Abstract
This cross-sectional study covered 301 individuals over 70 years of age—207 women (W) and 94 men (M)—living in the city of São Paulo, Brazil. Our aims were to evaluate the prevalence of low bone mineral density (BMD) in this population and the possible factors that influence BMD. The subjects were submitted to a bone densitometry scan (DXA) to evaluate the BMD at lumbar spine (LS), femoral neck (FN), trochanter (T), total femur (TF) and total body composition. At the time, the participants filled in a questionnaire about lifestyle habits, diet and medical history, as well as having blood samples taken to check hormone and biochemical levels. Anthropometric parameters were measured. Osteopenia and osteoporosis were defined in accordance with the criteria suggested by the World Health Organization. In the different sites studied, the prevalence of osteopenia and osteoporosis varied, in men ranging 33.3–57.4% and 6.4–16.1%, respectively, and in women ranging 36.6–56.5% and 22.2–33.2%, respectively. Weight was the variable that most strongly correlated with BMD at the proximal femur in both sexes (men, r =0.44–0.52; women, r =0.48–0.52) and with BMD at LS in women ( r =0.44). Height was the parameter that best correlated with BMD at LS in men ( r =0.34). In men follicle-stimulating hormone, growth hormone and glycemia correlated with BMD at T and TF, while plasma albumin only correlated with BMD at T. In women glycemia correlated with BMD at LS, and follicle-stimulating hormone correlated with BMD at FN, T and TF. In conclusion, we found a high prevalence of osteopenia and osteoporosis in this population, with weight being the best predictor of BMD. The prevalence of osteoporosis and osteopenia at FN was as high in men as that observed in women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is characterized by a decrease in bone mass and a deterioration of the microarchitecture of the bone, which leads to fragility and consequently an increase in the risk of fractures [1]. In 1994, the World Health Organization (WHO) defined osteoporosis as a condition in which bone mineral density (BMD) measures greater than 2.5 times the standard deviation (SD) below the mean for young people [1,2]. Studies showed that for each 1.0 SD below the mean BMD for young people, the risk of a vertebral fracture increases 2 to 2.4 times, and the risk of a nonvertebral fracture increases by 1.7 times [2]. Therefore, BMD measurement is an extremely useful parameter for classifying people who are susceptible to osteoporotic fractures, with bone densitometry being the best tool available for evaluating it [3,4].
Loss of bone mass is more common in women than in men, especially after menopause, although with age both sexes present bone mass loss and an increase in the risk of osteoporotic fractures [4]. The number of people over 60 in Latin America and especially in Brazil has been increasing rapidly due to the urbanization of the population and the decline in the mortality rate [5]. Along with this increment in the elderly population, we have also seen a dramatic increase worldwide in the number of osteoporotic fractures. It has been calculated that in 2050 there will be 6.3 million fractures per year, more than half of this total occurring in Asia and Latin America [6]. In Brazil there are approximately 14.5 million people over 65 years. It is estimated that by 2025 we will have the sixth largest population of elderly people in the world—more than 32 million—representing 15% of Brazil’s total population [5]. Despite this alarming data, we still do not know how prevalent osteoporosis is in our elderly population.
The aims of this work were to evaluate the prevalence of low BMD, by carrying out a cross-sectional study on a group of elderly residents in the city of São Paulo, Brazil, and to analyze the possible factors that have had an influence on the BMD of these individuals.
