Abstract
Introduction and hypothesis
To date, no study has investigated the correlation between pelvic floor muscle function and urinary incontinence in female runners. The aim of the study was to investigate the relationship between pelvic floor muscle function and to correlate urinary leakage as measured by the modified pad test with kinematic variables of running.
Methods
The sample consisted of 11 incontinent and 17 continent runners. On day 1, they performed a maximum velocity test on a treadmill using an incremental protocol. Their pelvic floor muscles were evaluated using vaginal palpation and manometry, and the adapted pad test was used to evaluate the severity of urinary incontinence. Then, running kinematics were evaluated on a treadmill using a circuit camera to capture vertical displacement, knee flexion during the load response phase, and the initial contact of the foot with the ground for subsequent analysis. The pad test was performed during the kinematic evaluation.
Results
The vaginal squeeze pressure of the continent group (mean = 43.40 mmHg, SD = 21.75) was higher in descriptive terms than that of the incontinent group (mean = 38.94 mmHg, SD = 31.08), but the difference was not statistically significant (p = 0.66). There was an association between the weekly training load and urinary leakage. No associations were found between pelvic floor muscle function or urinary leakage and the kinematic variables.
Conclusions
Despite the correlation between the weekly training load and the severity of urinary leakage, no relationships were found between pelvic floor muscle functioning and the kinematics of running.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physical exercise has health benefits [1, 2]. Because running is inexpensive and easy to access, it is becoming one of the most popular sports in the world, and the female presence in running is increasing [3]. However, although running has proven benefits to women’s quality of life, it is considered a high-impact form of exercise and may be a risk factor for the development of urinary incontinence (UI) [4].
The International Continence Society (ICS) defines UI as any involuntary leakage of urine; the most common form is stress-induced urinary incontinence (SUI), which is the leakage of urine during coughing, sneezing or physical exertion [5]. The main risk factors for UI are parity, childbirth lesions, perineal trauma, surgery, and age [6]. Currently, intense physical exercise is also considered a risk factor for this dysfunction [7]. Women who show symptoms of urinary leakage during exercise, but not during coughing or sneezing and have not gained weight gain are considered to have athletic incontinence (AI) [8].
During running specifically, the ground reaction force can increase corporeal weight by 1.6 to 2.5 times [9], and impact transmission via the foot strike can affect the continence mechanism by transmitting the change in force to the pelvic floor (PF) [3, 5]. Almeida et al. stated in their research that the prevalence of UI in long-distance runners is 62.2%. A high prevalence has been reported in several studies [6, 10], and UI leads many women to change their sport or abandon physical exercise because UI affects their sexual, social, domestic, and occupational activities [10]. In addition, strenuous physical exercise that increases intra-abdominal pressure can chronically overload and damage the pelvic floor muscles (PFMs), ligaments, and fasciae, and decrease the contraction force of these muscles [11].
Leitner et al. studied the electromyographic activity of the PFMs of continent and incontinent women during running and found no differences between the groups. However, the PFMs are activated during running, presumably because of the ground reaction force; hence, running demands stronger PFMs [1]. Given these results, it is a reasonable assumption that the PFMs are activated during running, but as UI is prevalent in female runners, it is important to establish which kinematic variables influence urinary leakage.
Kinematics is defined as a description of the movements of joints or body segments that occur independently of forces associated with the movement. The growing number of people taking up running has caused a significant increase in research and evaluation in this area, which has been further boosted by technical advances, including the availability of faster cameras and the use of specific markers that facilitate data analysis [11]. Kinematic analysis techniques make it possible to evaluate movement patterns during running. In this study, we sought to determine whether specific kinematic variables are related to urinary loss and a reduction in PFM function.
To date, there has been no study investigating the correlation between urinary leakage in runners and the kinematic variables of running. The central hypothesis of this study was that increased vertical displacement during a complete stride and reduced knee flexion during the load response phase would increase the vertical impact load, and consequently, greater overload would be dissipated to the PFMs, leading to increased urinary leakage. Furthermore, it is known that heel striking has a characteristic transient impact on the vertical ground reaction force; it is associated with high load rates [12] and could therefore also be related to lower PFM function.
