Abstract
Introduction and hypothesis
Data examining the effect of diabetes mellitus (DM) on prolapse recurrence after sacrocolpopexy (SCP) is limited. The primary objective of this study was to determine if DM affects prolapse recurrence after robotic SCP.
Methods
This was a retrospective cohort study of women who underwent robotic SCP between 2012 and 2019 at Kaiser Permanente Southern California. The cohort was divided into women with and without DM at the time of SCP. The primary outcome was composite failure. Secondary outcomes included recurrent compartment-specific prolapse, reoperation rates, and surgical complications.
Results
Of 547 patients included, 100 had DM. Women with DM were older, had higher BMI, higher parity, and were more likely to be nonwhite. Women with DM had more advanced prolapse at baseline but were not more likely to undergo concomitant procedures at the time of SCP. Over a median follow-up of 2.1 years (IQR 1.3, 3.4), women with DM had significantly increased risk of anterior vaginal prolapse (AVP) recurrence (13% vs 3%, p<0.01), but not composite failure (21% vs 14%, p=0.14). On multivariate regression, women with DM were almost 4 times as likely to experience AVP recurrence over time (AVP hazard ratio (HR) 3.93, 95% CI 1.29–12.03, p=0.02).
Conclusion
In our cohort, DM was a risk factor for AVP recurrence but not composite failure after robotic SCP.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
In the USA, an estimated 37.3 million people (11.3%) suffer from diabetes mellitus [1]. Diabetes is a known risk factor for pelvic organ prolapse [2,3,4,5], for which approximately 300,000 women undergo surgery in the USA each year [6].
In the past 20 years, minimally invasive sacrocolpopexy (SCP) performed using lightweight mesh has become the gold standard for advanced pelvic organ prolapse; however, diabetes has been shown to almost double the risk of mesh exposure (OR 1.87, 95% CI 1.35–2.57) [7]. It is thought that this is in part due to an increased inflammatory response to mesh in women with diabetes, which has been confirmed in murine models [8].
It is uncertain, however, if women with diabetes are also at increased risk for prolapse recurrence after SCP. Previous studies investigating risk factors for prolapse recurrence after SCP have been in predominantly white populations, where the rate of diabetes is significantly lower than that of the general population [9,10,11]. In reality, diabetes disproportionately affects racial/ethnic minority populations, who also suffer from some of the highest rates of pelvic organ prolapse [1, 4]. Thus, it is important to understand the impact of diabetes on prolapse recurrence to better care for patient populations with a higher prevalence of the disease.
The primary objective of our study was to determine if rates of prolapse recurrence after minimally invasive SCP are higher in women with diabetes. Secondary objectives included rates of recurrent compartment-specific prolapse, reoperation rates, and surgical complications in women with and without diabetes.
Materials and methods
This study, approved by the Southern California Kaiser Permanente Institutional Review Board, was a retrospective cohort analysis of women who underwent robotic SCP between 1 January 2012 and 30 August 2019 at Kaiser Permanente Southern California, with a total of eight different urogynecologists. Surgical case lists were reviewed to identify all female patients who had undergone robotic SCP during the specified time period. For all patients, SCP was performed using type 1 lightweight polypropylene mesh, delayed absorbable suture attachment to the vagina and cervix, if present, and permanent suture on the sacrum. No sacrohysteropexies were performed. If an anti-incontinence procedure was performed, it was by synthetic mid-urethral sling or urethral bulking. No concomitant Burch urethropexies were performed. Exclusion criteria were women under 18 years of age, pregnant women, and patients without pelvic organ prolapse examinations documented prior to or after surgical intervention.
Patient data including demographics, preoperative and postoperative examinations, surgical details, adverse events, and follow-up were abstracted from electronic medical records. The study cohort was divided into two groups for analysis: women with diabetes and women without diabetes. Diabetes was defined based on the American Diabetic Association’s 2021 guidelines as HbA1c of greater than or equal to 6.5% within the 3 months leading up to surgery or a diagnosis of diabetes in the patient’s chart [12]. Postoperative HbA1c was also collected within 3 months of their documented failure date or their most recent postoperative visit if they never met the criteria for failure.
