Abstract
The aim was to investigate whether the addition of supervised high intensity progressive resistance training to a moderate weight loss program (RT+WLoss) could maintain bone mineral density (BMD) and lean mass compared to moderate weight loss (WLoss) alone in older overweight adults with type 2 diabetes. We also investigated whether any benefits derived from a supervised RT program could be sustained through an additional home-based program. This was a 12-month trial in which 36 sedentary, overweight adults aged 60 to 80 years with type 2 diabetes were randomized to either a supervised gymnasium-based RT+WLoss or WLoss program for 6 months (phase 1). Thereafter, all participants completed an additional 6-month home-based training without further dietary modification (phase 2). Total body and regional BMD and bone mineral content (BMC), fat mass (FM) and lean mass (LM) were assessed by DXA every 6 months. Diet, muscle strength (1-RM) and serum total testosterone, estradiol, SHBG, insulin and IGF-1 were measured every 3 months. No between group differences were detected for changes in any of the hormonal parameters at any measurement point. In phase 1, after 6 months of gymnasium-based training, weight and FM decreased similarly in both groups (P<0.01), but LM tended to increase in the RT+WLoss (n=16) relative to the WLoss (n=13) group [net difference (95% CI), 1.8% (0.2, 3.5), P<0.05]. Total body BMD and BMC remained unchanged in the RT+WLoss group, but decreased by 0.9 and 1.5%, respectively, in the WLoss group (interaction, P<0.05). Similar, though non-significant, changes were detected at the femoral neck and lumbar spine (L2-L4). In phase 2, after a further 6 months of home-based training, weight and FM increased significantly in both the RT+WLoss (n=14) and WLoss (n=12) group, but there were no significant changes in LM or total body or regional BMD or BMC in either group from 6 to 12 months. These results indicate that in older, overweight adults with type 2 diabetes, dietary modification should be combined with progressive resistance training to optimize the effects on body composition without having a negative effect on bone health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes is typically associated with obesity, which has itself been associated with a higher bone mineral density (BMD) and may protect against osteoporosis and fractures. However, there are conflicting data with regard to the influence of type 2 diabetes on BMD and fracture risk. There are reports of increased, similar or decreased BMD in comparison to non-diabetic controls, independent of body composition [1, 2, 3, 4]. While data on fracture risk are less clear, there is evidence of an increased risk in older women with type 2 diabetes, even after adjusting for body mass or BMD [5]. For overweight patients with type 2 diabetes, dietary modification to induce weight loss is a key strategy to improve glycaemic control and reduce the risk of complications. However, weight loss in non-diabetic older adults has been shown to be associated with accelerated bone and muscle loss and an increased risk of fracture [6, 7, 8, 9, 10]. Epidemiological data show that fracture risk increases in older women who lose 10% or more of their body weight [8, 11]. While the mechanism underlying the reduction in BMD during weight loss remains uncertain, there is some evidence that it may be partly related to a decrease in body fat leading to a reduction in circulating sex hormone levels and insulin-like growth factor-I (IGF-I), and/or an increase in sex-hormone-binding globulin (SHBG) and parathyroid hormone (PTH) [2, 12, 13].
Exercise is widely recognized as having beneficial effects on BMD [14, 15], but there are conflicting reports as to whether exercise incorporated into weight loss programs can protect against bone loss and conserve lean mass. Some studies have reported an increase in total body and/or regional BMD [16], while others have reported no change [17, 18] or even a decrease [19, 20, 21] following exercise training. Most of these studies were short term and utilized aerobic or endurance exercise programs, which traditionally have been shown to have little or no effect on BMD or lean mass. In contrast, there is evidence that high-intensity progressive resistance training can lead to modest increases in BMD (1–3%), conserve or increase lean mass and contribute to increased muscle strength in older adults [14, 15, 22].
We examined whether the addition of high intensity progressive resistance training during moderate weight loss could protect against bone loss and conserve lean mass in older, sedentary overweight adults with established type 2 diabetes. We also examined whether any benefits achieved through a supervised resistance-training program could be maintained through a subsequent home-based program.