Subjects and methods
Subjects
In 1991, the Center for the Study of Ageing of the Federal University of São Paulo (CSA) began the first longitudinal, non-interventional, cohort study with a community-based older population in Brazil—the original “Epidemiologia do Idoso (EPIDOSO)” Study [5]. The EPIDOSO Study included only individuals aged 65 and over, living in the district of Saude, one of the 55 districts of the city of São Paulo, a large, industrialized urban center in southeastern Brazil. A previous census had established that this district had a population of more than 300,000 people, from different socioeconomic backgrounds, living in a middle-class residential area with a low rate of inward and outward migration. The sampling area was the catchment area of the CSA, with an estimated population of 30,000. A comprehensive door-to-door census survey was performed, and everyone aged 65 and older living in the catchment area was eligible for the study. Between the end of 1991 and the beginning of 1993, trained interviewers successfully interviewed 1,667 individuals at home. Between 1994 and 1995, 1,254 individuals (75%) of the original sample had a follow-up evaluation: 1,108 subjects were re-interviewed (the respondents) at home (66%); 146 died during the period (9%), and the last 413 (25%) were considered to be nonrespondents, because of refusal, change of address, or lack of contact after four attempts. A detailed description of the methodology is available elsewhere [5]. Between 1998 and 1999, 66% ( n =1,108) of the original sample (the respondents) had a follow-up evaluation: 441 subjects were re-interviewed at the CSA; 212 died during the period and 455 were considered to be nonrespondents, because of refusal, change of address, or lack of contact after four attempts by letter or by telephone. As we mentioned previously, 441 individuals visited the CSA and provided information about their medical history and their lifestyle. At the same time, they also gave blood samples for the laboratory tests that were of interest to the researchers. They were also invited to take part in a specific study on osteoporosis. A group of 207 women and 97 men—at this stage with a minimum age of 70—accepted the invitation to have a bone densitometry scan (BD). The remaining 137 individuals refused to take part in the study on osteoporosis. The study was authorized by the University’s Research Ethics Committee and written consent was obtained from all participants.
Methods
Lifestyle, diet and medical history
Lifestyle habits and medical history were evaluated using a standard questionnaire that was given by a previously trained doctor.
Physical exercise
Participants were categorized into those who engaged in some form of physical exercise and those who did not. Those who mentioned they did some type of physical exercise (for example, walking) at least three times a week for 30 min or more per session were considered to be people who engaged in exercise.
Smoking
Participants were classified as “non-smokers”, “former smokers” and “current smokers”. The group of non-smokers was made up of elderly people who said they had never smoked at any time in their lives. The group of former smokers was made up of elderly people who said they had smoked cigarettes at some stage in their lives. The group of current smokers was made up of people who said they smoked.
Medication
Participants were questioned about the regular use of medication, and medicine used continually was considered to be that which the elderly mentioned spontaneously. The elderly were not specifically asked about the use of drugs to treat osteoporosis; that is, the only information taken into account was who had spontaneously mentioned the use of these drugs. All the subjects were specifically asked about the use of anticonvulsant drugs and corticosteroids. Questions referring to the use of anticonvulsant drugs and corticosteroids led to the elderly being classified into three groups: “non-users”, “current users” and “former users.” We considered those participants who said they didn’t use any drugs as belonging to the group of non-users. The group of current users was made up of elderly people who mentioned they were using drugs at the time of the survey, and we included in the users-in-the-past group those participants who mentioned previous use of medication. All the women were questioned about the use of estrogen-replacement treatment during menopause, and they were also classified according to the criteria described above, that is, non-users, current users and former users.
Consumption of coffee and milk/cheese
The elderly people were divided into two groups according to their intake of coffee: non-drinkers and drinkers. The intake of milk and cheese led to a classification into three groups as far as the intake of calcium via food was concerned: non-consumers, consumers of 300–600 mg per day and those who consumed more than 600 mg per day.
Menstrual history
Women gave their menarche and menopause ages, based on which the duration of their reproductive years and time since menopause were calculated.
Bone mineral density
Between May 1998 and December 1999, we measured the bone mineral density (BMD) by means of dual energy X-ray absorptiometry (Hologic, model 4500 A, Waltham, MA, USA), evaluating the lumbar spine, the L2–L4 region (LS) and proximal femur in the regions of the femoral neck (FN), trochanter (T) and the total femur (TF). The variation coefficients for the lumbar spine and proximal femur were 0.6% and 1.5%, respectively [7]. In all the regions studied, we grouped the elderly based on WHO diagnostic criteria for osteoporosis:
-
Participants considered normal had BMD up to 1.0 SD below the sex-specific means for young adults
-
Participants considered to have osteopenia had BMD between 1.0 SD and 2.5 SD below the sex-specific means for young adults
-
Participants with osteoporosis had BMD more than 2.5 SD below the sex-specific means for young adults.
The reference standard used for the analysis of BMD at the lumbar spine was supplied by the manufacturer of the equipment [8]. As a reference for the analysis of BMD at the proximal femur we used data collected by the third National Health and Nutrition Examination Survey (NHANES III, 1984–1994) in accordance with the recommendations of the International Committee for Standards in Bone Measurement (1997) [9,10].