Primary outcome was composite failure, defined as the following: descent of the vaginal apex more than one-half of total vaginal length; vaginal descent in any compartment beyond the hymen; sensation of a vaginal bulge; or treatment for recurrent prolapse by pessary or surgery. Overall success was defined as absence of composite failure. Prolapse stage was measured using the pelvic organ quantification system (POP-Q). Secondary outcomes included intraoperative and postoperative complications, failure by compartment, reoperation rates, and the impact of clinical, demographic, and surgical characteristics on outcomes. Patients were included in outcome analysis if they had at least 12 months of follow-up or if they experienced failure prior to 12 months. Patients without 12-month follow-up were included in demographic and surgical data analysis only.
Continuous data that were normally distributed were analyzed using unpaired t tests and reported as mean and standard deviation. Continuous data that were not distributed normally were analyzed using the Wilcoxon rank sum test and reported as median and interquartile range. Categorical data were reported in absolute values and percentages and compared using the Chi-squared test. Kaplan–Meier plots were created for primary and secondary outcomes and tested using log-rank for the unadjusted comparison. Multivariate logistic regression was performed using Cox proportional hazard models to determine independent predictors of composite and individual compartment failure. Clinically relevant variables included in the model were BMI, parity, tobacco use, prior hysterectomy, prior prolapse repair, prior incontinence surgery, and advanced preoperative prolapse (stage 3 or 4). The proportional hazard assumption was satisfied using the cumulative sums of Martingale residuals and testing for interactions with time. A p value of 0.05 was considered significant. Data were analyzed using SAS version 9.4.
Results
A total of 547 charts were reviewed, including 100 patients with type 2 DM (18%) and 447 without (82%). Demographics and preoperative examination details are listed in Table 1. There were no patients with type 1 diabetes in the cohort. Overall, patients in the diabetic group were older, had a higher BMI, were more likely to be nonwhite, and were more likely to suffer from cardiovascular disease. Fifty-five percent of the patient population self-identified as nonwhite and represented 83% of patients with diabetes.
There was no difference in the rates of prior hysterectomy, prolapse surgery, or incontinence surgery. Diabetics were more likely to experience stage 3 or 4 prolapse in each compartment and overall. Average HbA1c was 6.5% preoperatively and 7.2% postoperatively, which was a statistically significant increase (p = 0.02); however, pre- and postoperative HbA1c values were not different between those with and without composite failure (pre: 6.3% vs 6.5%, p = 0.67; post 7.1% vs 7.2%, p = 0.72).
The majority of patients in both groups underwent supracervical hysterectomy over total hysterectomy at the time of SCP, and there was no difference between groups (65% vs 68%, p = 0.77). Diabetics were not more likely to undergo anterior or posterior colporrhaphy, perineorrhaphy, or an anti-incontinence procedure at the time of surgery. There was no difference in intraoperative or postoperative complications between groups, and no SCP mesh complications occurred in the diabetic group (Table 2). Median follow-up was 2.1 years and was not different between groups.
Postoperative success is described in Table 3. Overall composite failure rate was 15% and was not different between groups (14% vs 21%, p=0.14); however, anterior vaginal wall prolapse (AVP) beyond the hymen was significantly higher in diabetics (13% vs 3%, p<0.01). The same was true for apical prolapse greater than half of the total vaginal length (12% vs 2%, p<0.01). Overall prolapse stage was slightly more advanced in diabetics postoperatively (1.6 vs 1.3, p=0.02). Postoperative treatment for prolapse was not different between groups, and most women elected for surgical management of their recurrence (94%). There was no difference in recurrence rates across providers (p=0.14).
