Abstract
Introduction and hypothesis
Running is known to cause urinary leakage in women with stress urinary incontinence (SUI). Task-specific fiber-type recruitment while running can be estimated using wavelets. The aim of this study was to compare the effect of a new physiotherapy program including involuntary, reflexive training with a standard physiotherapy program on pelvic floor muscle (PFM) activation patterns and fiber-type recruitment behavior while running.
Methods
In this triple-blinded randomized controlled trial, women with SUI were randomly allocated to the control group (CON), which performed a standard physiotherapy program, or the experimental group (EXP), which received additional involuntary, reflexive training. PFM electromyography (EMG) was recorded during 10 s at three running speeds and analyzed using Morse wavelets. The relative distribution of power (%) over the frequencies from 20 to 200 Hz was extracted and analyzed within six-time intervals of 30 ms. Statistical nonparametric mapping was performed to identify power spectra differences.
Results
Thirty-nine (CON) and 38 (EXP) women were included. The power spectra showed no statistically significant group differences. The time intervals from 30 ms before to 30 ms after initial contact showed significantly lower intensities than the intervals from 30 to 150 ms after initial contact in the lowest and higher intensities in the highest frequencies for all running speeds and both groups.
Conclusions
Power spectra shifts toward higher frequency bands in the pre-initial contact phase could indicate a feed-forward anticipation and a muscle tuning for the expected impact of initial contact event in order to maintain continence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physiotherapy is recommended by the International Consultation on Incontinence (ICI) as first-line therapy in the treatment of all forms of female urinary incontinence [1, 2]. A recently updated Cochrane Review confirmed the effectiveness of pelvic floor muscle (PFM) interventions for women with stress urinary incontinence (SUI) [3]. However, also in the updated review, limitations such as unclear description of the training programs, great variation of interventions and outcome measures, and incompletely reported exercise regimen remained [3]. In the literature, PFM training is defined as a program of exercises to improve PFM strength, endurance, power and relaxation [1,2,3]. This theory was justified by increasing the urethral pressure, preventing urine leakage during effort or exertion, and a support of the bladder neck proximal urethra [4, 5]. However, skeletal muscle training theory regimens are not consequently considered and described in PFM research, as PFM research mainly focuses on voluntary activation of PFM. Although involuntary muscle activation is usually part of the training protocols in sports, especially when fast reflex activation is a specific requirement of the athletic discipline, the involuntary reflex activation of PFM is hardly paid any attention.
The ability to contract quickly (i.e., developing force at a high rate) in response to ground reaction forces, which rise up to 3.9 times the body weight force within 60 ms after a heel strike, is a key PFM requirement to guarantee continence during running [6, 7]. Thereby, PFM electromyographic (EMG) activity was shown to be significantly higher than during 100% of maximum voluntary contractions, increasing with speed [8]. Time to peak of fast voluntary contractions (FVC) was measured to be about 500 ms [9]. Therefore, FVC exercises seem to be too slow to provoke training stimuli that meet the muscle contraction characteristics necessary to counteract the high rates of force development during running [9]. To react to high rates of force development, fast type II fibers need to be activated [10]. As motor unit action potentials (MUAPs) are conducted faster along the large type II fibers, the EMG signal spectrum is shifted toward higher frequencies [11]. Large alpha-motoneurons and the related type II fibers generate frequencies around 100 Hz or more, small alpha-motoneurons and the related type I fibers less than 100 Hz [12]. Wavelet analysis of EMG signals is a method of estimating task-specific fiber-type recruitment in the range of a few milliseconds with high time resolution [13]. As women with SUI may lose urine during running, it can be assumed that rapid, involuntary muscle activation should be integrated into the PFM training protocols, especially because the fast, voluntary contractions with 500 ms time to peak are much too slow.
The aim of this study was to compare the effect of the new physiotherapy program with a standard physiotherapy program on PFM activation patterns and fiber-type recruitment behavior while running.