We are not aware of any previous research investigating urinary leakage during running or identifying specific factors related to this problem. This is a pioneering study whose objectives were to evaluate the PFM function by vaginal palpation and pressure manometry and to correlate urinary leakage as measured by the modified pad test with the kinematic variables of running in female runners.
Materials and methods
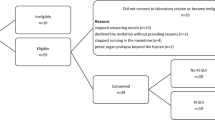
This cross-sectional, observational study was approved by the local institutional Ethics Committee (Federal University of Uberlândia—no. 1,451,984/2016). The sample consisted of 28 women divided into two groups: the incontinent group (IG) comprised 11 volunteers who reported SUI during sports practice, and the continent group (CG) comprised 17 women who reported that they did not lose urine during running. All volunteers were invited to participate by personal contact and provided written informed consent. Data were collected between November 2017 and August 2018 at the Faculty of Physical Education of Federal University of Uberlandia.
The G*Power program (power = 0.95; effect size = 1.35; α error = 0.05) was used to calculate the required sample size, which was estimated to be 26 volunteers [13].
Women were eligible for inclusion in the study if they ran at least 20 km per week, had been running for at least 6 months, had not been injured during the last 6 months (no acute joint or musculoskeletal problems and pain during running), had already had sexual intercourse, were aged over 18 years, could perform PFM contractions, and had never regularly performed pelvic floor exercises. Women were assigned to the IG group if they complained of urinary leakage only during running, not during other forms of exertion (cough, sneeze), confirmed by the International Consultation on Incontinence Questionnaire—Short Form (ICIQ-SF) [14]. The participants in the CG did not have symptoms of involuntary UI during any physical exertion, and they were completely continent.
Exclusion criteria for both groups were a history of urogynecological surgery, the presence of cooperation problems that would prevent successful participation in the assessments, urinary tract infection at the time of evaluation, and menstruation at the time of evaluation (Fig. 1.
The evaluation was spread across 2 days. On day 1, participants completed a questionnaire providing information about personal and gynecological history and running behavior. After verification of eligibility, participants performed the peak speed test on a treadmill. On day 2, the PFM evaluation and kinematic evaluation were performed.
Peak treadmill speed test
A peak treadmill speed test was conducted to determine the peak velocity for each participant, as kinematic analysis was carried out at 75% of maximum speed as a form of standardization. The tests were performed using an incremental treadmill protocol in the morning at the same time of the day for all participants and under normal laboratory conditions (temperature = 20–22 °C; relative humidity = 50–60%). The participants were instructed to refrain from training for 48 h beforehand and to be well fed and wearing light and comfortable running clothes on the day of the test [15]. All participants wore their own shoes.
The test was performed on a treadmill (model LX 250, Movement; Brudden, Sao Paulo, Brazil) with the gradient set at 1%. The protocol began with a 3-min warm-up at 6 km/h; thereafter, the treadmill speed was increased by 1 km/h every 2 min. The test continued until the participant reached exhaustion. Participants were given strong verbal encouragement to exert maximum effort during the test.
Peak speed was calculated using the formula: \( Vpeak= Vcomplete+\left( Inc\ x\frac{t}{T}\right) \), where Vpeak is the running speed during the last completed stage; Inc is the speed increment per stage (1 km), t is the duration of the incomplete stage in seconds and T is the duration of a completed stage (120 s) [16].
Exertion during the peak treadmill speed test was evaluated using the Borg scale [17] and heart rate measurements. Participants were familiarized with the Borg scale before the start of the test and asked to rate their exertion during the last 15 s of each stage and at exhaustion, i.e., at the end of the test. Heart rate was measured throughout the test using a frequency meter (Polar RS800CX; Polar, Kempele, Finland).