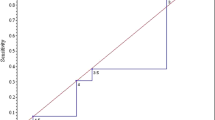
On univariate survival analysis with Kaplan–Meier, the presence of DM did significantly increase the risk of composite failure over time (p=0.05). This was also true for AVP (p≤0.001) and apical prolapse (AP) recurrence (p<0.01), but not for posterior vaginal wall recurrence (PVP; Fig. 1). However, on multivariate regression analysis, there was no longer a significant difference in composite failure, AP or PVP recurrence (Table 4). In contrast, diabetes carried an almost four times greater risk of AVP recurrence on multivariate analysis (hazard ratio [HR] 3.93, 95% CI 1.29–12.03, p = 0.02). BMI resulted in a small but significant increased risk for both anterior and composite failure (anterior: HR 1.14, 95% CI 1.03–1.26, p = 0.01; composite: HR 1.07, 95% CI 1.02–1.13, p = 0.01). Prior hysterectomy conferred an almost three-fold increased risk in overall prolapse recurrence over time (HR 2.94, 95% CI 1.16–7.44, p = 0.02).
Stress urinary incontinence (SUI) and urge urinary incontinence (UUI) affected 62% and 73% of patients preoperatively. Postoperatively, these numbers decreased to 17% and 38% respectively, and were not different between groups (Table 3).
As this was an analysis of retrospective data collected for a separate study, power calculations were not done for this analysis. However, post-hoc power calculation was performed. The study was adequately powered to detect a 10% difference in AVP recurrence and AP recurrence (82% and 87% respectively); however, it was not adequately powered to detect a difference in composite failure. A sample size of 199 per group would have been needed to detect a 10% difference in composite failure rates.
Discussion
In our study, we found that although diabetes did not increase the risk of composite failure after SCP, it did significantly increase the risk of AVP recurrence past the level of the hymen on both univariate and multivariate analysis. On multivariate regression, diabetes was associated with an almost four-fold risk of AVP recurrence over time. Fortunately, this did not translate to higher rates of re-operation over a median follow-up of 2.1 years. Prior hysterectomy and BMI were both independent risk factors for composite failure on multivariate analysis. Diabetics did not, however, suffer from increased rates of surgical complications or mesh exposure.
Eighteen percent of patients in our study had diabetes, higher than the 11% national average [1]. Eighty-three percent of patients with diabetes were nonwhite, highlighting the disproportionate impact of diabetes on racial/ethnic minority populations within the USA. These patients were more overweight, more likely to have cardiovascular disease, and had more advanced preoperative prolapse in each compartment. Women with diabetes also had more advanced overall postoperative prolapse stage at the time of most recent follow-up; however, this was a small and likely clinically insignificant difference given that both groups were found to have prolapse lower than stage 2.
Although glycemic control worsened from the pre- to the postoperative period, mean postoperative HbA1c (7.2%) fell just above the American Diabetic Association recommendation to maintain an HbA1c of 7% or less [13]. Although not statistically significant, mean postoperative HbA1c was slightly lower in the group that experienced failure (7.1% versus 7.2%). This highlights that even patients with relatively well-controlled diabetes are predisposed to recurrence. The pathophysiology behind this increased risk is unclear; however, it is well known that chronic hyperglycemia impairs wound healing, angiogenesis, and the immune response [14]. After surgery, insulin resistance increases significantly in both diabetic and nondiabetic patients, which can lead to a pronounced increase in hyperglycemia even in well-controlled diabetics, such as those in our study [15]. Postoperative hyperglycemia heightens the inflammatory response, which has been implicated as a risk factor for mesh complications after SCP in diabetic murine models [7, 15]. Theoretically, this inflammation and impaired angiogenesis may also impact postoperative healing and increase the risk of surgical failure. The anterior compartment has the highest risk of failure after SCP [16, 17], which may be why diabetics were particularly vulnerable to recurrence in this compartment. Apical descent and overall postoperative prolapse stage were also more advanced in diabetic patients. Although neither of these was clinically significant, they do lend support to the theory that impaired healing in diabetics may compromise surgical integrity.
