementation would increase the intake to close to the recommended levels. Previous studies have generally reported that calcium supplementation during adolescence is beneficial [30], particularly in Chinese children [31, 32] whose intake, as previously noted, is customarily lower than among Caucasian children. A co-twin study among girls with a mean age of 14 years found an increase in BMD at the spine and hip in the twin with calcium supplementation [33]. A study in Chinese children also supported these findings [31].
The majority of the observational studies based on dietary sources of calcium have also supported that increased calcium intake protects the skeleton [34]. However, for most of these studies, the calcium has mainly come from dairy sources [28, 30, 35, 36]. Our study is the first using calcium fortified into the traditional Chinese beverage of soymilk.
Bone modeling is a continuous process devoted to the development of the skeleton during the pubertal growth spurt. Previous studies revealed that bone turnover was maximal in mid-puberty, and decreased in late puberty [37]. NTx, a marker for bone resorption, correlates with the changes in growth rate during adolescence. Over 95% of the girls in both our study groups were already approaching the end stage of growth, as signified by a plateau or small height gain over the follow-up period. Accordingly, we found a significant decline in NTx levels over the follow-up period reflecting a decrease in bone modeling. A previous prospective study of bone resorption marker in adolescents also showed NTx excretion declines in girls after menarche [16]. Though the reduction was slightly higher in the intervention group, we have noted a similar decline in both our study groups. Therefore, the growth stage, a significant variable retained in the final stepwise multivariate regression models for all selected bone sites, had probably had a more dominant effect in the bone resorption marker. Our results also corroborate a previous study on adolescent girls [28], where NTx levels were similar in both the milk supplementation and control groups throughout the trial though there was a significant bone acquisition in the former group.
Other nutrients and lifestyle could have been potential confounders. We collected information on dietary intake and physical activity at baseline, as well as at one year. We found that baseline dietary intakes were similar between the intervention and control groups. However, the intervention group had a higher baseline intake of soy protein, but the mean difference was only about 1.5 g. The follow-up data showed there was a reduction of reported energy intake in both groups at follow-up. The control group also had slightly higher total protein and dietary calcium intakes at follow-up than the intervention group. The difference could have been due to a lower milk consumption in the intervention group, as they were receiving the soy milk supplementation.
We did not control the diet by providing the subjects with foods during the study period. Controlling for diet was unpractical given this was a 1-year study, and the target group was free-living adolescent girls. The uncontrolled diet and lifestyle are more likely to reflect the girls’ habitual situation and enhance compliance throughout the study, as well as, in the intervention group, indicate the feasibility of daily calcium-fortified soy milk as a potential and acceptable intervention strategy for this population group.
Based on this study result, we are unable to conclude which component(s) of the calcium-fortified soymilk, or their combined effect, resulted in the beneficial effect on bone health. Soy isoflavones in combination with fortified calcium, and possibly also a wide range of other nutrients or phytochemicals contained in soy milk, might have exerted the effect. For example, the conversion of isoflavones to equol metabolites enhances the endocrine effects of isoflavone ingestion, and studies [38] have shown that the ability to metabolize daidzein to equol is enhanced or better maintained with habitual intake of soy foods.
Though we are unable to delineate which is/are the effective component(s), we are able to conclude that supplementing the diet of adolescent girls with calcium-fortified soymilk was effective in increasing hip BMD and BMC. Daily consumption of soymilk, a culturally accepted food for Chinese youths as well as adult populations, is thus a practical approach for achieving optimal bone accretion.
In view of the fast-growing rate of hip fractures in Asia, a public health approach of prevention in early life should include targeting children and adolescents going through periods of fast growth and bone mineral accretion accounting for a large proportion of total bone mass gained over a lifetime. A plant-based diet together with adequate calcium intake has been recommended as a major strategy [39] for bone health. Thus, soy milk with calcium fortification is good food choice in the Asian context.
In conclusion, this study suggests that daily calcium-fortified soymilk consumption at less than two glasses per day may be an acceptable and effective strategy for maximizing peak bone mass in adolescent girls.