Anthropometric measurements
Weight and height
These were obtained using scales calibrated in kilograms and a stadiometer in centimeters. The measurements were carried out before the BD examination by the same examiner.
Body mass index (BMI)
The BMI calculation was carried out using the body weight and height (kg/m2) measurements.
Percentage of body fat and lean mass
These were obtained by examining body composition, using the dual energy X-ray absorptiometry technique (Hologic).
Lean mass index (LMI)
The LMI calculation was carried out using the measured values of lean mass and height (g/m2) [11].
Laboratory examinations
Between April 1998 and December 1999, during participant visits to the CSA, fasting blood was collected for hormonal and biochemical examinations. The immunoenzymatic method was used to measure growth hormone (GH), with normal values between 0–2.5 μg/l [12]. The immunoenzymatic method was also used for determinations of luteinizing hormone (LH) levels, considering as the reference value LH up to 10 IU/l for men and LH>15 IU/l for postmenopausal women [13]. The post-extraction immunoradiometric assay (Chiron Diagnostics, East Walpole, MA, USA) was used for determinations of insulin-like growth factor-1 levels (IGF-1), the reference value for the age band corresponding to 70–290 ng/ml. Radioimmunoassay was used for dehydroepiandrosterone sulfate (DHEAS) determinations, considering 10–160 μg/dl as normal for the age band [14]. Thyroid-stimulating-hormone (TSH) concentrations were obtained by the immunofluorometry method, with the reference value corresponding to 0.3–4.0 mIU/l [15]. Levels of follicle-stimulating hormone (FSH) were measured by immunofluorometric assay, considering the FSH reference value up to 10 IU/l for men and FSH>30 IU/l for postmenopausal women [16]. Competitive assay with luminometric readout was used for determinations of total testosterone (TT) levels, with normal values of 300–900 ng/dl and 15–75 ng/dl, for men and women, respectively [17].
In accordance with the serum level of total testosterone, the men were divided into two subgroups: normal (TT>300 ng/dl) and low (TT<300 ng/dl).
Glucose, creatinine and albumin were measured using an automatic colorimetric method. The normal value for glucose was 75–110 mg/dl. Reference values for creatinine and albumin were 0.8–1.2 mg/dl and 3.5–5.0 g/dl, respectively.
Statistical analysis
For all statistical analyses we used the Statistical Package for Social Sciences, version 11.0 (SPSS, Chicago) and analyzed men and women separately. We did a descriptive analysis for the whole group, including mean, standard deviation and minimum and maximum values. The chi-square association test was used to compare the distribution of BMD between men and women, in accordance with WHO diagnostic criteria for osteoporosis. The Student’s t -test, one way (ANOVA) or Mann–Whitney tests were used to do comparisons between subgroups with different lifestyles and eating habits, use of medication and serum level of testosterone. Subsequently, we used the Pearson correlation coefficients to evaluate the association between BMD and quantitative variables, that is, age, anthropometric parameters and laboratory examinations. To check the independent effect of the quantitative variables on BMD (dependent variable), we built four multiple-linear-regression models for each sex—a model for each site studied. We put together the multiple model using the stepwise forward-selection process. The variable remained in the multiple model when it was considered to be statistically significant or even if it was considered important for adjusting the statistical model. The level of statistical significance was defined as p <0.05 in all the analyses.
Results
Characterization of the population
We excluded from our study three men because of the lack of results for BMD at the proximal femur. For technical reasons we did not evaluate BMD at the LS for one male participant (M) and five female participants (F). We did not evaluate the total body composition of one female participant, also for technical reasons. The group was predominantly made up of Caucasians, who represented 90.3% of the individuals of both sexes. The rest of the group was made up of mulattos (2.3%) and subjects with Asian or African genetic backgrounds (3.0% and 4.3%, respectively). In Table 1, we summarize the characteristics of the population we studied. In both sexes the mean age was 78, with 62.3% ( n =129) of women and 68.1% ( n =64) of men being between 70–79 years old and 37.7% ( n =78) of women and 31.9% ( n =30) of men being over 80.
