Abstract
Introduction and hypothesis
To test in vitro and in vivo the capability of mesh materials to act as scaffolds for rat-derived mesenchymal stem cells (rMSCs) and to compare inflammatory response and collagen characteristics of implant materials, either seeded or not with rMSCs.
Methods
rMSCs isolated from rat bone marrow were seeded and cultured in vitro on four different implant materials. Implants showing the best rMSC proliferation rate were selected for the in vivo experiment. Forty-eight adult female Sprague–Dawley rats were randomly divided into two treatment groups. The implant of interest—either seeded or not with rMSCs—was laid and fixed over the muscular abdominal wall. Main outcome measures were: in vitro, proliferation of rMSCs on selected materials; in vivo, the occurrence of topical complications, the evaluation of systemic and local inflammatory response and examination of the biomechanical properties of explants.
Results
Surgisis and Pelvitex displayed the best cell growth in vitro. At 90 days in the rat model, rMSCs were related to a lower count of neutrophil cells for Pelvitex and a greater organisation and collagen amount for Surgisis. At 7 days Surgisis samples seeded with rMSCs displayed higher breaking force and stiffness.
Conclusions
The presence of rMSCs reduced the systemic inflammatory response on synthetic implants and improved collagen characteristics at the interface between biological grafts and native tissues. rMSCs enhanced the stripping force on biological explants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse (POP) is one of the most frequently occurring medical conditions in an aging population. About 50 % of women will develop POP, with a lifetime prevalence of 30–50 % [1, 2]. Surgical repair of fascial defects using native tissues is the mainstay of therapy, but since native tissues are by definition qualitatively weak, the failure rate is high. It has been estimated that about 29 % of women having undergone surgery for prolapse will require re-intervention within 1.5–12.5 years [3]. In an effort to reduce recurrence after pelvic reconstructive surgery with native tissues, synthetic or naturally-derived implant materials have increasingly been used. The aim of using meshes in pelvic reconstructive surgery is to reinforce weak supporting tissues and induce new growth of supporting tissue [4]. Although non-biodegradable synthetic polymers are widely used because of their superiority in terms of anatomical and biomechanical outcomes, they are associated with graft-related complications including infection, fibrosis, exposure and shrinkage [5, 6]. Biological materials have been introduced as an alternative because of their higher biocompatibility. Biological implants include autologous grafts, allografts and xenografts [7]. Bio-grafts, however, have potential limitations, including their limited availability, low mechanical strength and unpredictable behaviour in the medium and long term [5, 6].
Mesenchymal stem cells (MSCs) are an available cell source for tissue engineering, in particular for applications in skeletal and hard tissue repair [8]. MSCs possess multipotent differentiation capabilities and low immunogenicity, in addition to the fact that they can easily be harvested from multiple tissues (such as bone marrow and adipose tissue) and expanded in vitro. All these features make MSCs an attractive tool for tissue engineering applications [9]. An increasing number of biomaterials have been proposed as scaffolds for tissue regeneration [10]. The presence of stem cells on a prosthetic material surface can modulate the inflammatory response and promote a weaker foreign body reaction when implanted in vivo [11]. On collagen-based meshes, stem cells have the potential to decrease the degradation rate of the substrate [12].
The aims of this study were:
-
1.
To evaluate in vitro materials currently used in pelvic floor surgery and to select the best scaffolds for growth of MSCs
-
2.
To compare, in a rat model, the inflammatory response, collagen characteristics and mechanical properties of implant materials that are either seeded or not with homologous rat MSCs (rMSCs)
Materials and methods
Biomaterials tested
Four different commercially available implant materials were investigated:
-
1.
Non-cross-linked porcine small intestine submucosa (SIS), marketed as four-layer Surgisis ES (Cook Ireland, Limerick, Ireland). SIS contains collagen types I, III and V and growth factors such as transforming growth factor-β (TGF-β) and fibroblast growth factor 2 (FGF-2) [13].
-
2.
Cross-linked porcine acellular dermal collagen matrix, marketed as Pelvicol (Bard, Rome, Italy). Cross-linking by hexamethylene di-isocyanate (HMDI) prevents collagenolytic enzyme degradation. Pelvicol also contains elastic fibres, while cellular components are removed during manufacturing [14].
-
3.
Synthetic non-biodegradable polypropylene (PP) mesh marketed as Gynemesh PS (Ethicon, Rome, Italy). Gynemesh PS is a PP Amid type I material (45 g/m2, pore size 2.4 mm).