Materials and methods
Participants
The baseline characteristics of the participants for this study have been described in detail previously [23]. Thirty-six men and women aged 60 to 80 years with treated (diet and/or medication) type 2 diabetes were recruited from the clinics of the International Diabetes Institute and by a local media campaign. All participants were overweight (BMI >27 kg/m2 and ≤40 kg/m2), sedentary, had established (greater than 6 months) but not optimally controlled type 2 diabetes (HbA1c range 7–10%), were not taking insulin, were non-smokers and consumed on average fewer than two alcoholic beverages per day. Participants with the following characteristics were excluded during the initial screening: history or physical findings suggestive of ischemic heart disease, systemic diseases, hypertension (>160/90 mmHg), advanced diabetic neuropathy or retinopathy, severe orthopedic, cardiovascular or respiratory conditions that would preclude participation in an exercise program or those with a medical condition listed in the American College of Sports Medicine (ACSM) absolute exercise contraindications [24]. Of the 36 participants included into the study, 20 had a history of hypertension, 3 had a history of neuropathy, 1 had a history of retinopathy and 7 reported a history of arthritis/osteoarthritis. Four women had a history of HRT use, but none were current users (mean duration since use 14.9 years). One participant was taking a multivitamin supplement. Anti-diabetic and anti-hypertensive medications were continued during the study. The study was approved by the International Diabetes Institute and Deakin University Human Research Ethics Committee, and written consent was obtained from all participants.
Study design
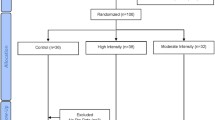
The study was a 12-month randomized controlled clinical trial, with repeated measurements performed at 3-month intervals. The intervention was divided into two distinct phases: Phase 1 (gymnasium-based), which consisted of 6 months of supervised gymnasium-based training, and phase 2 (home-based), which consisted of a further 6 months of training in the home-setting. Of the 36 participants initially randomized to either the high-intensity progressive resistance training plus moderate weight loss program (RT+WLoss, n=19) or the control program (moderate weight loss plus flexibility training, WLoss, n=17), 2 participants from the RT+WLoss group and 4 participants from the WLoss group withdrew from the study during the first 8 weeks. The reasons for withdrawal included health problems not related to the intervention (n=2) or other commitments that precluded ongoing participation (n=4). One participant from the RT+WLoss group was placed on insulin treatment within the first 6 weeks of the trial and was not included in any analysis. Thus, 29 participants [16 RT+WLoss (84%) and 13 WLoss (76%)] successfully completed phase 1 of the intervention, yielding a dropout rate of 19%. During the home-based training, a further three participants withdrew from the study (two from the RT+WLoss and one from the WLoss group). All withdrawals occurred within the first 2 weeks of the home-based training. Reasons for withdrawals included overseas travel, continued pain associated with osteoarthritis of the knee and personal problems unrelated to the study. Thus, 72% of the participants [14 RT+WLoss (74%) and 12 WLoss (71%)] completed the 12-month intervention.
Intervention
Prior to the commencement of phase 1 (gymnasium-based training), all participants were placed on a 4-week healthy eating plan supplying 30% or less of the total energy intake from fat (<10% from saturated fat), with the remainder distributed between carbohydrates and protein. The healthy eating plan was designed to elicit a moderate weight loss of 0.25 kg per week. All healthy eating plans were individually prescribed by a dietitian using two separate 3-day food records performed during the 4-week baseline period. Compliance with the healthy eating plan was assessed by interviews every 2 weeks with the dietitian and completion of a weekly food checklist. One 3-day food record was obtained every 3 months to assess changes in nutrient intake. During phase 2 (home-based training), participants were not required to follow a healthy eating plan and were not provided with dietary advice from the dietitian. All nutritional information obtained from food records was analyzed by a dietitian using the Foodworks nutrient analysis software program (Xyris Software, Brisbane, Queensland, Australia).
Phase 1: supervised gymnasium-based training
During the 6-month supervised gymnasium-based intervention, all participants attended the exercise laboratory on 3 non-consecutive days per week. Resistance training consisted of a 5-min warm-up and 5-min cool down period of low-intensity stationary cycling and approximately 45 min of high-intensity resistance training (dynamic exercise involving concentric and eccentric contractions). As previously reported [23], participants followed an individually monitored progressive resistance training program (three sets of eight to ten repetitions of nine exercises at 75 to 85% of their one repetition maximum strength, 1-RM) using free-weights and a multiple station weights machine. All sessions were supervised to ensure correct technique and to monitor the appropriate amount (and progression) of exercise and rest intervals. The control flexibility program (WLoss group) was designed to provide participative involvement, but not to elicit change in muscle strength or cardiovascular fitness. Each session involved stationary cycling with no workload for 5 min followed by a series of static stretching exercises. Participants were not blinded to treatment and were informed that improved flexibility was an expected outcome.