In our study, diabetic patients had more advanced prolapse at baseline, which is a known risk factor for recurrence [7, 8]; however, this was controlled for on multivariate analysis and was not found to be an independent risk factor. BMI, on the other hand, was found to be an independent risk factor for both AVP recurrence and composite failure, although the effect was small (HR 1.14 and 1.07 respectively). This is consistent with the literature, which suggests that there is minimal, if any, effect of BMI on prolapse recurrence after SCP [18]. Prior hysterectomy was also found to increase the risk of composite failure by three-fold. To date, there is only one study that has investigated the risk of prolapse recurrence in women with concomitant versus prior hysterectomy at the time of SCP, and the authors showed no difference between groups [19]. Similarly, a meta-analysis demonstrated no increased risk of prolapse recurrence in women with prior hysterectomy undergoing native tissue repair or repair with vaginal grafts [20]. Regardless of the effect prior hysterectomy may have on recurrence, SCP is the most effective surgical intervention for vaginal vault prolapse in a woman who wishes to preserve sexual function [21]. Thus, these findings are unlikely to impact clinical practice.
Limitations of our study include its retrospective nature. Additionally, it is well known that the risk of prolapse recurrence increases with time [22]; therefore, we may not have successfully captured the full extent of recurrences within the study population. Although we collected preoperative and postoperative HbA1c values at the time of failure and/or most recent postoperative visit, we did not trend values over time. Thus, it is possible that we did not capture periods of particularly poor glycemic control. Finally, these data were collected for a separate analysis; however, it represented a complete list of all patients who had undergone SCP within the specified time period. Thus, the risk of bias in data collection and patient selection was likely minimal. A power calculation was not performed for this specific research question. Consequently, the study may have been underpowered to detect a difference in composite failure between groups.
Our study has multiple strengths. The majority of patients in our study were from minority backgrounds, which allows the applicability of our findings to a more diverse population than traditionally represented in research. Our data came from a well-documented and highly organized medical record system through Kaiser Permanente. Active Kaiser members are unlikely to go outside of the system to receive care; thus, their retrospective data are more likely to be complete. Multivariate analysis controlled for multiple independent variables, and survival analysis was used to analyze outcomes over time given the wide follow-up period of 1 to 8.6 years.
In conclusion, even in well-controlled diabetics, diabetes increased the risk of anterior vaginal wall prolapse recurrence by approximately four-fold in our diverse patient population. This is an important consideration when counseling diabetic patients regarding postoperative expectations. Future studies should include larger cohorts and traditional laparoscopy, in addition to basic science research that furthers our understanding of the role that increased insulin resistance and hyperglycemia may play in prolapse recurrence.
References
National Diabetes Statistics Report [Internet]. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention; 2021. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed 3 June 2022.
Swift S, Woodman P, O’Boyle A, et al. Pelvic Organ Support Study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol. 2005;192(3):795–806. https://doi.org/10.1016/j.ajog.2004.10.602.
Nygaard I, Bradley C, Brandt D. Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol. 2004;104(3):489–97. https://doi.org/10.1097/01.AOG.0000136100.10818.d8.
Whitcomb EL, Rortveit G, Brown JS, et al. Racial differences in pelvic organ prolapse. Obstet Gynecol. 2009;114(6):1271–7. https://doi.org/10.1097/AOG.0b013e3181bf9cc8.
Kudish BI, Iglesia CB, Gutman RE, et al. Risk factors for prolapse development in white, Black, and Hispanic women. Female Pelvic Med Reconstr Surg. 2011;17(2):80–90. https://doi.org/10.1097/SPV.0b013e31820e5d06.
Smith FJ, Holman CD, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116(5):1096–100. https://doi.org/10.1097/AOG.0b013e3181f73729.
Deng T, Liao B, Luo D, Shen H, Wang K. Risk factors for mesh erosion after female pelvic floor reconstructive surgery: a systematic review and meta-analysis. BJU Int. 2016;117(2):323–43. https://doi.org/10.1111/bju.13158.
Liang R, Fisk A, King G, Meyn L, Xiao X, Moalli P. Characterization of vaginal immune response to a polypropylene mesh: diabetic vs. normoglycemic conditions. Acta Biomater. 2022;143:310–9. https://doi.org/10.1016/j.actbio.2022.03.007.
Padoa A, Shiber Y, Fligelman T, Tomashev R, Tsviban A, Smorgick N. Advanced cystocele is a risk factor for surgical failure after robotic-assisted laparoscopic sacrocolpopexy. J Minim Invasive Gynecol. 2022;29(3):409–15. https://doi.org/10.1016/j.jmig.2021.11.002.