Some of the elderly spontaneously mentioned the constant use of medication that could have an influence on bone metabolism. We emphasize that only the participants who spontaneously mentioned the use of drugs to treat osteoporosis were taken into account. The use of thiazidic diuretics was mentioned by 26.6% of women and 14.9% of men. The use of vitamin D was mentioned by 6.3% of women and 1.0% of men. Only women mentioned the use of levotiroxine (5.3%), bisphosphonates (1.9%) and medical calcium supplements (12.6%). Putting together the users of vitamin D, bisphosphonates and calcium supplements, 17.3% of the women referred to the use of some specific medication for the treatment of osteoporosis. We did not, however, exclude the users of medication from our analysis because, besides being small in number, their mean BMD (LS, FN, T, TF) did not differ statistically from that of other participants (data not shown).
Eleven male participants (12.0%) had below-normal testosterone levels. Of these, four individuals (4.3%) had high serum concentrations both of LH and FSH and two (2.1%) only had high serum levels of FSH.
One hundred and seventy women (82.2%) could recall their menstrual history. The mean time between the menopause and BD examination was 28.8 years (SD±7.7), and the mean duration of reproductive years was 35.8 years (SD±6.3).
Prevalence of osteoporosis
Figs. 1, 2 and 3 show the distribution of BMD at the LS and proximal femur in accordance with criteria suggested by the WHO. We found osteopenia and/or osteoporosis, in at least one of the sites studied, in 78.7% of the men. In the proximal femur, the FN region had the highest prevalence of osteopenia (57.4%) and osteoporosis (12.8%). Seventy five percent of cases of osteoporosis at the femoral neck were found among men over 80 years of age. Of the men who had osteoporosis at the trochanter and at the total femur, 44.4% and 50%, respectively, were over 80. Of the men who had osteoporosis at the lumbar spine, 53.3% were over 80. The majority of men with normal levels of total testosterone (79.0%) and men with low levels of total testosterone (72.7%) had osteopenia and/or osteoporosis in at least one of the sites studied. The mean BMD values between men with normal and low total testosterone levels did not differ.
We found osteopenia and/or osteoporosis in at least one of the sites studied in 92.8% of women. The trochanter was the region of the proximal femur with the highest prevalence of osteoporosis (25.6%). Of the women who had osteoporosis at the lumbar spine, 76.8% were between 70 and 79 and 33.2% were over 80. At the proximal femur there was similar distribution of cases of osteoporosis between the age bands 70–79 and over 80. The prevalence of osteoporosis and osteopenia was greater in women than in men for the lumbar spine ( p =0.001), trochanter ( p <0.001) and total femur ( p <0.001). For the femoral neck there was no significant difference between the sexes as far as the prevalence of osteoporosis and osteopenia was concerned ( p =0.083).
Lifestyle, diet, medical and menstrual history vs BMD
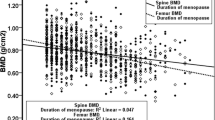
When we divided the participants into groups, taking into consideration their physical exercise, smoking, coffee and milk consumption, use of anticonvulsants and corticosteroids, and use of hormone replacement therapy, the mean BMD at the four sites studied did not differ statistically (data not shown). Time since menopause had a negative correlation with BMD at the trochanter ( r =−0.25, p =0.001) and at the total femur ( r =−0.28, p <0.001). The duration of the reproductive years did not correlate with BMD at any site studied.
Age, anthropometric parameters, laboratory results vs BMD
Table 2 shows only the variables that significantly correlated with BMD in at least one skeletal site in either sex. The variables that did not significantly correlate with BMD are not shown. The anthropometric parameters were the variables that best correlated with BMD in both sexes and in all the sites studied. Only BMI, LMI and body fat were not associated with BMD at the lumbar spine in men. Of the anthropometric measurements, body weight was the variable that best correlated with BMD at the proximal femur both in men and women. Weight was also the variable that most strongly correlated with BMD at the lumbar spine in women, while in men, height was the variable that best correlated with BMD at the lumbar spine.
In both sexes, we observed no correlation between age and BMD at the lumbar spine. However, at the proximal femur there was a negative and significant correlation between age and BMD in men and women (Table 2). In general, the laboratory parameters showed a weak correlation with BMD (Table 2). In men FSH and GH correlated negatively with BMD at the trochanter and total femur, while glycemia correlated positively at those sites. In men plasma albumin only correlated positively with BMD at the trochanter. In women glycemia also presented a positive correlation with BMD at the lumbar spine, and FSH correlated negatively with BMD at all the sites of the proximal femur (Table 2).
