Abstract
The diagnosis of osteoporosis is generally based on the assessment of bone mineral content with dual X-ray absorptiometry (DXA) but does not account for the spatial distribution and inherent material properties of the tissue. Peripheral quantitative computed tomography (pQCT) permits one to measure the compartment-specific density and geometry-based parameters of cortical bone. Quantitative ultrasound (QUS) parameters are associated with material properties of cortical bone. The purpose of this study was to test the hypothesis that pQCT and cortical QUS provide additional information to DXA in predicting structural strength of the distal radius. The intact right arm and the isolated left radius were harvested from 70 formalin-fixed cadavers (age 79±11 years). The bone mineral content (BMC) was assessed with DXA at the radial metaphysis and shaft. pQCT was also used at the metaphysis and the shaft, while QUS was employed only at the shaft. The failure loads of the radius were assessed by use of a 3-point bending test (isolated radius) and a complex fall simulation (intact arm). The BMC (DXA) displayed a correlation of r=0.96 with the failure moments in 3-point bending (P<0.001). The correlation between failure load and geometry-based parameters (pQCT) ranged from r=0.85 to r=0.96 and was r=0.64 for the speed of sound (QUS) (P <0.001). Cortical thickness (pQCT) improved the prediction marginally (r=0.964) in combination with DXA. For the fall simulation, the correlation coefficients were r=0.76 for BMC (DXA) of the shaft, r=0.83 for metaphyseal bone content (pQCT), r=0.55 for QUS, and ranged from r=0.59 to r=0.74 for geometry-based parameters at the shaft (pQCT). pQCT and QUS parameters provided no significant improvement versus DXA alone. Measurement of bone mass by DXA or pQCT thus appears to be sufficient as a surrogate of mechanical strength and fracture risk of the distal radius.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mechanical competence of bone as a structure (structural strength) is determined by the amount of material (bone mass), by its material properties, and by way the material is distributed in space in relation to the loading direction [1]. Currently, the clinical diagnosis of osteoporosis is based on the measurement of areal bone mineral density (BMD) alone using dual energy X-ray absorptiometry (DXA). This approach, however, does not take into account the spatial distribution and material properties of the tissue.
Recently, alternative diagnostic techniques have been developed, such as peripheral quantitative computed tomography (pQCT) [2, 3, 4, 5, 6] and quantitative ultrasound (QUS) [7, 8, 9, 10, 11, 12, 13, 14, 15]. These show potential for measuring the spatial distribution and material properties non-invasively in patients.
Specifically, pQCT permits the determination of geometry-based parameters of cortical bone in the peripheral skeleton that have been derived from engineering principles [1]. At metaphyseal sites, pQCT permits one to determine trabecular and subcortical volumetric bone density, whereas DXA provides only integral values for these compartments. QUS has been applied for some time in material research and has been shown to be associated with several aspects of bone tissue properties, including the trabecular micro-architecture and the material properties (Young’s modulus) of trabecular [14] and cortical [10] bone, cortical density [12, 13], cortical thickness [7, 12], and the configuration (i.e. orientation and number) of the Haversian channels in cortical bone [7]. Clinically, QUS has predominantly been applied to the calcaneus, but recently, new devices have been developed that permit the measurement of several skeletal sites throughout the body, including the distal radius [5, 9]. Although, recently, a large prospective clinical study has demonstrated that peripheral measurements of bone status are relevant predictors of fractures at these and other sites [16], it is currently unclear whether, in osteoporosis, measurement of cortical geometry and quantitative, cortical speed of sound (SOS) can significantly improve the prediction of structural bone strength versus measurement of bone mineral content alone. Because distal radius fractures occur earlier in life and represent a predictive sign for future fractures in the hip and spine [17], the current study focuses on the distal radius.
