Abstract
Introduction and hypothesis
The objective was to investigate the incidence and risk factors of postoperative de novo stress urinary incontinence (SUI) in stress-continent women following minimally invasive sacrocolpopexy without an anti-incontinence procedure.
Methods
We completed a multicenter, retrospective cohort study of women undergoing laparoscopic sacrocolpopexy without concurrent anti-incontinence procedures from October 2006 through January 2021.
Results
Of the 169 women who underwent minimally invasive sacrocolpopexy, 17.1% (n=30) developed de novo SUI, and 7.1% eventually underwent a midurethral sling placement. On logistic regression, BMI, preoperative urinary urgency, and history of transvaginal mesh repair were found to be significantly associated with and predictive of de novo SUI. When the concordance index (C-index) was calculated with the model published by Jelovsek et al. for women who developed de novo SUI within 12 months of the prolapse surgery, the current de novo SUI calculator was able to discriminate de novo SUI outcome (C-index = 0.71).
Conclusions
The incidence of de novo SUI after minimally invasive sacrocolpopexy without anti-incontinence procedure correlates directly with higher BMI, preoperative urinary urgency, and transvaginal mesh history for POP. Preoperative counseling for minimally invasive sacrocolpopexy should include discussing the risk of de novo SUI and preoperative factors that may increase this risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The lifetime risk of undergoing surgery for pelvic organ prolapse or stress urinary incontinence (SUI) is 11.1% [1]. Surgery for pelvic organ prolapse can unmask SUI in women without symptoms or worsen existing SUI [2,3,4]. Although women with preoperative symptoms of SUI or occult SUI are known to carry an increased risk of persistent or worsening postoperative SUI, the risk of developing de novo SUI for those who are stress-continent preoperatively is less well studied. [5,6,7,8]. Prophylactic surgical treatment for SUI in patients undergoing pelvic organ prolapse repair without symptoms of SUI is controversial. The options for this clinical scenario include universal, selective, or a staged treatment approach [4, 9,10,11,12,13]. Even though universally performing concomitant prophylactic incontinence surgery on all women undergoing pelvic organ prolapse repair has been shown to reduce the need for future SUI procedures, it is debated whether the benefit outweighs the risk of complications from an anti-incontinence procedure. Multiple randomized clinical trials have also demonstrated that 5–17% of women with de novo SUI elect to undergo an anti-incontinence procedure [10, 13, 14]. Surgeons must balance the risk and benefits of these approaches and involve patients in shared decision-making.
To aid in the shared decision-making process, several strategies have been developed. These include optimizing the sensitivity of preoperative prolapse reduction stress testing [4, 15], as well as using a preoperative incontinence risk calculator, which has been developed and externally validated using the two large clinical trials from the Pelvic Floor Disorders Network, the Outcomes Following Vaginal Prolapse Repair and Mid Urethral Sling (OPUS) and the Colpopexy and Urinary Reduction Efforts (CARE) trials [16]. However, although the CARE study included abdominal sacrocolpopexy, neither study included information from minimally invasive sacrocolpopexy, and the clinical reliability of the use of this calculator in this population is in question [17].
In this study, we define de novo SUI as postoperative SUI in previously stress-continent women with no reported symptoms of SUI and a negative preoperative stress test. The reported incidence of de novo SUI varies widely between 4 and 58% [2, 3, 10, 18,19,20,21,22]. The variability in the incidence of de novo SUI may be due to the difference in the ability to evaluate for occult SUI [4, 15] and the heterogeneity in the definition of de novo SUI [19, 23].
The primary objective of this study was to determine the incidence of de novo SUI in women undergoing laparoscopic sacrocolpopexy without concomitant anti-incontinent surgery in stress-continent women. Secondarily, we sought to identify potentially predictive preoperative clinical factors associated with the development of de novo SUI and test the predictability of the de novo SUI calculator developed by Jelovsek et al. using minimally invasive sacrocolpopexy data [16].