The results of the multiple linear-regression analysis are summarized in Table 3. In men, height was the best independent predictor of BMD at the lumbar spine, thereby explaining most of the variance of BMD at this site ( R 2=0.10). In women, body weight and level of glucose were included in the final LS statistical model, body weight being most responsible for BMD variance at this site ( R 2=0.19). In both sexes weight and age were predictors of BMD at the femoral neck, with weight again being the variable that best explained BMD variance at this site (men, R 2=0.19, women, R 2=0.23). In men the best explanatory model for BMD at the trochanter included the variables height, weight and FSH. In women the best explanatory model for BMD at the trochanter included weight, body fat, age and DHEAS. In both sexes, weight was the variable that best explained BMD variance at the trochanter (men, R 2=0.25; women, R 2=0.24).
In men the final TF model included weight, age and FSH as independent variables. In this case weight was also the independent predictor that best explained BMD variance at the total femur ( R 2=0.27). In women the independent predictors of BMD at the total femur were body fat, age, LMI and height, with body fat being the independent variable that best explained the BMD variance at the total femur ( R 2=0.19).
Discussion
This is the first extensive study that has evaluated BMD in the over-70 s group in Brazil, thereby increasing the knowledge about osteoporosis in our country. The city of São Paulo is situated in one of the richest regions in Brazil. With one of the highest life expectancies in the country—65 years for men and 73 years for women [19]—it is an ideal place to study osteoporosis in Brazil. Native Indians, Africans, Europeans and Asians have all contributed to the racial makeup of the Brazilian people, and this has led to great genetic and cultural diversity. Both the racial mixture and variations in climatic and economic conditions of the different regions have transformed Brazil into a heterogeneous country. Our research covered a population composed predominantly of Caucasians (90.3%)—different from the Brazilian population as a whole. The cohort also represented a middle-class, reasonably wealthy, area, with a lower proportion of illiterate elderly (21.5%) and a higher proportion of elderly who had reached high school and/or university (44.8%), when compared with the elderly living in the whole city of São Paulo (35% and 18%, respectively) [20]. The income distribution was also better, although the average income was still rather low by international standards. Sixty-eight percent of the studied group had a per capita monthly income of US $100 or more, while in a previous study only 29% of the elderly of São Paulo had a similar level of income [5,20].
The best tool available for evaluating the risk of fractures is the BMD measurement using the DXA scan [3, 4,10]. Currently, there is evidence that, for a proper analysis of the bone densitometry scan, the peak values of the bone mass specific to each population should be used as the standard of reference [10]. In Brazil we do not have these standard data; consequently, we compared our population with the reference data supplied by the manufacturer of the bone densitometry scanner [8] in the case of the lumbar spine. For the proximal femur, we compared it with the non-Hispanic white men and women from the NHANES III database [8,18]. However, other authors have compared a population of young Caucasian women living in São Paulo with American Caucasian women and found very similar BMD means [21]. The analysis of our data would be better if we had the fracture threshold of our population. Unfortunately, we still do not have data about the incidence rates for hip fractures in the elderly population of São Paulo, and this could be considered a limitation of our research. Recently, research conducted in a city located in the northeast of Brazil (Sobral) reported a low age-adjusted incidence of hip fracture, 20.7 and 8.9 hip fractures/10,000 inhabitants/year for women and men >60 years old, respectively [22]. However, as we mentioned, due to the heterogeneity of our country, it is not possible to consider these results as representing Brazil as a whole.