Specifically, we tested the hypothesis that pQCT and cortical QUS provide additional information to DXA in predicting bone strength of the distal radius. The more fundamental question was whether, in osteoporosis, the material properties and distribution of bone mass do or do not obey similar optimisation processes to mechanical function as observed in normal bone [18]. In other words, is it worthwhile for one to measure these parameters in addition to bone mass, in order to effectively improve the prediction of mechanical strength (as a surrogate of fracture risk)? To address these questions we related experimentally determined failure loads (an objective measure of bone strength) to quantitative measures of spatial distribution of cortical bone (geometry-based parameters), to compartment-specific values for trabecular and subcortical bone (pQCT), and to QUS parameters of the cortical shaft. One mechanical test was designed to apply controlled mechanical conditions to a specific site of the shaft (3-point bending test). The second mechanical test (contralateral side) was designed to simulate a fall on the outstretched hand and to create a fracture loco typico of the distal radial metaphysis, as seen in the clinical conditions of a Colles fracture.
Material and methods
Specimens
The left radius, right upper extremity and left pelvic bone were obtained from 74 formalin-fixed cadavers from two consecutive courses of macroscopic dissection. Prior to their death, when dedicating their bodies to the Institute of Anatomy the donors had given permission for these specimens to be used for scientific purposes. From the iliac crest, bone biopsies were taken at the site of clinical trans-iliac biopsies and prepared for routine histomorphometric assessment (embedding in methylmethacrylate, preparation of 5-µm sections, staining with Goldner’s, toluidine blue, and von Kossa’s) to exclude bone diseases other than osteopenia and osteoporosis. Four specimens with signs of malignancy were discarded from the study. Eventually, 70 subjects (age 79±10 years, range 52–100 years) were examined, 40 women (aged 81±10 years) and 30 men (aged 78±11 years). We cleaned the isolated radii from surrounding soft tissues, leaving the periosteum intact, and detached the right arms at the distal humerus, preserving the soft tissues (except for the skin and subcutaneus fat tissue), the elbow joint, the interosseous membrane, the wrist joint, and the hand. The bones were radiographed in anterior–posterior and lateral views, but none of the specimens contained osteosynthetic material or displayed signs of previous fracture. We degassed the isolated (left) radii in a vacuum chamber and sealed them under fluid in tight plastic bags, to avoid the formation of artefacts from entrapped air during densitometry and to facilitate coupling between the QUS transducer and the bone during the QUS measurements.
Bone densitometry
The bone mass [bone mineral content (BMC)] of the isolated left radius and right forearm was measured with a peripheral DXA scanner (pDXA; Norland/Stratec, Pforzheim, Germany), with one region of interest (ROI) at the distal metaphysis (1-cm length close to the wrist joint) and one ROI at the shaft (1-cm length at approximately 33% of bone length; Fig. 1). Based on the WHO definitions (technical report series 843, 1994) and the measurement at the metaphysis, 27 subjects showed normal results (T score >−1.0; 39%, ten women and 17 men), 29 subjects were osteopenic (T score −1.0 to −2.5; 41%, 19 women and ten men), and 14 subjects were osteoporotic (T score <−2.5; 20%, 11 women and three men). In 39 subjects, both the isolated radius and the forearm were measured twice on different days, with repositioning and recalibration of the system. The root mean square (r.m.s.) average coefficients of variation (CV%) for repeated DXA measurements are shown in Table 1.
Positioning of the ROIs in the DXA acquisitions. a Acquisition at the intact right arm. b Acquisition at the isolated radius. The ROI had a length of 1 cm in both cases. The metaphyseal/distal ROI was positioned close to the wrist joint space and the shaft/proximal ROI was positioned at 33% of the radius length (wrist joint space to radial head)
We applied pQCT (XCT 2000; Norland/Stratec, Pforzheim, Germany) to acquire cross-sectional images of the radius (both the isolated left radius and the intact right arm) at 5% bone length (wrist joint space to radial head) and at 33% bone length. These images were analysed with the software provided by the manufacturer, the precision in our hands [3] having been shown to be equivalent to other in vivo and in vitro studies [2]. Specifically, we determined the total bone content (g), total density (g/cm3) and total cross sectional area (cm2), and the trabecular and subcortical content/density/area at the metaphyseal site. At the shaft, we derived the total bone content, total density and total cross sectional area, the relative (% of total) cortical cross sectional area, the cortical thickness, and geometry-based parameters. The latter included the moment of inertia (mm4), the section modulus (moment of resistance in mm3), and the density-weighted section modulus (stress strain index, SSI, in mm3) [4, 6]. For these parameters, we determined the polar component as well as the X and Y components of the two-dimensional tensor [6]. The X component was aligned parallel and the Y component perpendicular to the loading direction of the 3-point bending test (see below).