Chang OH, Davidson ERW, Thomas TN, Paraiso MFR, Ferrando CA. Predictors for pelvic organ prolapse recurrence after sacrocolpopexy: a matched case-control study. Female Pelvic Med Reconstr Surg. 2021;27(1):e165–70. https://doi.org/10.1097/SPV.0000000000000874.
Aslam MF, Osmundsen B, Edwards SR, Matthews C, Gregory WT. Preoperative prolapse stage as predictor of failure of sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2016;22(3):156–60. https://doi.org/10.1097/SPV.0000000000000233.
American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15–33. https://doi.org/10.2337/dc21-S002 Erratum in: Diabetes Care. 2021;44(9):2182.
A1C and EAG [Internet]. A1C and eAG | ADA. Available from: https://www.diabetes.org/diabetes/a1c/a1c-and-eag. Accessed 23 June 2022.
Okonkwo UA, DiPietro LA. Diabetes and wound angiogenesis. Int J Mol Sci. 2017;18(7):1419. https://doi.org/10.3390/ijms18071419.
Ljungqvist O, Nygren J, Soop M, Thorell A. Metabolic perioperative management: novel concepts. Curr Opin Crit Care. 2005;11(4):295–9. https://doi.org/10.1097/01.ccx.0000166395.65764.71.
Yang J, He Y, Zhang X, et al. Robotic and laparoscopic sacrocolpopexy for pelvic organ prolapse: a systematic review and meta-analysis. Ann Transl Med. 2021;9(6):449. https://doi.org/10.21037/atm-20-4347.
Serati M, Bogani G, Sorice P, et al. Robot-assisted sacrocolpopexy for pelvic organ prolapse: a systematic review and meta-analysis of comparative studies. Eur Urol. 2014;66(2):303–18. https://doi.org/10.1016/j.eururo.2014.02.053.
Wen Q, Zhao Z, Wen J, et al. Impact of obesity on operative complications and outcome after sacrocolpopexy: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;258:309–16. https://doi.org/10.1016/j.ejogrb.2021.01.032.
Dubinskaya A, Hernandez-Aranda D, Wakefield DB, Shepherd JP. Comparing laparoscopic and robotic sacrocolpopexy surgical outcomes with prior versus concomitant hysterectomy. Int Urogynecol J. 2020;31(2):401–7. https://doi.org/10.1007/s00192-019-04017-5.
Friedman T, Eslick GD, Dietz HP. Risk factors for prolapse recurrence: systematic review and meta-analysis. Int Urogynecol J. 2018;29(1):13–21. https://doi.org/10.1007/s00192-017-3475-4.
Coolen AWM, Bui BN, Dietz V, et al. The treatment of post-hysterectomy vaginal vault prolapse: a systematic review and meta-analysis. Int Urogynecol J. 2017;28(12):1767–83. https://doi.org/10.1007/s00192-017-3493-2.
Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse.. JAMA. 2013;309(19):2016–24. https://doi.org/10.1001/jama.2013.4919. [published correction appears in JAMA. 2013 Sep 11;310(10):1076].
Acknowledgements
There are no funding sources for this study. Statistical analysis was provided through Kaiser Permanente Southern California, Department of Research and Evaluation. All individuals who have contributed significantly to the work are listed as authors. This work was presented as a short oral presentation at the American Urogynecologic Society Pelvic Floor Disorders Week on 17 June 2022.
Author information
Authors and Affiliations
Contributions
S. Eckhardt: project development, data collection, manuscript writing; K. Laus: manuscript writing; S. DeAndrade: manuscript writing; J. Lee: data analysis, manuscript writing; J. Nguyen: project development, data collection, manuscript writing.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eckhardt, S., Laus, K., DeAndrade, S. et al. The impact of diabetes mellitus on pelvic organ prolapse recurrence after robotic sacrocolpopexy. Int Urogynecol J 34, 1859–1866 (2023). https://doi.org/10.1007/s00192-023-05455-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-023-05455-y