The applicability of WHO diagnostic criteria to men is not certain, due to lack of sufficient data on the relation between BMD and fracture risk in men. When based on female cutoffs, we found osteopenia at the femoral neck, trochanter and total femur in 46.8%, 30.9% and 28.7% of men, respectively. When we defined osteoporosis based on female cutoffs, the diagnosis was found in 8.5%, 4.3% and 3.2% of men at the femoral neck, trochanter and total femur, respectively. But when based on male cutoffs, there were higher prevalence rates of osteopenia and osteoporosis in this cohort study; that is, the diagnosis of osteopenia was found in 57.4%, 37.2% and 36.2% of men at the femoral neck, trochanter and total femur, respectively. In addition, osteoporosis was noticed at the femoral neck, trochanter and total femur in 12.8%, 9.6% and 6.4% of men, respectively. This evident impact of cutoff points on prevalence estimates could be attributed to the fact that bone size creates an artifact that affects the measurement of areal BMD [10,18]. Since current DXA machines do not adjust BMD for body size, there is an overestimation of BMD in men relative to women [10,18]. Therefore, sex-specific cutoff values may be necessary to compensate for this artifact [18]. Regardless of which cutoffs are used for men, this issue will only be resolved with the establishment of appropriate cutoff points for the diagnosis of low bone mass in men [10,18]. When based on sex-specific cutoffs, the prevalence of osteoporosis of the lumbar spine was higher in women, whereas, at the femoral neck there was a similarity in prevalence between men and women. We must underline that 75% of the men who had osteoporosis of the femoral neck were over 80. These findings confirm the contribution that living longer plays in the etiology of osteoporosis in men [23]. Despite the high number of osteoporosis and osteopenia sufferers, only 17.3% of women and 1.0% of men spontaneously mentioned the specific use of some form of medication for treating these alterations. This may suggest that the therapeutic approach to osteoporosis and osteopenia still warrants more careful attention from the medical profession. However, it is important to clarify that the elderly were not specifically asked about the use of drugs to treat osteoporosis, and it is possible that some users were missed. All of this data should be considered by local public health authorities, since fractures occurring as a result of bone fragility can adversely affect the quality of life of the elderly and contribute to an increase in morbimortality in this age band [2, 4,24].
Zerbini and colleagues evaluated the BMD in another Brazilian male population [25], and for comparison they included the BMD data of a Brazilian female population [21]. These authors observed that both the male and female populations continued losing bone mass at the femoral neck as they aged, whereas, at the lumbar spine only the sample of women used for comparison [21] continued to lose it. These same findings were described in the Rancho Bernardo study [26], which analyzed a large American population made up of men and women between 50 and 98. In our analysis, the lack of correlation between age and BMD at the lumbar spine, seen in both men and women, suggests that at a more advanced age this site is not appropriate for evaluating BMD and, therefore, not appropriate for determining the risk of fractures. With age, individuals are more prone to developing degenerative alterations such as calcification of the aorta and osteophytosis of the lumbar spine [26–29]. These alterations interfere in the analysis of the bone densitometry scan of the lumbar spine and might overestimate the level of BMD in this region.
The loss of bone mass in women is strictly associated with menopause, a time when failure of ovarian function leads to rapid reduction of estrogen levels [30,31]. In this analysis, the duration of menopause and the serum levels of FSH correlated negatively with BMD at the proximal femur, therefore suggesting a link between ovarian failure and bone loss in this site.
In recent years, various studies have been developed with the aim of linking the reduction in circulating levels of testosterone with bone loss in elderly men. However, no consistent conclusion has been reached, because, while some authors have identified total testosterone as a strong predictor of BMD, others have not shown this same link [30,32–35]. Our studies also found no correlation between BMD and the serum level of total testosterone. Furthermore, we observed a similar proportion of low bone mass in men with low and normal levels of total testosterone (72.7% vs 79.0%). In fact, it is currently being suggested that estrogens have a more important influence on bone mass in men than testosterone does [30, 32, 35,40]. Despite this evidence, the role of testosterone cannot be ignored, because this androgen clearly contributes to maintaining bone mass in men. It has an effect on bone re-absorption and formation, as well as being the substance necessary for the aromatization and, consequently, the formation of estrogen in the testicles and surrounding tissue [30]. In this work, we were able to evaluate only serum concentrations of total testosterone, and it should be noted that, while on the one hand the average serum level of total testosterone stayed within the normal limits, on the other there was an increase in the average serum concentrations of gonadotrophins. This increase in gonadotrophins suggests a decline in the gonadal function of these individuals. Following this same reasoning, the negative correlation found between FSH serum level and BMD at the trochanter and total femur in men, as in women, shows the influence that gonadal dysfunction has on bone loss in these individuals.