The cortical speed of sound (SOS; longitudinal transmission) was measured with an Omnisense 7000 scanner (Sunlight Medical, Europe; Ravensburg, Germany) at 33% radius length (Fig. 2). QUS measurements were obtained only at the left isolated radius, not in the right forearm, because the fixed soft tissues and potential inclusions of small gas bubbles in the soft tissues of the cadavers prevented acquisition of appropriate ultrasound signals. The left radius was degassed and kept in fluid during the measurements so that optimal coupling conditions could be achieved between the transducer and the bone (Fig. 2). We tested the precision of this in vitro technique by obtaining three measurements in 26 bones on different days, the r.m.s. average (CV%) being 1.0% (inter-subject CV%=5.5) and the r.m.s. average SD being 43 m/ (inter-subject SD=224 m/s) for the SOS.
Mechanical testing
A uni-axial testing machine (Zwick 1445; Ulm, Germany) was used for the assessment of the maximum failure loads in both the isolated (left) radius and the intact (right) arm. The isolated radius was tested in a 3-point bending configuration at a loading rate of 5 mm/min. The load was applied at 33% length of the radius (the site where the cortical DXA, pQCT and QUS measurements were obtained), the supports being located at 16.5% and 49.5% bone length (Fig. 3). The clamping length was thus not kept constant but was adapted to each individual bone size, so that we could test identical anatomical regions. To prevent rotation of the bone during testing, a polyethylene wedge (Pattex hot sticks, Henkel, Düsseldorf, Germany) was made for each radius with a glue pistol (Fig. 3). To be able to compare the structural strength amongst the bones (despite different clamping length) we computed the maximum bending moments from the maximum failure loads and the individual lever arms (16.5% radius length).
The intact right arms were tested in a fall simulation at a loading rate of 100 mm/min, with the load being applied through the flexed elbow joint, and the hand being positioned on a wedge (Fig. 4). Note that the elbow joint, the interosseous membrane, the wrist joint, and the hand were left intact, so that normal load transfer through the distal radius during a fall could be simulated. The elbow was allowed to rotate within the loading device during load application, and the hand was fixed at 80° pronation and 70° dorsal flexion [4, 6]. The maximum failure load was defined as the peak of the force displacement curve, followed by a drop in registered load of >30%, and was used as a measure of structural bone strength.
After the test, the forearms were radiographed in two planes in anterior–posterior and lateral views, and fractures were classified according to the Frykman and AO classifications [19], which grade fractures based on the number of bone fragments and affection of the joint space. Only fractures at the distal radius (loco typico) were considered for further analysis, with 19 (five female, 14 male) out of 70 specimens being excluded because fracture occurred at another site.
Statistical analysis
The maximum moments of the 3-point bending test and the maximum failure loads derived from the fall simulation were correlated with densitometric variables and use of the Pearson correlation coefficient. Differences between groups were assessed with Student’s unpaired t-test. To answer whether pQCT and QUS provide significant additional information to DXA in predicting structural bone strength, we performed stepwise multiple regression analyses (forward mode), with maximum failure load (moment) as dependent variable and densitometric, geometric and QUS parameters as independent variables. For the 3-point bending test, the model included DXA at the shaft (forced), SOS (QUS) and the following pQCT variables: total cross-sectional area, absolute and relative (%) cortical area, cortical thickness, second moment of inertia, section modulus, and SSI in the direction of load application (X component of the tensors). For the fall simulation, a first model included densitometric variables from the ipsilateral arm (where QUS could not be measured), specifically DXA at the shaft (forced) and the following pQCT parameters: total cross-sectional area at the metaphysis and at the shaft, subcortical density and trabecular density at the metaphysis, absolute and relative cortical area at the shaft, and cortical thickness. From the geometry-based parameters, we selected the X components of the second moment of inertia, of the section modulus and of the SSI, as these displayed higher correlation coefficients than the polar and Y components of the tensors (see Results). The second model was derived from densitometric variables of the contralateral arm and included DXA of the shaft (forced), SOS (QUS) and the pQCT parameters listed above.