Materials and methods
We performed a multicenter, retrospective cohort study of stress continent women who underwent minimally invasive abdominal sacrocolpopexy with synthetic mesh without a concomitant anti-incontinence procedure from October 2006 through January 2021. The chart review included information obtained from the Research Patient Data Registry, a centralized clinical data registry that gathers clinical information from various hospitals, including Massachusetts General Hospital, Brigham and Women's Hospital, and other smaller academic and community hospitals in the Mass General Brigham health system [24]. Women were included if they underwent a minimally invasive abdominal sacrocolpopexy (laparoscopic or robot assisted). Patients were excluded if they had a prior history or concurrent anti-incontinence procedures for SUI. Patients were asked to answer the question of whether they have “leakage related to physical activity, coughing, or sneezing,” which is standard among all surgeons. Stress continence was defined as having no symptoms of SUI subjectively and objectively negative prolapse reduction testing (preoperative cough stress test or urodynamic testing). Eligible women were identified using the CPT code for laparoscopic sacrocolpopexy (57425) without a surgical procedure for SUI, and a chart review was performed to ensure eligibility. The study protocol was reviewed and exempted by the institutional review board (2021P000305).
To estimate the incidence and identify risk factors for de novo SUI, we defined the primary outcome of de novo SUI as newly developed postoperative SUI subjectively by symptoms and/or a positive cough stress test in the office or in urodynamic evaluations. No validated questionnaire was used for the postoperative evaluation. The secondary outcome was postoperative treatments for de novo SUI, categorized as surgical if the women ultimately underwent surgical treatment such as midurethral sling versus nonsurgical if the women did not seek treatment or elected for pelvic floor physical therapy. No data are available on whether periurethral bulking injection or treatment with a disposable incontinence device was offered to the patient. Demographics, as well as preoperative, intraoperative, and postoperative data, were gathered from the patient’s medical record. For the incontinence outcome, information was collected from all available electronic medical records between the time of minimally invasive sacrocolpopexy and the last visit recorded on the patient’s chart. Any self-report or new diagnoses of SUI after the index operation were marked as de novo SUI. The individualized prediction model for de novo SUI after vaginal prolapse surgery was utilized for the analysis of the correlation between minimally invasive sacrocolpopexy and de novo SUI [16].
Categorical data were compared using Chi-squared or Fisher’s exact tests and presented as frequency (proportion). Parametric continuous data were compared using Student’s t test and presented as means and standard deviations. Nonparametric continuous data were compared using Wilcoxon rank-sum test and presented as median and interquartile ranges. All tests except for the de novo SUI treatment group were two-sided. A p value <0.05 was considered statistically significant. Multivariate logistic regression was performed to examine the potential risk factors associated with de novo SUI, controlling for factors decided a priori, including age, body mass index (BMI), preoperative urinary urgency, history of transvaginal mesh to treat pelvic organ prolapse, and the Ba point of Pelvic Organ Prolapse Quantification (POP-Q). No vaginal mesh removal was performed at the time of the minimally invasive sacrocolpopexy. Because diabetes correlated highly with BMI, it was not used simultaneously in multivariate analysis because of collinearity concerns. Similarly, only Ba from the POP-Q measurements was used in the final modeling because of its known impact on de novo SUI [12, 23]. To test the predictive accuracy of the de novo SUI calculator, the concordance index (C-index) was calculated using receiver-operating characteristic analysis with the same elements of the multivariate logistic regression model published by Jelovsek et al. [16, 25] Data were stored using Research Electronic Data Capture (RedCap) [26, 27] and analyzed using Stata/IC 15 (Statacorp, College Station, TX, USA).
Results
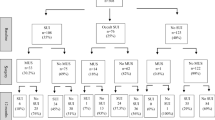
A total of 1,161 minimally invasive sacrocolpopexies were performed from October 2006 through January 2021. During the study period, 169 stress-continent women underwent a minimally invasive sacrocolpopexy without a concomitant or prior anti-incontinence procedure for SUI. The majority were white non-Hispanic women (94.1%) with a mean age of 59 (± 8.2 years). The average length of follow-up was 6.7 years ± 35 months. We defined the last day of their follow-up as the day that the patient was seen by any provider within the health system who addressed symptoms of SUI. All women either had a negative preoperative prolapse reduction cough stress test (57.3%) and/or no findings of SUI on multichannel urodynamic testing with prolapse reduction (83.4%). One hundred and twelve women (66.2%) underwent a laparoscopic sacrocolpopexy, and 57 women (33.7%) underwent a robot-assisted sacrocolpopexy. Ninety-nine women (58.6%) had had a prior hysterectomy, 57 (33.7%) underwent a concurrent supracervical hysterectomy, and 11 women (6.5%) had a total laparoscopic hysterectomy (Table 1). Two women had a sacrohysteropexy. Thirty women (17.8%) underwent anterior colporrhaphy, 48 (28.4%) had a posterior colporrhaphy, and 24 (14.2%) had both procedures.