Evaluation of PFMs
Participants were invited to return for the second stage of evaluation 48 h after the peak treadmill speed test. PFM function was evaluated by vaginal palpation and measurement of vaginal squeeze pressure. Both methods are widely used and cited and have proven reproducibility and validity [18]. All evaluations were carried out by the same examiner, who used standardized verbal commands during the examinations.
Intraexaminer reproducibility of vaginal palpation and manometry evaluations was assessed before the study using the intraclass correlation coefficient (ICC). The reproducibility test consisted of two evaluations of 11 random women (not necessarily runners) at 1-week intervals.
Participants were invited to empty their bladder before the PFM evaluation. The latter was performed with the participant lying on an appropriate stretcher in the supine position, with hips and knees flexed and feet supported. The participant was given instructions for performing the contractions and the appropriate pattern of breathing during the tests.
The evaluation began with bidigital vaginal palpation [19]. The physiotherapist introduced the index and middle fingers into the middle third of the vagina and asked the participant to perform three 5-s maximal contractions of the PFMs, with a 1-minute rest period between them. Participants were instructed to contract their muscles using an “inward and cranial” movement. Muscular strength was quantified using the six-point Modified Oxford Scale (0 = absence of muscle response; 5 = strong contraction with firm compression of the examiner’s fingers and positive movement toward the pubic symphysis). Contractions were considered valid if the examiner observed cranial elevation and an absence of visible contractions of the hip adductor, gluteal and abdominal muscles (ICC: 0.97), only an appropriate co-contraction of the deep abdominal muscles was accepted during PFM contraction [20]. Vaginal palpation was always carried out first, followed by evaluation of contraction pressure.
The PFM contraction pressure was evaluated using a Peritron™ (Cardio Design, Oakleigh, VIC, Australia) equipped with a vaginal probe that was entirely covered by an unlubricated condom and then lubricated with water-based gel. The probe sensor was connected to a handheld microprocessor with a latex tube that allows the pressure exerted by the muscular contraction to be measured in centimeters of water (cmH2O). The participant remained in the same position as for the previous test, and the vaginal sensor was introduced approximately 4 cm into the vaginal cavity. Then, the apparatus was inflated with a syringe to a pressure of 100 cmH2O (calibration). The participant was then instructed to perform three 5-s maximal contractions, with a 1-minute break between contractions. Participants were verbally encouraged to exert maximum contractile force. The average of the three peak pressures (ICC: 0.94) provided by the equipment was used in the statistical analyses [21].
Evaluation of running kinematics and urinary loss
After the evaluation of contraction pressure, the participant was given an absorbent pad that had been weighed on a high-precision scale (Mini Precision Digital Scale, 0.1 g). The participant was then instructed to ingest 500 ml of water within 15 min and place the absorbent pad in her underwear to absorb any urine lost during running. Thirty minutes after water intake, the participant returned to the treadmill for the evaluation of running kinematics.
After the evaluation of running kinematics, the participant was instructed to remove the pad, place it inside a plastic bag that had been provided by the examiner and return it to the examiner for weighing. Both groups performed the pad test.
The test was performed under normal laboratory conditions (temperature = 20–22 °C; relative humidity = 50–60%), ideally in the morning, and participants were instructed not to train during the 48 h before the test, to be well fed, and to wear dark, close-fitting clothes to facilitate attachment of the markers.
Twelve rounded Styrofoam markers (15-mm in circumference) were attached to the participant at key anatomical points with double-sided adhesive tape. Markers were placed at key anatomical points, as shown in Fig. 2.
The kinematic evaluation was based on recorded videos and images obtained by a circuit of cameras (right lateral view and back view). The data were collected while the participant was running on a treadmill (Movement, model LX 250) using two cameras: a 60-frame-per-second camera (Sony, model SD 60) positioned on a tripod placed at a distance of 2.50 m from the side of the treadmill and a 30-frame-per-second camera (Sony, model SD 44) positioned on a tripod 2.40 m behind the treadmill.
The running protocol for the kinematic evaluation began with a 3-min warm-up at 6 km/h, with the gradient set at 1%. The speed of the treadmill was then increased to 75% of the peak speed obtained by the participant in the peak treadmill speed test (day 1). This speed was maintained for 8 min, during which images were recorded for subsequent analysis.