Materials and methods
Study design
This study was based on a prospective, triple-blinded (participants investigators, statistician) randomized controlled trial (RCT) design. Both groups were informed that PFM training, including strength and power training, would be performed. The participants’ information sheet provided sufficient information for them to make an informed decision about their participation in the study. However, the participants were not informed about the exact exercises. The main difference was voluntary (fast voluntary contractions; control [CON] group) versus involuntary power training (e.g., running, jumping; experimental [EXP] group). Therefore, it was not possible for them to identify the group (or the differences between the groups). The investigators in the movement laboratory who performed the measurements were blinded concerning the group allocation of the participants. The study was approved by the Ethics Committee of the Canton of Bern, Switzerland (No. 249/14), in accordance with the Declaration of Helsinki, and registered at clinicaltrials.gov (www.clinicaltrials.gov/ study identifier: NCT02318251). The study protocol was published open access including the detailed therapy plan, provided as an “additional file” [14].
Participants
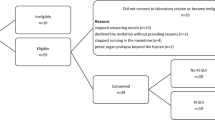
Ninety-six women with SUI were recruited and randomly allocated to the CON group or the EXP group. All participants signed written informed consent. Screening for inclusion was performed by an urogynecologist (AK). Inclusion criteria were SUI or mixed urinary incontinence with predominant SUI, age 18–70 years, BMI between 18 and 30 kg/m2, able to read and understand the German language, fit for exercises (running and jumping), negative pregnancy test, nulliparous or at least 12 months postpartum, finished lactation period, and in the case of systemic or local estrogen treatment, stable for the past 3 months prior to inclusion. Exclusion criteria were acute urinary tract or vaginal infections, tumor, fractures, predominant overactive bladder and urogenital prolapse grade > 1 POP-Q, de novo drug treatment with anticholinergics or other bladder-active substances [14].
Intervention
In the present study a new physiotherapy program was developed, focusing on involuntary reflexive PFM contractions, with a clear description of the training programs and completely reported exercise regimen [14]. Both protocols (CON and EXP group) included a standard physiotherapy program with educational background information and evident sensorimotor learning at the beginning, followed by voluntarily performed strength training concepts with progression of training for strength, power, and hypertrophy [2, 15]. The EXP group was additionally trained with whole body movements such as short running sequences and jumps, with the aim of triggering PFM reflex activity [8, 16]. The EXP group started after 6 weeks with the additional involuntary power training, building up to 3 × 15-s running sessions and following a maximum of 5 × 12 jumps. The intervention phase was 16 weeks, including nine personal physiotherapy consultations and 78 short home training sessions. EMG was measured at baseline and after a 16-week intervention phase [14]. More detailed information and the exactly described training parameters have been published before as an “additional file” [14].
Procedure and data collection
Demographics were collected at baseline. At baseline and after intervention vaginal palpation graded according to the modified Oxford grading system and a modified 20-min pad-test was performed [14]. The International Consultation on Incontinence Modular Questionnaire Urinary Incontinence short form (ICIQ-UIsf; scale 0 [not] to 21 [severely affected]) was measured at baseline, physiotherapy sessions 2 to 9 and post-intervention and defined as primary outcome [14]. Based on the primary outcome, sample size was calculated. However, sample size calculation was not included in this article, because the EMG data constituted one of the secondary outcomes, which were generally sampled for further exploratory analyses.Further results of the RCT are discussed elsewhere. A physiotherapist trained and experienced in PFM rehabilitation, first instructed and then controlled the correct voluntary PFM contraction by digital palpation in supine position. As soon as the correct PFM activation was guaranteed, a STIMPON™ (Innocept Biobedded System, Gladbeck, Germany) vaginal probe was carefully inserted into the vagina by the participants themselves. The correct vaginal probe placement was controlled by the physiotherapist before and after measurement. Participants received standardized shoes (Adidas, Duramo 6) for the treadmill running measurements. Initial contact of the right foot (T0) was captured by an instrumented treadmill (treadmill T7–0 Lifefitness, Rosemont, IL, USA) with two one-dimensional strain gauge force sensors (Modell KMB52K 10KN 000 D; Megatron Elektronik, Putzbrunn, Germany).