Results
The descriptive statistics of the mechanical data are shown in Table 2. Women displayed significantly lower failure moments of the radius (3-point bending −53%; P<0.001) and failure loads of the arm (fall simulation −41%; P<0.001) than men. Subjects that were classified as osteoporotic by DXA displayed substantially lower failure loads than normal specimens (Table 2).
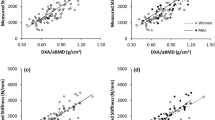
The BMC, as measured with DXA at the shaft (33%), displayed a correlation of r=0.96, with the failure moments of the radius in 3-point bending. Correlation coefficients for pQCT parameters were similar, with r=0.97 for cortical content, r=0.96 for cortical area, r=0.93 for cortical thickness, and values of r=0.85 (second moment of inertia perpendicular to the load direction) to r=0.91 (SSI in the loading direction) for geometry-based parameters. Values were similar for measurements at 33% of the contralateral arm (r=0.95 for BMC [DXA] and r=0.95 for cortical content [pQCT]), but were significantly lower (P<0.01; Fisher’s Z-transformation) when being measured at the distal metaphysis of either the ipsilateral or contralateral arm (data not shown). The SOS (QUS) displayed a significant correlation with the failure moment (r=0.64; P<0.001), but the correlation coefficient was significantly lower (P<0.01) than coefficients from DXA and pQCT. The SOS (QUS) did not provide significant additional information for the prediction of the structural strength of the distal radius in 3-point bending in combination with DXA (BMC) in a stepwise multiple regression model, while pQCT provided very marginal additional information with cortical thickness (multiple r=0.964).
The failure loads of the fall simulation displayed a correlation of r=0.73 with the BMC (DXA) of the metaphysis and of r=0.76 with the BMC of the shaft at the ipsilateral forearm (Table 3). The correlation with total bone content at the radial metaphysis was r=0.83 (pQCT), and that with the content at the shaft r=0.78. Specific values for the subcortical and trabecular compartment and geometry-based parameters did not show higher correlations than those for the total bone content in pQCT (Table 3).
Note that the SOS (QUS) could be measured only at the contralateral side (isolated radius), for methodological reasons (see above), and its correlation with the fall simulation should, therefore, be compared with DXA and pQCT at the contralateral radius. The correlation of DXA (BMC) and pQCT (total content) at the contralateral side with the fall simulation was somewhat lower than that of the ipsilateral side, the differences being higher for the metaphyseal than for the shaft location (Table 3). The SOS displayed a significant correlation (r=0.55, P<0.001) with the failure loads at the contralateral arm (fall simulation), but the coefficient was lower (although not significantly lower) than that obtained by DXA of this side.
In a multiple regression model from densitometric parameters measured at the ipsilateral arm, pQCT did not contribute independently to the prediction of failure loads in the fall simulation. The model included only the BMC (DXA, forced) and none of the independent variables offered. When we ran the model with densitometric variables from the contralateral radius (including QUS), pQCT and QUS, also, did not contribute independently to the prediction in the fall simulation.
Discussion
The objective of this study was to test the hypothesis that compartment-specific content and geometry-based parameters from pQCT, and cortical QUS, provide additional information to DXA in predicting bone strength of the distal radius. The more fundamental question was, whether—in osteoporosis—measurements of surrogates for site-specific material properties and bone geometry can improve the prediction of mechanical strength over that by bone mineral content (bone mass) alone.