The following kinematic variables were analyzed: vertical displacement during a complete stride was measured via the marker placed at the 5th lumbar vertebra (L5) in the dorsal plane (a); knee flexion during the load response phase was measured using the markers placed on the femur, greater trochanter (F), lateral condyle of the femur (G), and the sagittal plane of the lateral malleolus (H); foot strike (heel, midfoot or forefoot) was evaluated in the initial foot strike phase, looking at the sagittal plane. Images from the 6th minute of recordings were analyzed.
Data recorded by the cameras were analyzed using Kinovea version 0.8.24, free video analysis software that enables measurement of the range of motion of joints of the body. The videos were analyzed in slow motion, frame by frame. The software allows postures and movement speeds to be determined from captured images.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (V21; SPSS Chicago, IL, USA) software. ICCs were calculated to evaluate the reproducibility of the evaluation methods. ICC values above 0.75 were considered excellent [22].
The distribution of quantitative variables was assessed using the Kolmogorov–Smirnov test. This confirmed that the dependent variables were normally distributed, so group differences were assessed using Student’s t test. Group differences in categorical variables were assessed using Fisher’s exact test.
Pearson’s correlation coefficient was used to measure correlations between groups in the case of continuous variables, and boxplots and descriptive statistics were used to assess associations between categorical and continuous variables. A significance level of 5% (p ≤ 0.05) was adopted.
Results
Twenty-eight women (CG = 17; IG = 11) participated in the study. Table 1 shows the characteristics of the groups. The groups were homogeneous with respect to age, body mass index (BMI), running experience, and parity. The IG ran farther per week than the CG.
Kinematic variables are reported in Table 2. The results indicate that vertical displacement was greater in the IG, with no significant difference.
Comparisons of PFM function in terms of strength and endurance were measured by vaginal palpation, and contraction pressure was measured using a perineometer. Although the CG group presented higher PFM contraction pressure than the IG group, there were no statistically significant correlations between groups (Table 3).
There were no correlations, without significant difference, between the kinematic variables measured and urinary leakage as measured by the pad test (Table 4).
Whole-group (N = 28) correlations between the type of right foot strike and urinary leakage did not suggest any relationship between continence and foot strike (Fig. 3).
Whole-group correlations (N = 28) between urinary leakage during running (yes; no) and weekly distance revealed a positive association; the IG (n = 11) ran further (mean = 45 km/week, SD = 15.97; Fig. 4).
Discussion
To the best of our knowledge, and based on the literature, the present study is the first to investigate urinary leakage during running or to identify specific factors related to this problem. Weekly training load seems to be negatively associated with PFM strength, and correlations between urinary leakage and vertical displacement, knee flexion, and foot strike did not reveal any association, suggesting that there might not be a relationship between the kinematic variables we studied and urinary leakage, thus refuting our main hypothesis.
It has been reported that approximately 89% of runners are heel-strikers, and this method of landing is associated with a high vertical impact load and higher rates of stress fracture, plantar fasciitis, and joint cartilage damage than other methods of landing. The human musculoskeletal system consists of viscoelastic structures that are sensitive to load rates; the muscles stretch against the load and help to attenuate impact forces. It is possible that higher loading forces increase muscle deformation, thus predisposing the individual to injury [12]. The authors hypothesize that this vertical impact load of heel-strikers could also affect the PF, and like other muscles, could be overloaded by higher impacts, leading to weakness or early fatigue during running and resulting in loss of urine.
It is also known that during running, the load absorption phase is shorter and knee flexion is reduced. As long as the knee flexes after the initial contact, the quadriceps contract eccentrically to absorb energy, reflecting their importance as shock absorbers during running [11]. Our hypothesis was based on the fact that during the load response phase, knee flexion is used to cushion the impact; if flexion is reduced, then less impact is absorbed, and the PF could be affected by the increased load, resulting in loss of urine.