The participants first get used to the treadmill while walking. Afterwards, EMG was subsequently recorded for 10 s during running at 7, 9, and 11 km/h running speeds, without any voluntary PFM contraction.
For PFM EMG, a differentially configured tripolar STIMPON™ vaginal probe was used which was covered by a ProDry™ (Innocept Biobedded System GmbH) tampon soaked in a physiological saline-solution. This soft tampon adapted well to the individual vaginal cavities so that the probe did not slip during impact activities. A reference electrode (Ambu Blue Sensore N, Ballerup, Denmark) was fixed over the right anterior superior iliac spine after skin preparation following the SENIAM guidelines [17]. Electrodes were replaced when the impedance between the poles of the STIMPON™ electrode was greater than 2 kΩ (Digitimer model D175; Digitimer, Welwyn Garden City, UK). The electrodes were connected to the transmitter of the telemetric system via a pre-amplifier (baseline noise: <1 μV RMS; input impedance: <100 MΩ; CMMR: >100 dB; input range: ±10 mV; base gain: 500; integrated band-pass filter: 10–500 Hz). EMG was measured with a 16-channel Tele Myo 2400 G2 device (Noraxon USA, Scottsdale, AZ, USA [digital low-pass filter: 500 Hz; sampling rate: 3,000 Hz, gain: 1]). The EMG measurement and analysis software ADS (Analog-Digitale-Signalerfassung, uk-labs, Kempen, Germany) was used for visual plausibility control of the EMG signals (movement artifacts, baseline noise, baseline shift and baseline offset).
Data processing and analysis
Electromyography signals were processed and analyzed using custom-written software in MATLAB (version R2017b; MathWorks, Natick, MA, USA). The raw EMG signals were 20-Hz high-pass filtered to remove low-frequency components owing to possible movement artifacts and instability of the electrode–skin interface and 500 Hz low-pass filtered (2nd order Butterworth filter, zero-lag). Initial contact of the right foot (T0) was detected from the force signals of the instrumented treadmill by a thresholding algorithm, visually controlled and cut out within the 10 s running sequences. EMG signals of the right PFM encompassing 100 ms before to 300 ms after initial contact were used for analysis. A continuous wavelet transform (Morse wavelets: “cwt()” function from the Matlab Wavelet Toolbox) with a time-bandwidth product of 20 was used for PFM EMG analysis. Squared wavelet coefficients were considered as intensity patterns and were normalized to square root of the sum of all intensities [18]. Total intensity (sum of intensities among frequencies) and median frequency over time and the relative distribution of power (% of total power) over six frequency bands ([20–50], [50–80], [80–110], [110–40], [140–170], [170–200]) Hz were extracted (Figs. 1, 2). Frequencies between 0 and 20 Hz were not analyzed owing to possible movement artifacts. The relative distribution of power was extracted within six time intervals of 30 ms ([−30–0], [0–30], [30–60], [60–90], [90–120], [120–150]) ms in accordance with Fleischmann [19]. Missing data within a valid data set were replaced by the last valid data based on the intention-to-treat and last observation carried forward approach.