In the multiple linear-regression analysis, body weight was the most important independent predictor of bone mass at almost all the sites studied in both sexes, thereby explaining the 19–27% variation in BMD. This result is consistent with the study done by Edelstein and colleagues [41], who also found body weight to be the best marker of bone mass in 1,492 elderly residents of the community. In the Edelstein and colleagues’ study, body weight explained from 3.3% to 16.5% of the variation in BMD, depending on the bone site studied [41]. Height was the most important independent predictor of BMD at the lumbar spine in men, thereby explaining 10% of the BMD variation at this site. Height is related to skeleton size; that is, skeleton size is greater in tall individuals and can, therefore, become a factor when analyzing the bone densitometry scan (DXA) when BMD is measured only in two dimensions. Thus, the effect of skeleton size might have been more significant than the effect of body weight on bone mass at the lumbar spine in this male population [42]. Body fat was the most important predictor of BMD at the total femur in women, thereby explaining the 19% variation in BMD at this site. So, when placed in the multiple model, the percentage of body fat appears only as a predictor of bone mass in women and no longer in men. The stronger relation between BMD and fat mass in women as compared with men has also been reported by other authors [41,42].
In conclusion, our findings show that body weight was the best predictor of BMD in this population, principally at the proximal femur. We also noticed a high prevalence of osteoporosis and osteopenia in this population of elderly residents of the community in the largest city in Brazil. The prevalence of osteoporosis was greater in women than in men in the majority of sites analyzed. However, at the femoral neck, osteoporosis and osteopenia were as prevalent in men as in women, showing that, with the increase in life expectancy, osteoporosis also becomes relevant in men.
References
Kanis JA, Glüer CC (2000) An update on the diagnosis and assessment of osteoporosis with densitometry. Osteoporos Int 11:192–202
Ross PD (1996) Osteoporosis: frequency, consequences, and risk factors. Arch Intern Med 156:1399–1411
Eastell R (1999) Bone density can be used to assess fracture risk [comment]. BMJ 318:864–865
Hawker GA (1996) The epidemiology of osteoporosis. J Rheumatol 23 [Suppl 45]: 2–5
Ramos LR, Toniolo J, Cendoroglo MS et al (1998) Two-year follow-up study of elderly residents in S. Paulo, Brazil: methodology and preliminary results. Rev Saude Publica 32 (5): 397–407
Wark JD (1999) Osteoporosis: a global perspective. Bull World Health Organ 77(5): 424–426
Viana PRS (1999) Avaliação da densidade mineral óssea e da ocorrência de fratura vertebral em pacientes com lupus eritematoso sistêmico [master thesis]. Federal University of São Paulo, São Paulo, Brazil, p 18
Kelly TL (1990) Bone mineral density reference databases for American men and women. J Bone Miner Res 5 [Suppl 2]:S249
Looker AC, Wahner HW, Dunn WL et al (1998) Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8:468–489
Tenenhouse A, Joseph L, Kreiger N et al (2000) Estimation of the prevalence of low bone density in Canadian women and men using a population-specific DXA reference standard: the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos Int 11:897–904
VanItallie TB, Mei-Uih Y, Heymsfield SB et al (1990) Height-normalized indices of body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr 52:953–959
Vieira JGH, Lombardi MT, Nishida SK (1990) Monoclonal antibody-based immunoenzymometric assay for serum human growth hormone. Braz J Med Biol Res 23:293–296
Vieira JGH, Nishida SK, Lombardi MT et al (1990) Desenvolvimento de ensaio imunoenzimométrico em anticorpos monoclonais para a dosagem de hormônio luteotrófico (LH) no soro. Rev Bras Pat Clin 26(3):84–87
Vieira JGH, Furlanetto RP, Russo EMK et al (1980) Radioimunoensaio do sulfato de deidroepiandrosterona: metodologia e padronização de valores normais. Arq Bras Endocrinol Metabol 24(2):79–83
Vieira JGH, Kunii IS, Nishida SK et al (1992) Desenvolvimento e aplicação de um método imunofluorométrico para a dosagem de tirotrofina humana (TSH) no soro e em sangue total colhido em papel de filtro. Arq Bras Endocrinol Metabol 36(1):7–12