Within the follow-up period, 9 (5.3%) women had a vaginal mesh exposure. Five women were diagnosed with mesh exposure within 12 months of surgery. Perioperative complications within 30 days of operation include 1 woman with an intraoperative ureteral injury, 1 cystotomy, 1 woman who had immediate postoperative hemorrhage reoperation and blood transfusion, and 3 women needing treatment for surgical site infections treated with oral antibiotics.
During the study period, 30 women (17.8%) developed de novo SUI following minimally invasive sacrocolpopexy (Table 1). Twenty women (66.7%) were diagnosed after developing bothersome SUI symptoms, and 10 (33.3%) demonstrated objective SUI on a cough stress test or urodynamic testing (Table 2). Of the 30 women who developed de novo SUI, more than half of these (n=20) developed SUI symptoms within 6 months of surgery. Among women who developed de novo postoperative SUI, 13 (43.33%) selected expectant management, 9 (30%) chose to pursue pelvic floor physical therapy, and the remaining 12 women (40%) underwent a midurethral sling. Among this group, a total of 4 women opted for a combination of pelvic floor physical therapy and a midurethral sling. There were no women who elected for a pessary. No Burch colposuspension was performed in these patients during the study period. Women who underwent a midurethral sling were more likely to have preoperative detrusor overactivity on urodynamics and objective postoperative de novo SUI compared with the nonsurgical group (p=0.05 and p<0.001 respectively; Table 2). However, on univariate analysis, only objective postoperative de novo SUI was significantly associated with undergoing a midurethral sling (OR 76, 95% CI 6.0–962, p=0.001).
Compared with women who remained stress continent, women who developed de novo SUI had a significantly higher preoperative BMI (29.6 ± 7.2 vs 25.8 ± 4.2, p<0.001), higher total and vaginal parity (p<0.04), and preoperative urinary urgency (p=0.02; Table 1). Furthermore, more women in the de novo SUI group (13.3%) had a history of transvaginal mesh prolapse surgery than the stress-continent women (2.2%; p<0.005). Otherwise, the two groups were similar regarding age, preoperative pelvic organ prolapse stage, and multichannel urodynamic testing parameters, including preoperative maximum flow rate, maximum cystometric capacity, maximum urethral closure pressure, and presence of detrusor overactivity (all p>0.05; Table 1).
On univariate analysis, preoperative BMI, urinary urgency symptoms, and prior transvaginal mesh prolapse surgery were associated with a higher risk of de novo SUI following minimally invasive sacrocolpopexy. Multivariate logistic regression analysis confirmed that BMI (aOR 1.13; 95% CI 1.05–1.20), preoperative urinary urgency (aOR 2.82; 95% CI 1.10–7.19), and prior transvaginal mesh surgery (aOR 8.92; 95% CI 1.50–52.78) were independent predictors of the development of de novo SUI (Table 3).
We tested the functionality of the online de novo SUI calculator developed and published by Jelovsek et al. [16], using the receiver-operating characteristic analysis from the multivariate logistic regression models built from the same covariate element of the online calculator. In women who developed de novo SUI during the first 12 postoperative months, the analysis showed the C-index of 0.71 (p=0.07), which is moderate discriminatory ability per the classification of the C-index used in Ross et al. [17] for the entirety of our cohort of women who developed de novo SUI at any point during their follow-up, the C-index was 0.69 (p=0.004). The mean predicted risk of developing de novo SUI was higher in women who developed de novo SUI following minimally invasive sacrocolpopexy (0.42 ± 0.02) than in those who remained stress continent (0.35 ± 0.01, p=0.003).