Exemplary illustration of a wavelet analysis, control group, 9 km/h running speed. Pelvic floor muscle electromyography was analyzed using Morse wavelets. Red line: initial contact. Values summarized from top to bottom: tot. intensity total intensity, MDF median frequency. Distribution of frequencies: values summarized from right to left. Gray illustrations: global and time windows spectra, time intervals of 30 ms, from 30 ms before to 150 ms after initial contact according to Fleischmann ([−30–0], [0–30], [30–60], [60–90], [90–120], [120–150]) ms [19]. Colored illustrations: relative distribution of power within six frequency bands, from 20 Hz to 200 Hz ([20–50], [50–80], [80–110], [110–140], [140–170], [170–200]) Hz. The missing percentages are because frequencies of only up to 200 Hz were analyzed
Exemplary illustration of a wavelet analysis, experimental group, 9 km/h running speed. Pelvic floor muscle electromyography was analyzed using Morse wavelets. Red line: initial contact. Values summarized from top to bottom: tot. intensity total intensity, MDF median frequency. Distribution of frequencies: values summarized from right to left. Gray illustrations: global and time windows spectra, time intervals of 30 ms, from 30 ms before to 150 ms after initial contact according to Fleischmann ([−30–0], [0–30], [30–60], [60–90], [90–120], [120–150]) ms [19]. Colored illustrations: relative distribution of power within six frequency bands, from 20 Hz to 200 Hz ([20–50], [50–80], [80–110], [110–140], [140–170], [170–200]) Hz. The missing percentages are because frequencies of only up to 200 Hz were analyzed
Statistical analysis
Statistical analysis was performed using SPSS (IBM SPSS Statistics for Macintosh, Version 25.0). A t test to evaluate group differences for demographics, a nonparametric Mann–Whitney U (between group comparison) and a Wilcoxon test (within group comparison) for the Oxford grading and the 20-min pad test were applied. EMG data were checked for normal distribution with statistical parametric mapping (SPM). Total intensity, median frequency waveforms and time window spectra were compared between groups and pre- with post-intervention (within groups) was calculated using statistical nonparametric mapping (SnPM; SPM: spm1D 0.4, Matlab). A variance analysis was performed to compare differences between the 30 ms time windows within groups. The significance level was set at p ≤ 0.05.
Results
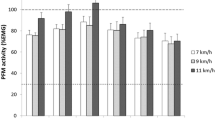
Ninety-six participants could be assigned to randomization and group allocation (EXP, n = 48; CON, n = 48). Baseline assessments of 45 (EXP) and 47 (CON) participants were completed, 4 participants were lost after randomization. After EMG plausibility check, 77 EMG data sets could be analyzed (EXP, n = 38; CON, n = 39). Table 1 depicts the demographics of the participants. There was no statistically significant between group difference, except that the Oxford grading differed pre-intervention (p = 0.010). Within groups, the Oxford grading increased significantly pre- to post-intervention in the EXP group (p < 0.001), but not in the CON group, the 20-min Pad test decreased significantly pre to post intervention in both groups (EXP, p = 0.015; CON, p = 0.005), and the ICIQ-UIsf also decreased significantly in both groups (p < 0.001; Table 1). As the sample was not normally distributed, nonparametric statistical tests were applied. The EMG analyses of intensities, median frequencies and power spectra of each time interval showed no statistically significant group differences and no difference pre- and post-intervention. A sample of the results was presented in the example of a running speed of 9 km/h (Figs. 1, 2, 3, 4, and 5). The results of the CON group pre-/post-intervention are depicted in Fig. 3, those of the EXP group pre-/post-intervention in Fig. 4 and the group comparison (CON/EXP) post-intervention in Fig. 5. However, the lowest frequency band (20–50 Hz) in the time intervals from 30 ms before to 30 ms after initial contact contained significantly less signal power than the intervals from 30 to 150 ms after initial contact for all running speeds and within both groups. In contrast, the highest frequency band (140–200 Hz) in the time intervals from 30 ms before to 30 ms after initial contact showed significantly more signal power than the time intervals from 30 to 150 ms after initial contact for all running speeds and within both groups (Figs. 1, 2, 3, 4, and 5, time windows spectra ([−30–0] and [0–30] vs [30–60], [60–90], [90–120], [120–150]) ms).
Exemplary illustration of statistical parametric mapping, control (CON) group pre- (red line)/post-intervention (blue line), 9 km/h running speed; median (interquartile range) intensity and frequency, differences pre-/post-intervention, statistical nonparametric mapping (SnPM) significance level p ≤ 0.05 displayed as dashed red lines (no significant values). Bottom median frequencies within the time intervals of 30 ms, from 30 ms before to 150 ms after initial contact, differences pre-/post-intervention, and SnPM (no significant values)
Exemplary illustration of statistical parametric mapping, EXP group pre- (red line)/post intervention (blue line), 9 km/h running speed; median (interquartile range) intensity and frequency, differences pre-/post-intervention, statistical nonparametric mapping (SnPM). significance level p ≤ 0.05 displayed as dashed red lines (no significant values). Bottom median frequencies within the time intervals of 30 ms, from 30 ms before to 150 ms after initial contact, differences pre-/post-intervention, and SnPM (no significant values)
Exemplary illustration of statistical parametric mapping (SPM), group CON post- (red line)/group EXP post-intervention (blue line), 9 km/h running speed; median (interquartile range) intensity and frequency, differences pre-/post-intervention, statistical nonparametric mapping (SnPM) significance level p ≤ 0.05 displayed as dashed red lines (no significant values). Bottom median frequencies within the time intervals of 30 ms, from 30 ms before to 150 ms after initial contact, differences pre-/post-intervention, and SnPM (no significant values)
Discussion
The purpose of this study was to evaluate the effect of a new physiotherapy program compared with a standard physiotherapy program on PFM EMG activation patterns and fiber-type recruitment behavior while running.