Vieira JGH, Nishida SK, Lombardi MT et al (1994) Emprego de anticorpos monoclonais no desenvolvimento de um ensaio imunofluorométrico de alta sensibilidade para a dosagem de FSH. Estudo dos níveis séricos em meninos normais nos diferentes estádios puberais. Arq Bras Endocrinol Metabol 38(2):76–81
Wheeler MJ, D’Souza A, Matadeen J et al (1996) Ciba Corning ACS: 189 testosterone assay evaluated. Clin Chem 42:1445–1449
Looker AC, Orwoll ES, Johnston CC et al (1997) Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res 12(11):1761–1768
IBGE: Instituto Brasileiro de Geografia e Estatística (2000) Censo demográfico 2000. Available from: http://www.ibge.gov.br/ibgeteen/noticias/brasileirosvivemmais.html
Ramos LR, Rosa TEC, Oliveira ZM, Medina MCG (1993) Perfil do idoso em área metropolitana na região sudeste do Brasil: resultados de inquérito domiciliar. Rev Saude Publica 27(2):87–94
Szejnfeld VL, Atra E, Baracat EC et al (1995) Bone density in white Brazilian women: rapid loss at the time around the menopause. Calcif Tissue Int 56(3):186–191
Castro da Rocha FA, Ribeiro AR (2003) Low incidence of hip fractures in an equatorial area. Osteoporos Int 14(6):496–499
Orwoll ES, Klein R (1995) Osteoporosis in men. Endocr Rev 16(1):87–116
Yang S, Taxel P (2002) Osteoporosis in older men: an emerging clinical problem. Clin Geriatrics 10(8):28–37
Zerbini CAF, Latorre MRO, Jaime PC et al (2000) Bone mineral density in Brazilian men 50 years and older. Braz J Med Biol Res 33(12):1429–1435
Blunt BA, Klauber MR, Barret-Connor EL et al (1994) Sex differences in bone mineral density in 1,653 men and women in the sixth through tenth decades of life: the Rancho Bernardo study. J Bone Miner Res 9(9):1333–1338
Orwoll ES, Oviatt SK, Mann T (1990) The impact of osteophytic and vascular calcifications on vertebral mineral density measurements in men. J Clin Endocrinol Metab 70(4):1202–1207
Jones G, White C, Nguyen T, Sambrook PN, Kelly PJ, Eisman JA (1996) Prevalent vertebral deformities: relationship to bone mineral density and spinal osteophytosis in elderly men and women. Osteoporos Int 6(3):233–239
Hannan MT, Felson DT, Dawson-Hughes B et al (2000) Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 15(4):710–720
Khosla S, Melton LJ III, Riggs BL (2002) Estrogen and the male skeleton. J Clin Endocrinol Metab 87(4):1443–1450
Hui SL, Perkins AJ, Zhou L et al (2002) Bone loss at the femoral neck in premenopausal white women: effects of weight change and sex-hormone levels. J Clin Endocrinol Metab 87(4):1539–1543
Riggs BL, Khosla S, Melton LJ III (2002) Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23(3):279–302
Harman SM, Metter EJ, Tobin JD et al (2001) Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Encocrinol Metab 86(2):724–731
Seeman E (1999) Osteoporosis in men. Osteoporos Int [Suppl 2]:S97–S110
Greendale GA, Edelstein S, Barrett-Connor E (1997) Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res 12(11):1833–1843
Rudman D, Drinka PJ, Wilson CR et al (1994) Relations of endogenous anabolic hormones and physical activity to bone mineral density and lean body mass in elderly men. Clin Endocrinol (Oxf) 40(5):653–661
Center JR, Nguyen TV, Sambrook PN, Eisman JA (1999) Hormonal and biochemical parameters in the determination of osteoporosis in elderly men. J Clin Endocrinol Metab 84(10):3626–3635
Martínez Díaz-Guerra G, Hawkins F, Rapado A, Ruiz Díaz MA, Díaz-Curiel M (2001) Hormonal and anthropometric predictors of bone mass in healthy elderly men: major effect of sex hormone binding globulin, parathyroid hormone and body weight. Osteoporos Int 12:178–184
Smerdely P, Seller M, Smith A et al (2000) Predictors of bone mass in healthy older men in the community. Med J Aust 173:183–186
Khosla S, Melton LJ III, Atkinson EJ et al (1998) Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83(7):2266–2274
Edelstein SL, Barrett-Connor E (1993) Relation between body size and bone mineral density in elderly men and women. Am J Epidemiol 138(3):160–169
Reid IR (2002) Relationships among body mass, its components, and bone. Bone 31 (5): 547–555
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues Camargo, M.B., Cendoroglo, M.S., Ramos, L.R. et al. Bone mineral density and osteoporosis among a predominantly Caucasian elderly population in the city of São Paulo, Brazil. Osteoporos Int 16, 1451–1460 (2005). https://doi.org/10.1007/s00198-004-1831-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-004-1831-y