Discussion
To our knowledge, this is the largest study investigating the incidence of de novo SUI in stress-continent women both by symptom and by objective evaluation undergoing minimally invasive sacrocolpopexy. In our study, the incidence of de novo SUI in previously stress continent women following minimally invasive sacrocolpopexy was 17.7%, and 40% of these women underwent a midurethral sling. Our findings are supported by two small retrospective cohort studies. In a retrospective analysis of 15 women with no subjective or objective findings of SUI who underwent a laparoscopic sacrocolpopexy or sacrohysteropexy, the incidence of de novo SUI was 13.3% [19]. Additionally, Leclaire and colleagues in a retrospective cohort study of 77 women reported a 15% rate of de novo SUI after a laparoscopic sacrocolpopexy compared with 45% in women who underwent an abdominal sacrocolpopexy [20].
There have been two large multicenter randomized controlled trials that evaluated the incidence of postoperative SUI following pelvic organ prolapse repairs, the Colpopexy and Urinary Reduction Efforts (CARE) trial and the Outcomes Following Vaginal Prolapse Repair and Midurethral Sling (OPUS) trial [10, 14]. In both studies, women without symptomatic SUI were randomized to apical prolapse repair with or without concomitant anti-incontinence procedure regardless of a positive or negative prolapse reduction stress test—abdominal sacrocolpopexy with or without concomitant Burch in the CARE trial and vaginal apical repair with or without midurethral sling in the OPUS trial [10, 14]. In the CARE trial, the rate of postoperative SUI was significantly higher in women who did not have a Burch than in those who did, 57.4% and 33.6% (p<0.001) respectively [10]. In the subset of women in the CARE trial who had a negative prolapse reduction stress test prior to abdominal sacrocolpopexy, the incidence of de novo SUI was 38%, which is more than double the incidence we found in women undergoing a minimally invasive sacrocolpopexy [10]. Similarly, in the OPUS trial, 38% of women with a negative prolapse reduction stress test developed postoperative SUI [10, 14]. The online de novo SUI calculator by Jelovsek et al. [16] was designed and validated from the CARE and OPUS trial data and did not include data from minimally invasive sacrocolpopexy. This model has also been validated in a Dutch population undergoing vaginal surgery [28]. Ross et al. generated a receiver operating curve using the regression model created by the de novo SUI calculator from 428 women who underwent a minimally invasive sacrocolpopexy [17]. In this study, they concluded that the calculator was unable to reliably predict postoperative SUI when the apical suspension was performed without a sling, but noted that they relied on a nonvalidated patient-reported symptom survey to determine postoperative stress continence [17]. However, when we applied our data to this calculator, the model was able to maintain the moderate discriminatory ability for the continence outcome with a C-index comparable with the previously published C-index of 0.73 and 0.62 for the OPUS and CARE trial data respectively [16, 17]. In our study, an important consideration is that the de novo SUI model appears to have a moderate discriminatory ability. Still, it is unclear whether it is appropriately calibrated in this laparoscopic sacrocolpopexy population. Future studies with a larger number of patients may shed light and help to answer this question.
Our study found that a higher BMI, preoperative urinary urgency symptoms, and a history of transvaginal mesh prolapse surgery were risk factors for developing de novo SUI. The association of BMI, preoperative urinary urgency and de novo SUI has been reported in both the OPUS and the CARE trial [10, 11]. However, the history of transvaginal mesh prolapse surgery as a risk factor for de novo SUI after minimally invasive sacrocolpopexy is unique. We hypothesize that in a woman who undergoes a sacrocolpopexy after transvaginal mesh, additional tensioning may be placed on the anterior vaginal wall causing over-tension of the urethrovesical angle. This over-tension might not be exactly seen in native-tissue anterior vaginal wall repairs. It is worthwhile mentioning that the confidence interval for prior transvaginal mesh use is wide, likely as a result of the small sample size.
Strengths of this present study include that it is the largest retrospective cohort study investigating the incidence of de novo SUI in women undergoing minimally invasive sacrocolpopexy. Additionally, the long-term mean follow-up time of 81 months allowed us to capture additional cases of de novo SUI. The limitations included those inherent to the retrospective design, including only being able to assess symptoms and findings documented in medical charts. Specifically for this study, there was variability in preoperative evaluation and the diagnosis of preoperative SUI, which could negatively impact generalizability of the study. Additionally, the liberal use of the definition of postoperative SUI without a validated questionnaire or standardized examination could have led to over-reporting of the incidence. However, our observed rate of SUI following colpopexy is less than previously reported in the literature. Finally, although our study is multicenter, which can certainly help the generalizability of our findings, we acknowledge the lack of racial diversity, as our study population consisted of primarily white women to reflect the patient population seen in our practice and our data were taken from a center specializing in the treatment of pelvic floor disorders in the New England area.