It seems likely that the failure to detect correlations between kinematic variables and urinary leakage was due to the short duration of our runner-adapted pad test (8 min). At 75% of maximum speed, an 8-min running test was not sufficient to lead to loss of urine in our sample of experienced runners.
The pad test is a validated measure of the amount of urinary leakage. It involves measuring the difference between the weight of the pad at the beginning and end of the test [23]. There are two evaluation protocols. The standardized 1-h pad test protocol requires women to perform a series of activities likely to provoke urinary leakage, whereas the 24-h pad test involves women wearing a pad for 24 h during which they carry out their normal daily routine and is therefore regarded as a more representative measure of the true severity of urinary leakage. The repeatability of the two protocols is still in question [23, 24].
The most widely used protocol is the 1-h pad test, standardized by the International Continence Society (ICS pad test) [25]. There is as yet no standardized and validated pad test protocol for athletes, and we believe that the existing validated protocols would not be sufficient to produce urinary leakage in athletes; thus, we developed our own protocol. We attempted to reproduce the conditions during which urinary leakage occurs in runners who suffer from SUI during sports practice; hence, the pad test was performed during the evaluation of running kinematics. The mean urinary leakage in the IG (3.17 g) represents only mild incontinence. The test could also be criticized on the grounds that excessive sweating as a result of exercise could affect the outcome [3]. Studies have shown that the pad test has low negative predictive value and low reproducibility and is more useful in cases of urethral sphincter deficiency than as a method of screening for UI [3, 26].
With regard to the possibility that a high volume of the exercise has repercussions for the PF, it is known that increases in exercise volume may lead to damage to the musculoskeletal system if it is not prepared for the increase in training load [2], but the repercussions for the PF remain unclear. Exercise increases intra-abdominal pressure, and the increases in pressure resulting from repeated, intense exercise may negatively affect the PFM support structures [5, 27] with adverse consequences for function. Da Roza et al. related UI frequency to physical activity level, dividing their sample into quartiles based on the weekly training load, with the first quartile classed as sedentary and the fourth quartile as athletic. The athletes experienced UI more frequently [28]. The findings of our study are consistent with the literature, since we found an association between weekly running distance and UI, suggesting that women who experience urinary leakage during running tend to have a higher weekly training load. The more frequent the impact associated with increased intra-abdominal pressure, the greater the need for pelvic organ restraint and support, and the PFMs must be trained to preserve their function [6].
We measured peak speed using an incremental protocol, which is a good predictor of resistance performance in runners and does not require expensive metabolic equipment or invasive techniques [15]. We decided to measure running kinematics and urinary leakage at 75% of peak speed, as we believed that this would be close to the speed runners would achieve under normal road running conditions. Respiratory gases and blood lactate were not monitored during the test, as such interventions can impair performance [29].
Some studies have found that athletes show satisfactory PFM strength in functional evaluations. Araújo et al. evaluated female athletes with a mean age of 20 years (SD = 3) who were in menacme, nulliparous, and eumenorrhoeic. They found a mean contractile pressure of 70.1 (2.4) cmH2O using perineometry and a strength greater than 3 on the Oxford Scale [3] using vaginal palpation. In comparison, the IG group in our study had a mean age of 41.91 years (SD = 11.56) years, a mean PFM strength score of 2.91 (SD = 1.14) on the Oxford Scale, and a mean maximal contractile pressure of 38.94 cmH2O (SD = 31.08) measured using a perineometer. Our sample was not limited to nulliparous women, as other studies have shown that women may not begin running until later in life and many have already given birth when they take up running [30]; thus, our sample is more representative of the population of female runners.
Reduced PFM strength is widely recognized as a risk factor for pelvic dysfunction, including UI and sexual disorders [19]. During running, vertical ground reaction forces can increase significantly; therefore, runners need a stronger PF than the general population to absorb these forces [31]. Hence, the reduction in PFM function observed in our IG group may have contributed to their SUI during sports practice.