The Oxford grading, the 20-min pad test and the ICIQ-UIsf improved statistically significantly in both groups, whereas the change in the ICIQ-UIsf of about 3 points is considered clinically relevant [20]. However, SUI was not completely restored post-intervention. This finding was comparable with other programs, evaluated in a systematic review where in 41.2 to 95% SUI has not been completely restored after the applied PFM training [21]. However, the improvement was not represented by the EMG measurements. No statistically significant differences in median frequencies, EMG intensity, and EMG power spectra were found neither within nor between groups. A possible reason could be that the intervention phase of 16 weeks was rather short for a change of alpha-motoneuron discharge behavior and related muscle fiber recruitment, especially because the involuntary part of the training program started after 6 weeks as all participants received basic sensorimotor and behavioral training at the beginning [14]. However, a systematic review evaluating sprinting has shown an alteration of nerve conduction velocity (NCV), maximum EMG intensity, and motor unit recruitment strategies in lower extremity muscles during high-intensity sprint trainings [22]. A longitudinal study reported an increased NCV in response to 14 weeks of repeated 10-s cycling sprint trainings at 48-h intervals [23]. Training programs in sports usually are targeted and include exhausting exercises. Therefore, the training can lead to structural and physiological adjustment of the muscles, the nervous system, and the noncontractile structures such as tendons and connective tissue [24]. The challenge in the present RCT was that compared with training methods in sports, parameters such as training intensity and maximum exhaustion are not as easy applicable to PFM as to skeletal muscles of the extremities. In addition, the boundary between too much stress (see high prevalence rates among female athletes [25]) and insufficient training stimulus for PFM is difficult to assess. Therefore, the ethical consideration was to use conservative training parameters (involuntary power training, building up to 3 × 15 s running sessions and following a maximum of 5 × 12 jumps) [14]. This may have resulted in a training stimulus that was probably too low to yield a change in fiber recruitment. However, comparative values have so far been lacking. Reasons are, that training parameters are still described inconsistently or not at all, and that involuntary power training is not integrated into standard PFM training programs [3]. No change may indicate that the SUI problem in women did not get worse with jumping and running training.
However, differences in the EMG power spectra in the pre- and post-initial contact phase could be extracted. Power spectra shifts toward higher frequencies in the pre-initial contact phase could indicate a feed-forward anticipation and a muscle preparation for the expected ground reaction forces. This corroborates the findings from Constantinou and Govan [26]. These observed power shifts in the pre-initial contact phase were comparable with those in a study comparing PFM fiber-type recruitment of women with and without SUI while running [27]. Women with SUI presented relatively less intensity in high-frequency bands and more at the lower frequencies. It was supposed that the specific differences in mean median frequencies and EMG power spectra during the pre- and post-initial contact phase was due to different fiber-type recruitment [27]. In a systematic review of different EMG running studies of lower extremity muscles it was evaluated that fiber types are recruited selectively in order to adapt the contractile properties to the mechanical requirements of initial contact [28]. Depending on the muscle measured, a preparation (pre-activation) or a reaction (reflex activation) of initial contact usually takes place.