In the present study, the incidence of de novo SUI after laparoscopic sacrocolpopexy without concomitant anti-incontinence procedure was approximately 1 in 5, which is lower than reported in stress-continent women undergoing abdominal sacrocolpopexy and vaginal pelvic organ prolapse surgery. Understanding these risk factors will guide future research about the development of de novo SUI after vaginal apical suspension.
In conclusion, the development of de novo SUI after undergoing surgery for pelvic organ prolapse can be distressing for both the patient and the surgeon. A higher BMI, preoperative symptoms of urinary urgency, and a prior history of transvaginal mesh repair were identified as risk factors for developing de novo SUI following minimally invasive abdominal sacrocolpopexy. Refining the de novo SUI calculator to include laparoscopic sacrocolpopexy will aid preoperative counseling and guide clinical management.
References
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–6. https://doi.org/10.1016/S0029-7844(97)00058-6.
Ennemoser S, Schönfeld M, von Bodungen V, Dian D, Friese K, Jundt K. Clinical relevance of occult stress urinary incontinence (OSUI) following vaginal prolapse surgery: long-term follow-up. Int Urogynecol J. 2012;23(7):851–5. https://doi.org/10.1007/s00192-012-1765-4.
Park J, McDermott CD, Terry CL, Bump RC, Woodman PJ, Hale DS. Use of preoperative prolapse reduction stress testing and the risk of a second surgery for urinary symptoms following laparoscopic sacral colpoperineopexy. Int Urogynecol J. 2012;23(7):857–64. https://doi.org/10.1007/s00192-011-1648-0.
Visco AG, Brubaker L, Nygaard I, et al. The role of preoperative urodynamic testing in stress-continent women undergoing sacrocolpopexy: the Colpopexy and Urinary Reduction Efforts (CARE) randomized surgical trial. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(5):607–14. https://doi.org/10.1007/s00192-007-0498-2.
Han EJ, Kim SR, Kim SK, Bai SW. Comparison of midurethral sling outcomes with and without concomitant prolapse repair. Obstet Gynecol Sci. 2014;57(1):50–8. https://doi.org/10.5468/ogs.2014.57.1.50.
Ellerkmann RM, Cundiff GW, Melick CF, Nihira MA, Leffler K, Bent AE. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185(6):1332–7; discussion 1337–8. https://doi.org/10.1067/mob.2001.119078.
Gutman RE, Ford DE, Quiroz LH, Shippey SH, Handa VL. Is there a pelvic organ prolapse threshold that predicts pelvic floor symptoms? Am J Obstet Gynecol. 2008;199(6):683.e1–7. https://doi.org/10.1016/j.ajog.2008.07.028.
Swift S, Woodman P, O’Boyle A, et al. Pelvic Organ Support Study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol. 2005;192(3):795–806. https://doi.org/10.1016/j.ajog.2004.10.602.
Borstad E, Abdelnoor M, Staff AC, Kulseng-Hanssen S. Surgical strategies for women with pelvic organ prolapse and urinary stress incontinence. Int Urogynecol J. 2010;21(2):179–86. https://doi.org/10.1007/s00192-009-1007-6.
Brubaker L, Cundiff GW, Fine P, et al. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med. 2006;354(15):1557–66. https://doi.org/10.1056/NEJMoa054208.
Wei J, Nygaard I, Richter H, et al. Outcomes following vaginal prolapse repair and mid urethral sling (OPUS) trial—design and methods. Clin Trials. 2009;6(2):162–71. https://doi.org/10.1177/1740774509102605.
Lo TS, Bt Karim N, Nawawi EA, Wu PY, Nusee Z. Predictors for de novo stress urinary incontinence following extensive pelvic reconstructive surgery. Int Urogynecol J. 2015;26(9):1313–9. https://doi.org/10.1007/s00192-015-2685-x.
Van der Ploeg JM, van der Steen A, Zwolsman S, van der Vaart CH, Roovers J. Prolapse surgery with or without incontinence procedure: a systematic review and meta-analysis. BJOG Int J Obstet Gynaecol. 2018;125(3):289–97. https://doi.org/10.1111/1471-0528.14943.