We chose an experimental (biomechanical) study design because bone strength cannot be objectively determined in vivo. A strength of the present study is that it included a relatively large sample size and that —in the intact forearm—DXA and pQCT were acquired in situ, including soft tissue artefacts as they occur under clinical conditions [20]. Another strength is that the radius was tested both under very controlled mechanical conditions (3-point bending) and in a clinically realistic configuration (fall simulation with preservation of an intact elbow joint, interosseous membrane, wrist joint and hand), and that the pQCT measurements in the radius were aligned with the direction of load application in the 3-point bending test. A very recent study by Muller et al. also examined the relationship of radial DXA, pQCT and QUS with radial failure loads, with a more modest sample size (n=21) [21]. However, the isolated radius was examined in a much more controlled fashion than in our fall configuration, without intact wrist, elbow and soft tissues. Moreover, our 3-point bending test, performed in the contralateral arm, assessed bone strength precisely at the point where densitometric measurements were made.
Limitations of the current study include the lack of detailed medical history. However, in contrast to specimens from pathological dissection, the sample did not include a pre-selection of highly pathological cases because the subjects had dedicated their bodies to the Institute of Anatomy many years prior to death. By obtaining radiographs of the bones before densitometry and mechanical testing, and by performing a histological analysis of the iliac crest, we were able to apply exclusion criteria similar to those of clinical studies. Another potential limitation is the use of fixed specimens. Edmondston et al. [22], however, reported that mechanical strength of entire bones was only minimally affected and that the correlation between bone mineral status and mechanical strength, as investigated in this current study, was unchanged. We have shown previously that prolonged (10-month) formalin fixation had no significant effect on DXA [20]. In this context it is also important to note that the correlation between DXA and failure loads were in the range of those reported in previous experimental studies in fresh cadavers [6, 15]. When testing the isolated radius Muller et al. [21] found a slightly higher correlation between DXA and failure load than we did in the fall simulation of the total forearm, but a lower one in the 3-point bending test of the radius. QUS values (particularly SOS) of trabecular specimens have been shown to change during the course of 18 months’ fixation with the same solution, as used in this study, but the values maintained a linear relationship (high correlation) with values before embalmment [11]. In this context, it is also important that bone densitometry (DXA, pQCT and QUS) was shown to be as reproducible in the specimens as under clinical conditions in vivo. Therefore we believe that the results of this study should not be critically affected by fixation. However, cortical bone is a micro-porous elastic solid with a very different SOS from the highly porous trabecular bone, and the effect of fixation on cortical SOS has, so far, not been formally tested in an experimental study. Therefore, we cannot exclude that the fixation might have had a non-linear effect on cortical SOS, and this represents a limitation of this study. The correlation between DXA and bone failure loads in a fall simulation in our study was similar to those reported by other experimental investigations [6, 15, 21]. We found that pQCT permitted only marginal improvement of strength prediction for either the 3-point bending test or fall configuration versus DXA. In particular, compartment-specific and geometry-based parameters were not better predictors than bone mass from DXA or pQCT. This also applied for very controlled mechanical conditions (3-point bending), in which pQCT derivates of geometry-based parameters were precisely aligned with the loading direction. This finding is in principle agreement with a clinical study [5], in which pQCT did not show better discrimination between subjects with and without distal radius fractures than DXA, but is in slight contrast with the experimental finding of Muller et al. [21], who reported a significant improvement of failure load prediction by geometric variables (specifically SSI). This is surprising, because they assessed only the polar moment of the SSI, whereas we also assessed the tensor components precisely in the direction of load application. The very high correlation between bone mass (DXA or pQCT) and mechanical failure, and the inability of geometry-based parameters to improve the prediction of failure loads, might be explained by the fact that—also at states of bone loss (osteoporosis)—the spatial distribution of bone mass is subject to an optimisation process. This process might warrant that the existing material is used in a mechanically useful (or even optimal) way. This “optimisation” process of bone to mechanical usage has been historically addressed as Wolff’s law [18]. For these reasons, measurement of bone mass alone (by either DXA or pQCT) might be sufficient as a surrogate of mechanical strength of fracture risk, despite the theoretical advantages in measuring bone geometry. It should be emphasised that the findings here apply only to the distal radius and that results might be different at other skeletal sites.