Phase 2: home-based training
At the completion of the gymnasium-based training, all participants were provided with individualized instructions, training and equipment (dumbbells/ankle weights or a flexibility wall chart) to perform resistance training or light flexibility training 3 days per week at home and/or within a commercial/community center. To accommodate the transition to home-based training, participants in the RT+WLoss group completed the home-based exercise program within the supervised gymnasium-based setting during the final 4 weeks of the gymnasium-based program to become familiar with the new exercises and ensure correct technique. The home-based exercises were similar to those used in the gymnasium-based program, with the exception that dumbbells (DB) and ankle weights replaced the exercises performed on the multi-station weights machine. The nine home-based exercises included: lying DB flies, seated single leg extension (ankle weights), DB shoulder press, DB bent over row, standing leg curl (ankle weights), DB upright row, DB bicep curls, DB triceps kickbacks and abdominal curls. Participants were also provided with detailed instruction booklets describing each resistance training exercise and were asked to follow an individually developed training program (three sets of eight to ten repetitions with the goal to exercise at an intensity corresponding to 60–80% of their current 1 RM). During the first week of the home-based training, home visits were conducted to ensure that the home training environment was appropriate and safe. The control (flexibility) group was provided with a stretching wall chart and was asked to continue with the stretching program at home. Participants were telephoned weekly for the first 4 weeks and every 2 weeks thereafter to monitor compliance, answer questions and provide individualized feedback. Participants also completed weekly training diaries and were required to attend Deakin University monthly to perform the home-based training so that technique and progression could be monitored.
Testing procedures
Anthropometry, bone density and body composition
Height (cm) was measured using a Holtain stadiometer (Holtain Ltd, Crosswell, Wales). Body weight (kg) was assessed using SECA electronic scales to the nearest 0.1 kg. Total body, femoral neck and lumbar spine (L2-L4) bone mineral content (BMC), bone mineral density (BMD) and bone area, and total body lean mass (LBM, kg), fat mass (FM, kg) and percentage body fat were assessed by dual energy X-ray absorptiometry (DPX-L, Lunar Corp, Madison, WI) at baseline, 6 and 12 months. All scanning and analyses were performed by the same operator (RD), and follow-up scans were analyzed using the compare mode function. Total body scans were analyzed using the extended analysis mode. The coefficient of variation (CV) for repeated measurement of BMC and BMD ranged from 0.9 to 1.9%, and 1.2% for FM and 1.7% for LBM.
Muscle strength
Prior to the initial assessment of one-repetition maximum strength (1-RM), participants attended two separate familiarization sessions where they were shown proper exercise techniques by a trained instructor and given the opportunity to become accustomed to the selected exercises. To determine the 1-RM, each participant initially performed a warm-up set of eight repetitions with a light weight. Following the successful completion of a further three to five repetitions at a moderate to heavy weight selected by the instructor, and after a brief rest (1–2 min), the workload was increased incrementally until only one repetition with correct technique could be completed. The 1-RM testing on the bench press and leg extension exercises were used to document the respective changes in upper body and lower body strength at baseline and every 3 months.
Clinical and laboratory measurements
Fasted, resting morning (8–10 a.m.) blood samples were obtained from each participant’s antecubital vein at baseline and every 3 months throughout the study. All samples were collected 48 h post-exercise and were stored at −80°C until assayed. All assays were performed in duplicate and each participant’s samples (baseline, 3, 6, 9 and 12 months) were assessed in a single batch. Glycosylated hemoglobin was measured with the Roche Unimate 5 HbA1c kit using the Olympus AU600 automated analyzer. Serum insulin was measured using a human insulin-specific radioimmunoassay kit (Linco Research, Inc., St Charles, MO). Serum total testosterone and estradiol were analyzed using coated tube radioimmunoassay kits (Orion Diagnostic, Espoo, Finland). Sex hormone-binding globulin (SHBG) was measured using a coated tube immunoradiometric assay (Orion Diagnostic, Espoo, Finland). The free androgen index (FAI) was calculated as the ratio testosterone/SHBG ×100. Serum insulin-like growth factor-1 (IGF-1) was measured using a commercial preextraction radioimmunoassay with a polyethylene glycol-enhanced second antibody separation step (Bioclone Australia Pty, Ltd., Australia). The interassay coefficients of variation (CV) for these assays ranged from 2.5 to 8.6%. Routine biochemical parameters (serum calcium, phosphorus, albumin, creatinine, alkaline phosphatase and 24-h urinary calcium) were assessed using standard methods (interassay CVs ranged from 1.5 to 4.8%).