An often-discussed limitation of PFM EMG is the potential occurrence of crosstalk. Crosstalk occurs when signals from nearby muscles rather than the muscle of interest are recorded [29]. Longitudinal electrodes and a differential configuration are recommended to provide a specific representation of each side of PFM and are therefore less likely to record crosstalk [30]. As the STIMPON™ vaginal probe met these requirements, the likelihood of crosstalk was limited. A scoping review evaluating crosstalk considerations in PFM EMG studies found no study that could identify and no study that could exclude crosstalk during PFM EMG [29]. Another advantage of the STIMPON™ vaginal probe used was that the soft tampon adapted well to the individual vaginal cavities; thus, the probe was quite resistant to movement during impact activities. The data that nevertheless showed motion artifacts were identified by careful visual inspection of raw EMG data. Finally, 15 data sets (EXP, n = 7; CON, n = 8) were excluded because of motion artifacts or measurement errors.
Conclusion
Although it is known that physiotherapy is effective at treating SUI, women are often not completely cured. Training parameters should carefully be described in PFM training protocols. Training methods should maximally load PFM without overloading. The pre-activation of PFM while running can at best be used in addition to standard PFM training programs. Further analyses are needed to evaluate the specific PFM activation patterns during SUI triggering activities.
References
Dumoulin C, Hay-Smith EJ, MacHabee-Seguin G (2014) Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev (5):Cd005654.
Bo K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J Urol. 2012;30(4):437–43.
Cacciari LP, Dumoulin C, Hay-Smith EJ. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women: a Cochrane systematic review abridged republication. Braz J Phys Ther. 2019;23(2):93–107.
Baessler K, Miska K, Draths R, Schuessler B. Effects of voluntary pelvic floor contraction and relaxation on the urethral closure pressure. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(3):187–90; discussion 190–191.
Shishido K, Peng Q, Jones R, Omata S, Constantinou CE. Influence of pelvic floor muscle contraction on the profile of vaginal closure pressure in continent and stress urinary incontinent women. J Urol. 2008;179(5):1917–22.
Hay JG. Citius, altius, longius (faster, higher, longer): the biomechanics of jumping for distance. J Biomech. 1993;26(Suppl 1):7–21.
Luginbuehl H, Naeff R, Zahnd A, Baeyens JP, Kuhn A, Radlinger L. Pelvic floor muscle electromyography during different running speeds: an exploratory and reliability study. Arch Gynecol Obstet. 2016;293(1):117–24.
Leitner M, Moser H, Eichelberger P, Kuhn A, Radlinger L. Evaluation of pelvic floor muscle activity during running in continent and incontinent women: an exploratory study. Neurourol Urodyn. 2017;36(6):1570–6.
Leitner M, Moser H, Eichelberger P, Kuhn A, Radlinger L. Pelvic floor muscle activity during fast voluntary contractions in continent and incontinent women. Neurourol Urodyn. 2019;38(2):625–31.
Wakeling JM, Pascual SA, Nigg BM, von Tscharner V. Surface EMG shows distinct populations of muscle activity when measured during sustained sub-maximal exercise. Eur J Appl Physiol. 2001;86(1):40–7.
Von Tscharner V. Intensity analysis in time-frequency space of surface myoelectric signals by wavelets of specified resolution. J Electromyogr Kinesiol. 2000;10(6):433–45.
Lindstrom L, Kadefors R, Petersen I. An electromyographic index for localized muscle fatigue. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(4):750–4.
Von Tscharner V, Goepfert B. Estimation of the interplay between groups of fast and slow muscle fibers of the tibialis anterior and gastrocnemius muscle while running. J Electromyogr Kinesiol. 2006;16(2):188–97.
Luginbuehl H, Lehmann C, Baeyens J-P, Kuhn A, Radlinger L. Involuntary reflexive pelvic floor muscle training in addition to standard training versus standard training alone for women with stress urinary incontinence: study protocol for a randomized controlled trial. Trials. 2015;16:524.
Winstein CJ. Knowledge of results and motor learning—implications for physical therapy. Phys Ther. 1991;71(2):140–9.
Moser H, Leitner M, Eichelberger P, Kuhn A, Baeyens JP, Radlinger L. Pelvic floor muscle activity during jumps in continent and incontinent women: an exploratory study. Arch Gynecol Obstet. 2018;297(6):1455–63.