With regard to the predictive ability of QUS, one previous experimental study has addressed the correlation of phalangeal ultrasound with failure of the distal radius [15], and another one, that of calcaneal ultrasound [4]. Whereas calcaneal ultrasound displayed a significantly lower correlation with radial failure loads than did site-specific measurements [4], the study by Wu et al. [15] found phalangeal ultrasound to display similar correlations with radial failure loads as radial DXA and pQCT. However, the latter study involved only 13 specimens, and the confidence levels of the correlation coefficients were therefore relatively large.
Radial QUS measurements, as performed in this current study, differ principally from those at the phalanges and at the calcaneus, as they do not employ transverse transmission through the object of interest but use longitudinal transmission along the cortical bone. With these measurements, the ultrasound waves partially run along the cortical shell, and the transmission time is registered by the detector. The SOS has been found to be dependent on the cortical thickness [7, 12], the cortical density [12, 13], and the configuration (i.e. orientation and number) of the Haversian channels of the cortical bone [7]. In this context it is of interest that the SOS displayed a similar correlation with bone failure loads as cortical density. Recent studies have suggested that QUS measurements at the distal radius can discriminate as effectively between subjects with and without fractures as DXA of the distal radius [7, 8, 9]. However, to date, only one experimental study has related radial QUS to mechanical strength of the distal radius [21]. This study reported a similar correlation coefficient between the SOS and failure load of the isolated radius but did not report whether SOS was able to contribute independent information in a stepwise multiple regression model. We find that SOS of the radial cortex displays a significant correlation with mechanical strength, both in a 3-point bending test and in a fall configuration, but that the correlations are significantly lower than those for site-specific DXA and pQCT (P<0.01). QUS did not contribute additional information to these methods for the 3-point bending test or for the fall simulation. As a clinical consequence, it may be useful for one to apply radial QUS to estimate fracture risk of the distal radius if no other densitometric method is available. However, if radiographic methods, such as DXA or pQCT, can be employed, these appear to permit significantly better prediction of the mechanical strength of the distal radius. Whether the material properties of the cortical bone are not sufficiently different amongst individuals to improve the prediction of mechanical failure in conjunction with bone mass (DXA), or whether QUS is currently unable to capture relevant differences in material properties of cortical bone, remains an open question and will have to be addressed in further studies.
In conclusion, this paper suggests that material-based parameters of cortical bone (QUS) and geometry-based parameters of cortical bone (pQCT) do not significantly improve the prediction of mechanical strength of the radius versus measurement of bone mass (by either DXA or pQCT) in either 3-point bending or a fall configuration. Geometry-based parameters of cortical bone (pQCT) only very marginally improve the prediction of failure loads in combination with DXA. We hypothesise that, also in osteoporosis, the spatial distribution of bone mass is subject to an optimisation process, which warrants that the existing material is used in a mechanically useful way (Wolff’s law). Therefore, measurement of bone mass appears to be sufficient as a surrogate of mechanical strength and fracture risk of the distal radius.