Statistical analysis
Statistical analysis was conducted using SPSS version 11.5.2.1 for Windows (SPSS, Inc, Chicago, Ill.). Baseline characteristics between groups were compared by independent t-tests for continuous variables and chi-square tests for categorical variables. Time, group and interaction effects were examined using a two-way analysis of variance (ANOVA) or analysis of covariance (ANCOVA), adjusting for gender, age, use of oral hypoglycaemic medication, change in HbA1c and body composition (weight, fat mass or lean mass) from baseline, and baseline bone or hormonal results. There were no group-by-gender interactions for any of the bone or hormonal measurements, so the pooled data for males and females were presented in Figs. 1 and 2. All BMD and BMC results are presented as the percentage change from baseline or 6 months. All hormonal results were log transformed. Percentage change in the hormonal parameters represents the absolute differences from baseline in the log-transformed data multiplied by 100. All other data were normally distributed. Linear regression analysis was used to examine the relationships between variables. ANCOVA was used to evaluate slope differences between the groups in the regression curves for the changes in fat mass with changes in BMC.
Percent change (unadjusted means, 95% CI) for BMD in the resistance training+weight loss (RT+WLoss) and weight loss (WLoss) group after gymnasium- and home-based training. P=0.05 between group diffrence after adjusting for gender, age, use of oral hypoglycaemic medication, baseline BMD, and change in HbA1c, weight, lean or fat mass
Results
Participant characteristics
At the start of the program, there were no differences in any of the characteristics between the RT+WLoss or WLoss groups (Tables 1 and 2). The mean age of men (67.3 years) and women (67.4 years) was no different. As previously reported [23], four subjects (three RT+WLoss and one WLoss) decreased their oral hypoglycaemic medication dosage, and two subjects from each group increased their medication during the gymnasium-based training. During the home-based training, one subject decreased and another increased their oral hypoglycaemic medication dosage (both RT+WLoss), and one subject from the RT+WLoss group was placed on insulin treatment.
Exercise adherence
In the 29 men and women who completed the gymnasium-based training, compliance with the exercise program averaged 88% (95% CI: 82, 94) for the RT+WLoss, and 85% (95% CI: 78, 92) for the WLoss group. During the home-based program, compliance decreased similarly in both groups (P<0.05), averaging 73% (95% CI: 57, 88) for the RT+WLoss group and 78% (95% CI: 65, 91) for the WLoss group.
Changes in muscle strength
Upper and lower body muscle strength increased, on average, by 43 and 33%, respectively, in the RT+WLoss group (both P<0.01) following the gymnasium-based training; no changes were detected in the WLoss group (1.5 and 5.0%, respectively). The increases in muscle strength from baseline remained greater in the RT+WLoss compared to the WLoss group (upper body 38 vs. 3%; lower body 17 vs. −0.3%, respectively, both P<0.05) following the home-based training. As previously reported, habitual physical activity did not change from baseline in either group following either phase of training [23, 25].
Changes in nutrient intake
Analysis of the food records revealed that there was a similar and significant decrease in the mean total energy intake for both the RT+WLoss [mean change 16% (95% CI: -28, -5); P<0.05] and WLoss groups [21% (95% CI: -30, -12); P<0.01] during the gymnasium-based training. After 12 months, there was a trend for energy intake to remain lower than baseline values in both groups [RT+WLoss, 14% (95% CI: -31, 2); WLoss 15% (95% CI: -21, -9)], but there were no between-group differences. There were no significant changes in the percentage of kilocalories from protein, fat and carbohydrate in either group at any time after the initial dietary stabilization period. Dietary calcium intakes did not change significantly in either group throughout the intervention.
Change in weight and body composition
Both the RT+WLoss and WLoss group experienced similar significant reductions in weight and fat mass during the 6-month gymnasium-based training (Table 3). In contrast, lean mass tended to increase in the RT+WLoss group [mean absolute change 0.5 kg, (95% CI: −0.1, 1.1), P=0.09], but remained unchanged in the WLoss group [−0.4 kg, (95% CI: −1.1, 0.3)] resulting in a significant interaction [net percent difference (95% CI), 1.8% (0.2, 3.5), P<0.05]. During the home-based training, both groups experienced similar increments in weight and fat mass (Table 3, P ranging <0.05 to <0.01). While weight remained significantly lower than baseline values in both groups after 12 months (P<0.01), fat mass had returned to baseline levels in the WLoss group. Lean mass did not change significantly in either the RT+WLoss or WLoss group following the home-based training, but relative to baseline the WLoss group experienced a significant reduction after 12 months [0.6 kg (95% CI: -1.2, -0.1), P<0.05].