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361–74. https://doi.org/10.1016/s1050-6411(00)00027-4.
Von Tscharner V, Goepfert B, Nigg BM. Changes in EMG signals for the muscle tibialis anterior while running barefoot or with shoes resolved by non-linearly scaled wavelets. J Biomech. 2003;36(8):1169–76.
Fleischmann J, Gehring D, Mornieux G, Gollhofer A. Task-specific initial impact phase adjustments in lateral jumps and lateral landings. Eur J Appl Physiol. 2011;111(9):2327–37.
Nystrom E, Sjöström M, Stenlund H, Samuelsson E. ICIQ symptom and quality of life instruments measure clinically relevant improvements in women with stress urinary incontinence. Neurourol Urodyn. 2015;34(8):747–51.
Riemsma R, Hagen S, Kirschner-Hermanns R, et al. Can incontinence be cured? A systematic review of cure rates. BMC Med. 2017;15(1):63.
Ross A, Leveritt M, Riek S. Neural influences on sprint running: training adaptations and acute responses. Sports Med. 2001;31(6):409–25. https://doi.org/10.2165/00007256-200131060-00002.
Sleivert GG, Backus RD, Wenger HA. The influence of a strength-sprint training sequence on multi-joint power output. Med Sci Sports Exerc. 1995;27(12):1655–65.
Hartmann H, Wirth K, Keiner M, Mickel C, Sander A, Szilvas E. Short-term periodization models: effects on strength and speed-strength performance. Sports Med. 2015;45(10):1373–86. https://doi.org/10.1007/s40279-015-0355-2.
Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311–6. https://doi.org/10.1001/jama.300.11.1311.
Constantinou CE, Govan DE. Spatial distribution and timing of transmitted and reflexly generated urethral pressures in healthy women. J Urol. 1982;127(5):964–9.
Koenig I, Eichelberger P, Leitner M, et al. Pelvic floor muscle activity patterns in women with and without stress urinary incontinence while running. Ann Phys Rehabil Med. 2019;13(19):30160–5.
Koenig I, Eichelberger P, Blasimann A, Hauswirth A, Baeyens JP, Radlinger L. Wavelet analyses of electromyographic signals derived from lower extremity muscles while walking or running: a systematic review. PLoS One. 2018;13(11):e0206549.
Flury N, Koenig I, Radlinger L. Crosstalk considerations in studies evaluating pelvic floor muscles using surface electromyography in women: a scoping review. Arch Gynecol Obstet. 2017;295(4):799–809.
Keshwani N, McLean L. State of the art review: intravaginal probes for recording electromyography from the pelvic floor muscles. Neurourol Urodyn. 2013;34(2):104–12.
Authors’ contribution to the manuscript
All listed authors have made the required substantive intellectual contributions and meet the criteria for authorship. I. Koenig: project development, conception, and design, data extraction and analyses, manuscript writing, article revision, final approval; P. Eichelberger: data analyses, article revision, final approval; H. Luginbuehl: project development, data collection, article revision, final approval; A. Kuhn: project development, data collection, article revision, and final approval; C. Lehmann: project development, article revision, and final approval; J. Taeymans: manuscript writing, article revision, and final approval; L. Radlinger: project development, conception, and design, data analyses, manuscript writing, article revision, and final approval.
Funding
The RCT was funded by the Swiss National Science Foundation, Division III (Medicine & Biology; 320030_153424/1). The granting organization was not involved in the development and conduction of the present study.
The protocol was registered at Clinical Trials.gov (NCT02318251).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of interest
None.
Ethics
Ethics committee approval (Ethics Committee of the Canton of Bern, reference number 249/14), in accordance with the Declaration of Helsinki and the Swiss Human Research Act, and written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koenig, I., Eichelberger, P., Luginbuehl, H. et al. Activation patterns of pelvic floor muscles in women with incontinence while running: a randomized controlled trial. Int Urogynecol J 32, 335–343 (2021). https://doi.org/10.1007/s00192-020-04334-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-020-04334-0