References
Hayes WC, Bouxsein ML (1997) Biomechanics of cortical and trabecular bone: implications for assessment of fracture risk. In: Mow VC, Hayes WC (eds) Basic orthopaedic biomechanics, 2nd edn. Lippincott-Raven, Philadelphia, pp 69–111
Guglielmi G, Schneider P, Lang TF, et al (1997) Quantitative computed tomography at the axial and peripheral skeleton. Eur Radiol 7:32–42
Groll O, Lochmuller EM, Bachmeier M, et al (1999) Precision and intersite correlation of bone densitometry at the radius, tibia and femur with peripheral quantitative CT. Skeletal Radiol 28:696–702
Lochmuller EM, Lill CA, Kuhn V, et al (2002) Radius bone strength in bending, compression, and falling and its correlation with clinical densitometry at multiple sites. J Bone Miner Res 17:1629–1638
Formica CA, Nieves JW, Cosman F, et al (1998) Comparative assessment of bone mineral measurements using dual X-ray absorptiometry and peripheral quantitative computed tomography. Osteoporos Int 8:460–467
Augat P, Iida H, Jiang Y, et al (1998) Distal radius fractures: mechanisms of injury and strength prediction by bone mineral assessment. J Orthop Res 16:629–635
Barkmann R, Kantorovich E, Singal C, et al (2000) A new method for quantitative ultrasound measurements at multiple skeletal sites: first results of precision and fracture discrimination. J Clin Densitom 3:1–7
Gnudi S, Ripamonti C, Malavolta N (2000) Quantitative ultrasound and bone densitometry to evaluate the risk of nonspine fractures: a prospective study. Osteoporos Int 11:518–523
Hans D, Srivastav SK, Singal C, et al (1999) Does combining the results from multiple bone sites measured by a new quantitative ultrasound device improve discrimination of hip fracture? J Bone Miner Res 14:644–651
Pithioux M, Lasaygues P, Chabrand P (2002) An alternative ultrasonic method for measuring the elastic properties of cortical bone. J Biomech 35:961–968
Pöpperl G, Lochmüller EM, Becker H, et al (1999) Determination of calcaneal ultrasound properties ex situ: reproducibility, effects of storage, formalin fixation, maceration, and changes in anatomic measurement site. Calcif Tissue Int 65:192–197
Prevrhal S, Fuerst T, Fan B, et al (2001) Quantitative ultrasound of the tibia depends on both cortical density and thickness. Osteoporos Int 12:28–34
Sievanen H, Cheng S, Ollikainen S, et al (2001) Ultrasound velocity and cortical bone characteristics in vivo. Osteoporos Int 12:399–405
van den Bergh JP, van Lenthe GH, Hermus AR, et al (2000) Speed of sound reflects Young’s modulus as assessed by microstructural finite element analysis. Bone 26:519–524
Wu C, Hans D, He Y, et al (2000) Prediction of bone strength of distal forearm using radius bone mineral density and phalangeal speed of sound. Bone 26:529–533
Miller PD, Siris ES, Barrett-Connor E, et al (2002) Prediction of fracture risk in postmenopausal white women with peripheral bone densitometry: evidence from the National Osteoporosis Risk Assessment. J Bone Miner Res 17:2222–2230
Cuddihy MT, Gabriel SE, Crowson CS, et al (1999) Forearm fractures as predictors of subsequent osteoporotic fractures. Osteoporos Int 9:469–475
Huiskes R (2000) If bone is the answer, then what is the question? J Anat 197:145–156
Lill CA, Goldhahn J, Albrecht A, et al (2003) Impact of bone density on distal radius fracture patterns and comparison between five different fracture classifications. J Orthop Trauma 17:271–278
Lochmüller EM, Krefting N, Bürklein D, et al (2001) Effect of fixation, soft-tissues, and scan projection on bone mineral measurements with dual energy X-ray absorptiometry (DXA). Calcif Tissue Int 68:140–145
Muller ME, Webber CE, Bouxsein ML (2003) Predicting the failure load of the distal radius. Osteoporos Int 14:345–352
Edmondston SJ, Singer KP, Day RE, et al (1994) Formalin fixation effects on vertebral bone density and failure mechanics: an in vitro study of human and sheep vertebrae. Clin Biomech 9:175–179
Acknowledgements
We would like to thank Stratec Medizintechnik (Pforzheim, Germany) for providing the XCT 2000 pQCT scanner and Sunlight Medical GmbH, Europe (Ravensburg, Germany) for providing the Omnisense ultrasound system (Omnisense 7000). Gudrun Goldmann is to be thanked for her help with the ultrasound and DXA measurements and Dr. Stephan Metz for his help with obtaining the radiographs of the forearms after mechanical testing.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Hudelmaier and V. Kuhn contributed equally to this study
Rights and permissions
About this article
Cite this article
Hudelmaier, M., Kuhn, V., Lochmüller, E.M. et al. Can geometry-based parameters from pQCT and material parameters from quantitative ultrasound (QUS) improve the prediction of radial bone strength over that by bone mass (DXA)?. Osteoporos Int 15, 375–381 (2004). https://doi.org/10.1007/s00198-003-1551-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-003-1551-8