Changes in bone mineral density, bone mineral content and bone area
Total body bone mineral density (BMD) and bone mineral content (BMC) did not change in the RT+WLoss group after 6 months of gymnasium-based training, but was decreased by 0.9 and 1.5%, respectively, in the WLoss group (P<0.05), which resulted in a significant group-by-time interaction (BMD: P=0.05; BMC P<0.05; adjusted for gender, age, use of oral hypoglycaemic medication, baseline BMC or BMD, and change in HbA1c, weight, lean mass or fat mass; Fig. 1 and Table 3). There were no between-group differences nor within group changes from baseline for either group for femoral neck or lumbar spine BMC and BMD after 6 months (Table 3). During the home-based training (6 to 12 months), total body and regional BMD and BMC did not change in either group, with the exception of a reduction in total body BMD in the WL group (P<0.05). However, from baseline to 12 months there was a trend to a greater reduction in femoral neck BMD and BMC (interaction P=0.10 and P=0.12, respectively) and total body BMD (interaction P=0.07) in the WLoss compared to RT+WLoss group. Within-group analysis revealed that total body BMD in both groups and lumbar spine BMD and femoral neck BMD and BMC in the WLoss group were significantly lower than baseline levels (P ranging <0.05 to <0.01). Bone area at all three skeletal sites did not change significantly in either group throughout the study.
Hormonal and metabolic changes
For all hormonal measurements, there were no between-group differences for the changes after 3, 6, 9 or 12 months (Fig. 2). However, SHBG levels increased by 18.1% in the RT+WLoss group (P<0.05) after 6 months, which led to a trend for the free androgen index (FAI) to decrease (19.6%, P=0.08) during this period. Estradiol and the FAI increased in the RT+WLoss group from 6 to 12 months (P<0.05), whereas insulin levels decreased in the WLoss group during this period (P<0.05). Over 12 months, both insulin (P<0.001) and IGF-1 levels (P<0.05) decreased from baseline in the WLoss group, whereas total testosterone increased in the RT+WLoss group (P<0.01). For all serum biochemical [calcium, phosphorus, albumin, creatinine and alkaline phosphatase (ALP)] and urinary indices (24-h urinary calcium), there were no significant group, time or interaction effects after the gymnasium-based or home-based training. Changes in HbA1c have been reported in detail separately [23].
Determinants of changes in bone traits
Absolute and percent changes (baseline to 6 months) in body weight and lean mass did not correlate with changes in TB BMC or BMD. In contrast, absolute change in fat mass was correlated with the absolute changes in total body BMC (r=0.38, P<0.05). These results remained unchanged after adjusting for gender, baseline fat mass and BMC. When expressed as TB BMC loss per kilogram of fat loss, participants lost an average of 8.7 g of total body BMC/kg fat loss. There were no significant differences between the RT+WLoss (9.6 g BMC/kg fat loss) and WLoss group (8.0 g BMC/kg fat loss). Changes in body composition following the home-based training were not significantly related to the changes in total body BMC or BMD. Furthermore, no relationships were detected for the changes in HbA1c with changes in total body (or regional) BMC or BMD at any time
Discussion
In this study of older sedentary overweight adults with type 2 diabetes, we have demonstrated that the addition of 6 months of supervised high intensity progressive resistance training to a moderate weight loss program prevented the loss of total body BMC and BMD, and increased lean mass and muscle strength. Furthermore, an additional 6 months of home-based resistance training without further dietary modification was effective for maintaining the gymnasium-based improvements in bone and lean mass and muscle strength.
There are limited and conflicting reports relating to whether the inclusion of exercise into a weight loss program can offset the loss of bone mineral and lean mass typically associated with weight loss alone [16, 18, 19, 20, 21]. This study is the first to report that 6 months of supervised high intensity progressive resistance training added to a moderate weight loss program was effective for maintaining total body bone and lean mass compared to moderate weight loss alone in older adults with type 2 diabetes. However, no significant differences were detected between the groups for the change in femoral neck or lumbar spine BMC and BMD. There are several factors that may account for the positive findings at the total body in relation to previous work in non-diabetic adults. First, this was a randomized controlled trial and 81% of the 36 subjects completed the study, with exercise compliance averaging 88% in the RT+WLoss group over the 6-month gymnasium-based training period. Second, the training program consisted of progressive high intensity (75 to 85% of maximum strength) resistance training, which has been shown to be more osteogenic in healthy older adults than low to moderate intensity resistance training or aerobic-based exercise programs [14, 22, 26]. Third, the magnitude of weight loss in this study was less than that reported in three previous studies examining the effects of exercise on changes in BMD during weight loss (~3 kg compared 7 to 19 kg) [16, 19, 21]. A greater weight loss has been shown to be associated with a greater bone loss [7, 10, 27], and thus it is possible that the beneficial effects of exercise on the skeleton in these earlier studies may have been countered, to some extent, by the increased weight loss. In the present study, both groups experienced a similar reduction in body weight and habitual physical activity did not change throughout the intervention, and thus the maintenance of total body BMD and BMC in the RT+WLoss can be attributed to the high intensity resistance training program.