Wei JT, Nygaard I, Richter HE, et al. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366(25):2358–67. https://doi.org/10.1056/NEJMoa1111967.
Svenningsen R, Borstad E, Spydslaug AE, Sandvik L, Staff AC. Occult incontinence as predictor for postoperative stress urinary incontinence following pelvic organ prolapse surgery. Int Urogynecol J. 2012;23(7):843–9. https://doi.org/10.1007/s00192-012-1764-5.
Jelovsek JE, Chagin K, Brubaker L, et al. A model for predicting the risk of de novo stress urinary incontinence in women undergoing pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(2 Pt 1):279–87. https://doi.org/10.1097/AOG.0000000000000094.
Ross JH, Carter-Brooks CM, Ruppert KM, Giugale LE, Shepherd JP, Zyczynski HM. Assessing the performance of the de novo postoperative stress urinary incontinence calculator. Female Pelvic Med Reconstr Surg. 2021;27(1):23–7. https://doi.org/10.1097/SPV.0000000000000717.
Leruth J, Fillet M, Waltregny D. Incidence and risk factors of postoperative stress urinary incontinence following laparoscopic sacrocolpopexy in patients with negative preoperative prolapse reduction stress testing. Int Urogynecol J. 2013;24(3):485–91. https://doi.org/10.1007/s00192-012-1888-7.
Alas AN, Chinthakanan O, Espaillat L, Plowright L, Davila GW, Aguilar VC. De novo stress urinary incontinence after pelvic organ prolapse surgery in women without occult incontinence. Int Urogynecol J. 2017;28(4):583–90. https://doi.org/10.1007/s00192-016-3149-7.
LeClaire EL, Mukati MS, Juarez D, White D, Quiroz LH. Is de novo stress incontinence after sacrocolpopexy related to anatomical changes and surgical approach? Int Urogynecol J. 2014;25(9):1201–6. https://doi.org/10.1007/s00192-014-2366-1.
Wille S, Braun M, Heidenreich A, Hofmann R, Engelmann U. Sacral colpopexy with concurrent Burch colposuspension in patients with vaginal vault prolapse. Urol Int. 2006;76(4):339–44. https://doi.org/10.1159/000092059.
Klutke JJ, Ramos S. Urodynamic outcome after surgery for severe prolapse and potential stress incontinence. Am J Obstet Gynecol. 2000;182(6):1378–81. https://doi.org/10.1067/mob.2000.106176.
Davenport MT, Sokol ER, Comiter CV, Elliott CS. Does the degree of cystocele predict de novo stress urinary incontinence after prolapse repair? Further analysis of the colpopexy and urinary reduction efforts trial. Female Pelvic Med Reconstr Surg. 2018;24(4):292–4. https://doi.org/10.1097/SPV.0000000000000487.
Wattanasin N, Peng Z, Raine C, Mitchell M, Wang C, Murphy SN. E-facts: business process management in clinical data repositories. AMIA Annu Symp Proc AMIA Symp. Published online 6 November 2008:1170.
Vickers AJ, Cronin AM. Everything you always wanted to know about evaluating prediction models (but were too afraid to ask). Urology. 2010;76(6):1298–301. https://doi.org/10.1016/j.urology.2010.06.019.
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. https://doi.org/10.1016/j.jbi.2019.103208.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010.
Jelovsek JE, van der Ploeg JM, Roovers JP, Barber MD. Validation of a model predicting de novo stress urinary incontinence in women undergoing pelvic organ prolapse surgery. Obstet Gynecol. 2019;133(4):683–90. https://doi.org/10.1097/AOG.0000000000003158.
Author information
Authors and Affiliations
Contributions
Youngwu Kim: study design and data gathering, statistical analysis, manuscript writing; Jennifer Rowley: study design and data gathering, manuscript writing; Marcus Ortega: study design and data gathering, and manuscript writing; Kaitlyn James: study design, statistical analysis, and manuscript writing; Emily Von Bargen: study design and manuscript writing.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, Y., Rowley, J.E., Ortega, M.V. et al. Incidence of de novo stress urinary incontinence following minimally invasive sacrocolpopexy. Int Urogynecol J 34, 1599–1605 (2023). https://doi.org/10.1007/s00192-022-05434-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-022-05434-9