References
Schwartz AV, Kelsey JL, Maggi S, Tuttleman M, Ho S C, Jonsson PV, Poor G, Sisson de Castro JA, Xu, L, Matkin CC, Nelson LM, Heyse SP (1999) International variation in the incidence of hip fractures: cross-national project on osteoporosis for the World Health Organization Program for research on aging. Osteoporosis Int 9:242–253
Ho SC, Lau EMC, Woo J, Sham A, Chan KM, Lee S, Leung PC (1999) The prevalence of osteoporosis in Hong Kong Chinese female population. Maturitas 32:171–178
Ho SC (1997) Attainment of peak bone mass and dietary factors in Chinese women. In: Lau MC, Ho SC, Leung S, Woo J (eds) Osteoporosis in Asia: crossing the frontiers. World Scientific Publishing Co PTE Ltd
Ho SC, Leung PC (1995) Determinants of peak bone mass in Chinese and Caucasian populations. HKMJ 1:38–42
Messina V, Setchell K (1994) The simple soybean and your health. Avery, New York
Messina MJ, Persky V, Setchell KDR, Barnes S (1994) Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer 21:113–121
Anderson JW, Johnstone BM, Cook-Newell ME (1995) Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med 333:276–282
Potter SM, Baum JA, Teng H, Stillman RJ, Erdman JW Jr (1998) Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr 68:1375S–1379S
Alekel DL, Germain AS, Peterson CT, Hanson KB, Stewart JW, Toda T (2000) Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr 72:844–852
Ho SC, Chan SG, Yi Q, Wong E (2001) Soy intake and the maintenance of peak bone mass in Hong Kong Chinese Women. J Bone Miner Res 16:1363–1369
Ho SC, Woo J, Chen YM, Sham A, Lau J (2003) Soy protein consumption and bone mineral density in early postmenopausal Chinese women. Osteoporos Int 14:835–842
Chen YM, Ho SC, Lam SSH, Ho SSS, Woo JLF (2003) Soy isoflavones have a favorable effect on bone loss in Chinese postmenopausal women with lower bone mass: a double-blind randomized-controlled trial. J Clin Endoc Metab 88:4740–4747
Anderson JJ, Chen X, Boass A, Symons M, Kohlmeier M, Renner JB, Garner SC (2002) Soy isoflavones: no effects on bone mineral content and bone mineral density in healthy, menstruating young adult women after one year. J Am Coll Nutr 21:388–393
Ho SC, Leung PC, Swaminathan R, Chan C, Chan SG, Lindsay R (1994) Determinants of bone mass in Chinese women aged 21–40. II. Pattern of dietary calcium intake and association with bone mineral density. Osteoporosis Int 4:167–175
Ho SC, Eric Wong, Chan SG, Lau J, Chan C, Leung P.C (1997) Determinants of peak bone mass in Chinese women aged 21–40 years. III. Physical activity and bone mineral density. J Bone Miner Res 12:1262–1271
McArdle WD, Katch FI, Katch VI (1981) Exercise physiology. Lea and Febiger, Philadephia, pp 486–493
Bollen AMA (2000) A prospective longitudinal study of urinary excretion of a bone resorption marker in adolescents. Ann Hum Biol 27:199–211
Lydeking-Olsen E, Jensen JEB, Setchell KDR, Damhus M, Jensen TH (2002) Isoflavone-rich soymilk prevents bone loss in the lumbar spine of postmenopausal women: a 2-year study. J Nutr 132:581S (abstract)
Petrakis N, Barnes S, King EB, Lowenstain J, Wiencke J, Lee MM, et al (1996). Stimulatory influence of soy protein isolate on breast secretion in pre- and postmenopausal women. Cancer Epidemiol Biomarkers Prev 5:785–794
Nagata C, Takatsuka N, Inaba S, Kawakami N, Shimizu H (1998) Effect of soymilk consumption on serum estrogen concentrations in premenopausal Japanese women. J Nat Cancer I 90:1830–1835
Verkasalo PK, Appleby PN, Davey GK, Key TJ (2001) Soy milk intake and plasma sex hormones: a cross-sectional study in pre- and postmenopausal women (EPIC-Oxford). Nutr Cancer 40:79–86
Anderson JJB, Miller CP (1998) Lower lifetime estrogen exposure among vegetarians as a possible risk factor for osteoporosis: a hypothesis. Vegetarian Nutr 2:4–12