At the femoral neck and lumbar spine, there were no significant between-group differences for the change in either BMC or BMD after the gymnasium-based training. The major mechanism by which resistance training contributes to an osteogenic response is by the action of muscle pull mediated through the force of muscular contraction at the site of attachment of the tendon on the bone [14]. While all participants in the RT+WLoss group were involved in a high intensity resistance-training program that resulted in large increases in muscle strength, the upper and lower body exercises utilized were not specific to the hip or lumbar spine region. Thus, it is likely that they did not provide a sufficient stimulus to induce an osteogenic response at these sites. Furthermore, the femoral neck is a site that does not have muscle attachments, and thus the lack of a significant effect following the resistance-training program is consistent with the site-specific response of bone to exercise.
Although previous studies in healthy older adults have reported a positive effect of supervised progressive resistance training on bone density [14, 15, 22], few studies have assessed the long-term influence of home-based training on bone and muscle traits. In the present study, we demonstrated that the skeletal and muscle benefits obtained from a supervised gymnasium-based resistance training program were maintained following a further 6-month home-based training, despite a lower adherence to exercise and a reduction in the volume and intensity of resistance training. We estimated that training volume (calculated as the total amount of weight lifted multiplied by the number of repetitions completed) decreased initially by 52% from the gymnasium-based to home-based training [25]. This was largely due to a reduction in the amount of weight lifted because various exercises performed using the machine weights during the gymnasium-based training could not be replicated with similar absolute weights using the hand or leg weights. While it is important to acknowledge that the study design does not allow a direct comparison of the effectiveness of gymnasium-based versus home-based resistance training, these findings highlight that a home-based resistance training program using hand and leg weights was sufficient to maintain the initial skeletal and muscle benefits obtained from the gymnasium-based program.
The observation that a 6-month program of moderate dietary-induced weight loss in older overweight adults with type 2 diabetes was associated with increased rates of bone loss is consistent with the findings from several intervention studies that have measured changes in weight and bone density over a similar time interval [10, 16]. Several mechanisms have been proposed to explain the loss in BMD following weight loss. A reduction in body weight leads to a decline in the mechanical loads imparted to the skeleton, which may result in increased bone remodeling and subsequent bone loss [7, 20]. While there are data showing that weight loss is associated with increased bone resorption [20, 28], the reduction in body weight in the present study was relatively small (~3 kg) and similar between the groups. Thus, it is unlikely that reduced loading contributed directly to the reduction in BMD or BMC in the WLoss group and the differential skeletal responses between groups.
A common concern regarding dietary-induced weight loss programs is the concurrent loss of lean mass, which is an important determinant of bone mass. We found that supervised high-intensity resistance training was effective for increasing lean mass in the presence of weight loss, but no relationship was detected between changes in lean mass and changes in any of the bone traits. While this could be due to the small changes observed in lean mass, others have also failed to detect a significant relationship between changes in BMD (or BMC) and lean mass in adults [18, 29, 30]. In contrast, there is evidence that age- and weight loss-induced changes in bone mineral in older adults are more strongly related to changes in fat mass [29, 30]. In overweight men randomized to either weight loss through dietary restriction or exercise, Pritchard et al. [18] reported a loss of 11.5 g of total body bone mineral for each 1-kg reduction in fat mass in both groups. In the present study, we also detected a significant relationship between the 6-month absolute changes in fat mass and total body BMC following the gymnasium-based training. On average, participants lost 8.7 g of bone mineral per kg of fat loss.
The mechanism underlying the relationship between changes in body composition (weight or fat mass) and bone mineral has not been fully elucidated. There is some evidence that a reduction in body fat associated with weight loss may alter circulating sex steroid and SHBG levels, both of which play an important role in bone metabolism [12, 13, 28]. We observed a significant increase in SHBG levels in the RT+WLoss group after 6 months, but there were no between-group differences for the change in SHBG, testosterone, estradiol or the FAI at any time. It has also been suggested that dietary-induced weight loss, with a resultant decrease in nutrient intake, may adversely affect bone metabolism by altering circulating levels of insulin and insulin-like growth factor-1 (IGF-1) [13, 28]. Conversely, it has been proposed that an increase in lean mass associated with resistance training may lead to an increase in IGF-1 levels [22]. In the present study, we did not observe any changes in IGF-1 in the RT+WLoss group, nor could we detect any between group differences for either insulin or IGF-1. Others have indicated that weight loss may lead to a rise in PTH levels and subsequent increase in bone resorption because of a reduction in dietary calcium associated with decreased nutrient intake [13]. Although we did not measure serum PTH levels or biochemical markers of bone turnover, dietary calcium intakes were similar between the groups and did not change throughout the intervention. Thus, it would appear that changes in circulating sex hormones or IGF-1 did not mediate the differential effects of weight loss with and without resistance training on changes in BMC or BMD. However, we acknowledge that a limitation of this study was the small heterogeneous sample and subsequent potential lack of power to detect significant changes or differences between groups.