It is important to emphasize that UI is a multifactorial condition and may not be related to the kinematics of running or an individual’s training load. The main risk factors for UI are age, previous pelvic surgery, pregnancy, instrumental vaginal delivery, obesity, menopause, and constipation [32]. Eating disorders and hypothalamic amenorrhea due to intense physical exercise may also trigger UI in athletes owing to a lack of estrogens, as estrogen is essential for urethral coaptation, one of the intrinsic mechanisms of urinary continence [3].
The strengths of this study are the attempts to quantify urinary leakage during running and to find the cause of the high prevalence of UI in runners through kinematic analysis of running style and functional evaluation of the PFMs. We found that urinary leakage appears to be related to increased vertical displacement, heel striking and knee flexion during the load response phase. Given that manometry and vaginal palpation provided evidence of reduced PMF function in our sample of volunteers, further studies are needed to elucidate the real cause of UI in runners. The limitations of our study include two-way analysis. We know that three-way analyses would have provided more information, and the same examiner interviewed the participants, assessed the function of the PFMs, and performed the allocation of the groups without blindness. Furthermore, we did not ask the participants at what stage of their running they lost urine, which may have been far later into their running than we tested, or the accumulated small leaks throughout the run may have been greater. Additionally, we did not ask about their normal drinking in relation to training, which may have differed considerably from the research conditions. We suggest that future studies investigate these aspects to improve results.
Weekly training load seems to be negatively associated with PFM strength. We did not find any relationship between PFM strength and vertical displacement or knee flexion during the response to load or foot strike. We suggest that further research might use kinematic analysis combined with real-time electromyography assessment of PFMs.
Abbreviations
- AI:
-
Athletic incontinence
- BMI:
-
Body mass index
- CG:
-
Continent group
- ICC:
-
Intraclass correlation coefficient
- IG:
-
Incontinent group
- ICIQ-SF:
-
International Consultation on Incontinence Questionnaire—Short Form
- ICS:
-
International Continence Society
- IU:
-
Urinary incontinence
- PF:
-
Pelvic floor
- PFMs:
-
Pelvic floor muscles
- SUI:
-
Stress urinary incontinence
References
Leitner M, Moser H, Eichelberger P, et al. Evaluation of pelvic floor muscle activity during running in continent and incontinent women: an exploratory study. Neurourol Urodyn. 2017;36(6):1570–6.
Haskell W, Lee I-M, Pate R, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–93.
Araújo M, Oliveira E, Zucchi E, et al. Relação entre incontinência urinária em mulheres atletas corredoras de longa distância e distúrbio alimentar. Rev Assoc Med Bras. 2008;54(2):146–9.
Fozzatti C, Riccetto C, Herrmann V, et al. Prevalence study of stress urinary incontinence in women who perform high-impact exercises. Int Urogynecol J Pelvic Floor Dysfunct. 2012;23(12):1687–91.
Haylen BT, Freeman RM, Swift SE, Cosson M, Davila GW, et al. An international Urogynecological association (IUGA) / international continence society (ICS)joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) & grafts in female pelvic floor surgery. Int Urogynecol J. 2011;22:3–15. https://doi.org/10.1007/s00192-010-1324-9.
Almeida M, Barra A, Saltiel F, et al. Urinary incontinence and other pelvic floor dysfunctions in female athletes in Brazil: a cross-sectional study. Scand J Med Sci Sports. 2015;26(9):1109–16.
Ree M, Nygaard I, Bo K. Muscular fatigue in the pelvic floor muscles after strenuous physical activity. Acta Obstet Gynecol Scand. 2007;86(7):870–6.
Araujo MP, Sartori MG, Girão MJ. Athletic incontinence: proposal of a new term for a new woman. Rev Bras Ginecol Obstet 2017;39(9):441–2. https://doi.org/10.1055/s-0037-1605370.
Gottschall JS, Kram R. Ground reaction forces during downhill and uphill running. J Biomech. 2005;38(3):445–52.
Nygaard I, Delancey J, Arnsdorf L, Murphy E. Exercise and incontinence. Obstet Gynecol. 1990;75(5):848–51.