Arjmandi BH, Getlinger MJ, Goyal NV, Alekel L, Hasler CM, Juma S, Drum ML, Hollis BW, Kukreja SC (1998) Role of soy protein with normal or reduced isoflavone content in reversing bone loss induced by ovarian hormone deficiency in rats. Am J Clin Nutr 68:1358S–63S
Anderson JJB, Thomsen K, Christiansen C (1987) High protein meals, insular hormones and urinary calcium excretion in human subjects. In: Christiansen C, Johansen JS, Riis BJ (eds) Osteoporosis. Nørhaven A/S, Viborg, Denmark, pp 240–245
Arjmandi BH, Khalil DA, Smith BJ, Lucas EA, Juma S, Payton ME, Wild RA (2003) Soy protein has a greater effect on bone in postmenopausal women not on hormone replacement therapy, as evidenced by reducing bone resorption and urinary calcium excretion. J Clin Endocrinol Metab 88:1048–1054
Boonen S, Lesaffre E, Dequeker J, Aerssens J, Nijs J, Pelemans W, Bouillon R (1996) Relationship between baseline insulin-like growth factor-I (IGF-I) and femoral bone density in women aged over 70 years: potential implications for the prevention of age-related bone loss. J Am Geriatr Soc 44:1301–1306
Sugimoto T, Nishiyama K, Kuribayashi F, Chihara K (1997) Serum levels of insulin-like growth factor (IGF) I, IGF-binding protein (IGFBP)-2, and IGFBP-3 in osteoporotic patients with and without spinal fractures. J Bone Miner Res 12:1272–1279
Cadogan J, Eastell R, Jones N, Barker ME (1997) Milk intake and bone mineral acquisition in adolescent girls: randomized, controlled intervention trial. BMJ 315:1255–1260
China Nutrition Association (2000) Reference dietary intake of nutrients of a Chinese population. Beijing. China Light Industry Press
Kristinsson JO, Valdimarsson O, Steingrimsdottir L, Sigurdsson G (1994). Relation between calcium intake, grip strength and bone mineral density in the forearms of girls aged 13 and 15. J Int Med 236:385–390
Lee WTK, Leung SSF, Leung DMY, Tsang HSY, Lau J, Cheng JCY (1995) A randomized double-blind controlled calcium supplementation trial, and bone and height acquisition in children. Brit J Nutr 74:125–139
Lee WTK, Leung SSF, Wang SH, Xu YC, Zang WP, Lau J, Oppenheimer SJ, Cheng JCY (1994) Double-blind, controlled calcium supplementation and bone mineral accretion in children accustomed to a low-calcium diet. Am J Clin Nutr 60:744–750
Nowson CA, Green RM, Hopper JL, et al. (1997) A co-twin study of the effect of calcium supplementation on bone density during adolescence. Osteoporos Int 7:219–225
Heaney RP (2000) Calcium, dairy products and osteoporosis. J Am Coll Nutr 19:83S–99S
Chan GM, Hoffman K, McMurry M (1995) Effects of dairy products on bone and body composition in pubertal girls. J Pediatr 126:551–556
Renner E, Hermes M, Stracke H (1998) Bone mineral density of adolescents as affected by calcium intake through milk and milk products. Int Dairy J 8:759–764
Blumsohn A. Hannon RA, Wrate R, Barton J, al-Dehaimi AW, Colwell A, Eastell R (1994). Biochemical markers of bone turnover in girls during puberty. Clin Endocrinol 40:663–670
Lu LJW, Anderson KE (1998) Sex and long-term soy diets affect the metabolism and excretion of soy isoflavones in humans. Am J Clin Nutr [Suppl] 68:1500S–1504S
Anderson JJB (1999). Plant-based diets and bone health: nutritional implications. Am J Clin Nutr [Suppl] 70:539S–5342S
Acknowledgements
Calcium-fortified soymilk (Calciplus) was supplied by Vitasoy International Holdings (Ltd.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ho, S.C., Guldan, G.S., Woo, J. et al. A prospective study of the effects of 1-year calcium-fortified soy milk supplementation on dietary calcium intake and bone health in Chinese adolescent girls aged 14 to 16. Osteoporos Int 16, 1907–1916 (2005). https://doi.org/10.1007/s00198-005-1963-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-005-1963-8