Previous research has shown that a reduction in BMD with diet-induced weight loss is reversible following a subsequent regain in weight [31, 32]. In the present study, weight regain during the home-based training was not associated with similar increments in BMC or BMD. While the reasons for these findings are not clear, it has been suggested that small changes in bone density associated with weight loss may be attributable to measurement artifacts associated with changes in the amount of soft tissue surrounding the bone between scans [33]. The finding that total body BMD, but not BMC, decreased in both groups over 12 months indicates that changes in body composition may have influenced the ability of the DXA to detect bone edges accurately, thus changing the estimation of bone area and subsequently BMC and BMD. However, since both groups lost and subsequently regained a similar, though small amount of weight (and fat mass) throughout the intervention, it is unlikely that the differential changes in BMD or BMC between groups were due to errors in edge detection by DXA alone.
In summary, we have shown in previously sedentary overweight adults with type 2 diabetes that supervised high intensity progressive resistance training added to a moderate weight loss program was safe, well tolerated and effective for maintaining bone and muscle mass and for improving muscle strength compared to moderate weight loss alone. Furthermore, home-based resistance training without dietary modification maintained the initial benefits to muscle and bone mass. Although these findings may not be generalizable to other population groups, they highlight that a high intensity progressive resistance training program can be recommended as a practical approach to prevent bone and muscle loss during weight reduction in older overweight adults with type 2 diabetes. While further research is needed into the clinical significance of bone loss associated with dieting in patients with type 2 diabetes, many of whom may have ‘normal’ BMD, clinicians need to consider the overall risks and benefits of weight loss when discussing treatment strategies for older adults with type 2 diabetes.
References
Barrett-Connor E, Holbrook TL (1992) Sex differences in osteoporosis in older adults with non-insulin-dependent diabetes mellitus. JAMA 268:3333–3337
Gregorio F, Cristallini S, Santeusanio F, Filipponi P, Fumelli P (1994) Osteopenia associated with non-insulin-dependent diabetes mellitus: what are the causes? Diabetes Res Clin Pract 23:43–54
Tuominen JT, Impivaara O, Puukka P, Ronnemaa T (1999) Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care 22:1196–1200
Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Zmuda JM, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB (2004) Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: The Health, Aging, and Body Composition Study. J Bone Miner Res 19:1084–1091
Schwartz AV, Hillier TA, Sellmeyer DE, Resnick HE, Gregg E, Ensrud KE, Schreiner PJ, Margolis KL, Cauley JA, Nevitt MC, Black DM, Cummings SR (2002) Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 25:1749–1754
Chao D, Espeland MA, Farmer D, Register TC, Lenchik L, Applegate WB, Ettinger WH Jr (2000) Effect of voluntary weight loss on bone mineral density in older overweight women. J Am Geriatr Soc 48:753–759
Jensen LB, Quaade F, Sorensen OH (1994) Bone loss accompanying voluntary weight loss in obese humans. J Bone Miner Res 9:459–463
Ensrud KE, Cauley J, Lipschutz R, Cummings SR (1997) Weight change and fractures in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med 157:857–863
Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR (2003) Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc 51:1740–1747
Ramsdale SJ, Bassey EJ (1994) Changes in bone mineral density associated with dietary-induced loss of body mass in young women. Clin Sci (London) 87:343–348
Langlois JA, Harris T, Looker AC, Madans J (1996) Weight change between age 50 years and old age is associated with risk of hip fracture in white women aged 67 years and older. Arch Intern Med 156:989–994
O’Dea JP, Wieland RG, Hallberg MC, Llerena LA, Zorn EM, Genuth SM (1979) Effect of dietary weight loss on sex steroid binding sex steroids, and gonadotropins in obese postmenopausal women. J Lab Clin Med 93:1004–1008
Ricci TA, Heymsfield SB, Pierson RN, Jr., Stahl T, Chowdhury HA, Shapses SA (2001) Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr 73:347–352
Kerr D, Morton A, Dick I, Prince R (1996) Exercise effects on bone mass in postmenopausal women are site-specific and load-dependent. J Bone Miner Res 11:218–225