Novacheck T. The biomechanics of running. Gait Posture. 1998;7(1):77–95.
Davis IS, Bowser BJ, Mullineaux DR. Greater vertical impact loading in female runners with medically diagnosed injuries: a prospective investigation. Br J Sports Med. 2016;50(14):887–92.
Cohen J. Statistical power analysis for the behavioral sciences, 2nd edition. Hillsdale: Lawrence Erlbaum; 1988.
Tamanini JT, Dambros M, D’Ancona CA, et al. Validação para o português do International Consultation on Incontinence Questionnaire—Short Form (ICIQ-SF). Rev Saúde Pública. 2004;38(3):438–44.
Peserico CS, Zagatto AM, Machado FA. Evaluation of the best-designed graded exercise test to assess peak treadmill speed. Int J Sports Med. 2015;36(9):729–34.
Kuipers H, Rietjens G, Verstappen F, et al. Effects of stage duration in incremental running tests on physiological variables. Int J Sports Med. 2006;24(7):486–91.
Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81.
Bø K, Finckenhagen HB. Vaginal palpation of pelvic floor muscle strength: inter-test reproducibility and comparison between palpation and vaginal squeeze pressure. Acta Obstet Gynecol Scand. 2001;80(10):883–7.
Bø K, Sherburn M. Evaluation of female pelvic-floor muscle function and strength. Phys Ther. 2005;85(3):269–82.
Bø K. Pelvic floor muscle training is effective in treatment of female stress urinary incontinence, but how does it work? Int Urogynecol J. 2004;15:76–84.
Frawley HC, Galea MP, Phillips BA, Sherburn M, Bo K. Reliability of pelvic floor muscle strength assessment using different test positions and tools. Neurourol Urodyn. 2006;25:236–42.
Fleiss J. The measurement of interrater agreement: statistical methods for rates and proportions. New York: Wiley; 1981.
Dumoulin C, Morin M, Mayrand M, et al. Group physiotherapy compared to individual physiotherapy to treat urinary incontinence in aging women: study protocol for a randomized controlled trial. Trials. 2017;18:544.
Karantanis E, Allen W, Stervermuer T, et al. The repeatability of 24-hour pad test. Int Urogynecol J. 2005;16:63–8.
Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: report from the standardization sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–78.
Siltberg H, Victor A, Larsson G. Pad weighing tests: the best way to quantify urine loss in patients with incontinence. Acta Obstet Gynecol Scand Suppl. 1997;16(6):28–32.
Da Roza T, Brandao S, Mascarenhas T, et al. Volume of training and the ranking level are associated with the leakage of urine in young female trampolinists. Clin J Sport Med. 2015;25(3):270–5.
Da Roza T, Brandao S, Mascarenhas T, et al. Urinary incontinence and levels of regular physical exercise in young women. Int J Sports Med. 2015;36(9):776–80.
Schabort EJ, Hopkins WG, Hawley JA. Reproducibility of self-paced treadmill performance of trained endurance runners. Int J Sports Med. 1998;19(1):48–51.
Iguana Sports. (2014) A primeira pesquisa sobre a corrida feminina no Brasil. Available at: <http://www.suacorrida.com.br/dnadecorredora/apres/dna_de_corredora_2014.pdf>. Accessed on: 20 August 2017.
Da Roza T, de Araujo MP, Viana R, et al. Pelvic floor muscle training to improve urinary incontinence in young, nulliparous sport students: a pilot study. Int Urogynecol J. 2012;23(8):1069–73.
Bø K. Urinary incontinence, pelvic floor dysfunction, exercise and sport. Sports Med. 2004;34(7):451–64.
Funding
National Council for Scientific and Technological Development (CNPq), process number: 134141/2017-2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Melo Silva, R., Rodrigues, M.E.S., Puga, G.M. et al. The relationship between running kinematics and the pelvic floor muscle function of female runners. Int Urogynecol J 31, 155–163 (2020). https://doi.org/10.1007/s00192-019-03968-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-019-03968-z