Nelson ME, Fiatarone MA, Morganti CM, Trice I, Greenberg RA, Evans WJ (1994) Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. A randomized controlled trial. JAMA 272:1909–1914
Ryan AS, Nicklas BJ, Dennis KE (1998) Aerobic exercise maintains regional bone mineral density during weight loss in postmenopausal women. J Appl Physiol 84:1305–1310
Fogelholm GM, Sievanen HT, Kukkonen-Harjula TK, Pasanen ME (2001) Bone mineral density during reduction, maintenance and regain of body weight in premenopausal, obese women. Osteoporos Int 12:199–206
Pritchard JE, Nowson CA, Wark JD (1996) Bone loss accompanying diet-induced or exercise-induced weight loss: a randomised controlled study. Int J Obes Relat Metab Disord 20:513–520
Svendsen OL, Hassager C, Christiansen C (1993) Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med 95:131–140
Salamone LM, Cauley JA, Black DM, Simkin-Silverman L, Lang W, Gregg E, Palermo L, Epstein RS, Kuller LH, Wing R (1999) Effect of a lifestyle intervention on bone mineral density in premenopausal women: a randomized trial. Am J Clin Nutr 70:97–103
Andersen RE, Wadden TA, Herzog RJ (1997) Changes in bone mineral content in obese dieting women. Metabolism 46:857–861
Maddalozzo GF, Snow CM (2000) High intensity resistance training: Effects on bone in older men and women. Calcif Tissue Int 66:399–404
Dunstan DW, Daly RM, Owen N, Jolley D, De Courten M, Shaw J, Zimmet P (2002) High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care 25:1729–1736
American College of Sports Medicine (2000) ACSM guidelines for exercise testing and prescription. Lippincott Williams and Wilkins, Philadelphia
Dunstan DW, Daly RM, Owen N, Jolley D, Vulikh E, Shaw J, Zimmet P (2005) Home-based resistance training is not sufficient to maintain improved glycemic control following supervised training in older individuals with type 2 diabetes. Diabetes Care 28:3–9
Snow Harter C, Bouxsein ML, Lewis BT, Carter DR, Marcus R (1992) Effects of resistance and endurance exercise on bone mineral status of young women: a randomized exercise intervention trial. J Bone Miner Res 7:761–769
Van Loan MD, Johnson HL, Barbieri TF (1998) Effect of weight loss on bone mineral content and bone mineral density in obese women. Am J Clin Nutr 67:734–738
Ricci TA, Chowdhury HA, Heymsfield SB, Stahl T, Pierson RN Jr, Shapses SA (1998) Calcium supplementation suppresses bone turnover during weight reduction in postmenopausal women. J Bone Miner Res 13:1045–1050
Chen Z, Lohman TG, Stini WA, Ritenbaugh C, Aickin M (1997) Fat or lean tissue mass: which one is the major determinant of bone mineral mass in healthy postmenopausal women? J Bone Miner Res 12:144–151
Reid IR, Ames RW, Evans MC, Sharpe SJ, Gamble GD (1994) Determinants of the rate of bone loss in normal postmenopausal women. J Clin Endocrinol Metab 79:950–954
Hui SL, Perkins AJ, Zhou L, Longcope C, Econs MJ, Peacock M, McClintock C, Johnston CC, Jr. (2002) Bone loss at the femoral neck in premenopausal white women: effects of weight change and sex-hormone levels. J Clin Endocrinol Metab 87:1539–1543
Compston JE, Laskey MA, Croucher PI, Coxon A, Kreitzman S (1992) Effect of diet-induced weight loss on total body bone mass. Clin Sci (London) 82:429–432
Tothill P, Pye DW (1992) Errors due to non-uniform distribution of fat in dual X-ray absorptiometry of the lumbar spine. Br J Radiol 65:807–813
Acknowledgements
Dr. Robin Daly and Dr. David Dunstan are both supported by National Health and Medical Research Council (NHMRC) Research Fellowships. Professor Owen is supported by Queensland Health and by the National Health and Medical Research Council. This study was financially supported by a grant from the Victorian Health Promotion Foundation (VicHealth). Funds for the purchase of exercise equipment were kindly provided by the Rotary Club of Kew, Victoria, and the Soroptimist International, Brighton Division. We wish to thank Lucy Robinson and Elena Vulikh for their kind assistance in the clinical management of our volunteers. We are also grateful for the dietary assistance of Kathy McConell and Helen Bauzon. Most importantly, the authors are indebted to the volunteers whose cooperation and dedication made this study possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daly, R.M., Dunstan, D.W., Owen, N. et al. Does high-intensity resistance training maintain bone mass during moderate weight loss in older overweight adults with type 2 diabetes?. Osteoporos Int 16, 1703–1712 (2005). https://doi.org/10.1007/s00198-005-1906-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-005-1906-4