-
4.
Hybrid collagen-coated polypropylene (PP) mesh marketed as Pelvitex (Bard). Pelvitex is a PP-derived Amid type I implant material (38 g/m2, pore size 1.7 mm) coated with a biodegradable, hydrophilic film of porcine collagen [15]. The collagen film is reabsorbed within 2 weeks [16].
Cell preparation and seeding on scaffolds
All materials were provided in a sterile condition by the manufacturer. Materials tested as scaffolds for in vitro study were cut into square samples measuring 4.0 × 4.0 mm and placed in 24-well cell culture plates under sterile conditions. Heterologous MSCs were obtained from donator rats’ bone marrow as previously described [17]. Cells were cultured in Eagle’s alpha minimum essential medium containing 2 mM of L-glutamine, 100 U/ml of penicillin, 100 mg/ml of streptomycin and 250 mg/ml of Fungizone (BioWhittaker, Bergamo, Italy) plus 20 % defined foetal bovine serum (Hyclone, Logan, UT, USA). rMSCs were suspended at a concentration of 5 × 106 cells/ml and 106 cells were poured onto each scaffold through pipetting. In addition, drop seeding with a 25-gauge needle was performed for SIS and Pelvicol because of their sponge-like texture. After 4 h at 37 °C the medium was replaced with fresh medium and the scaffolds were maintained in a humidified atmosphere at 37 °C with 5 % CO2 in air. The medium was changed every 3–4 days. For each time point, an unseeded scaffold incubated with culture medium alone was used as a control. Scaffolds used for in vivo implantation were cut into square samples measuring 20 × 20 mm samples and seeded in analogous conditions with 25 × 106 cells each for 7 days before implantation in the animals.
Staining and microscopic analysis
Specimens derived from Surgisis ES and Pelvicol were washed twice with phosphate buffered saline, fixed with 4 % paraformaldehyde for 1 h at room temperature, embedded in paraffin with standard methods and cut into 7-μm sections. Sections were deparaffinised with xylene, hydrated with 70 % alcohol and stained according to standard haematoxylin/eosin (H&E) staining protocols and mounted in permanent medium (Permanent DPX Mountant for histology; Sigma-Aldrich).
rMSC seeded scaffold sections derived from Gynemesh PS and Pelvitex were incubated for 1 h with culture medium containing the fluorescent dye DiI (30 μg/ml) before being fixed with 4 % paraformaldehyde for 1 h at room temperature, washed with phosphate buffered saline and mounted. Samples were analysed using confocal fluorescent microscopy (Radiance 2100; Biorad Laboratories, Hercules, CA, USA).
The aforementioned histological analyses were performed at days 7, 14 and 21 after culture. Microscopic analysis for cell growth was performed semi-quantitatively. The proliferation rate was classified as high, intermediate or low. Furthermore, cellular viability was evaluated by studying cell and nuclear morphology and in cases where there were signs of degeneration.
Experimental surgery
Adult female Sprague–Dawley rats (Harlan, Udine, Italy) were used in the animal study. Animals were housed in the animal facility of the Faculty of Medicine of the University of Milano-Bicocca, Monza, Italy, and were treated in accordance with current national guidelines on animal welfare. The study was approved by the Ethics Committee for Animal Experimentation of the Faculty of Medicine of the University of Milano-Bicocca. The rats were randomly divided into two treatment groups of 24 rats each to receive either Pelvitex or Surgisis implants, either seeded (PS and SS groups, 12 animals in each) or not (PN and SN groups, 12 animals in each) with rMSCs. The rats were anaesthetised with 2.5 % isoflurane mask inhalation with oxygen (0.5 L/min). The abdomen was shaved, disinfected with povidone iodine (Betadine; Meda Pharma, Milan, Italy) and covered with sterile draping. A vertical midline skin incision was made and skin flaps were raised.
The implant of interest was laid over the right side of the abdominal wall. It was fixed without tension to the muscular layer using four non-absorbable polypropylene 4/0 stitches (Prolene, Ethicon) at its four corners. Finally, subcutaneous tissues and skin were closed using interrupted absorbable 3/0 polyglactin (Vicryl, Ethicon).
Following recovery, the rats were returned to their cages with free access to food and drink. Animals were clinically checked weekly for local and systemic complications. Samples of venous blood were collected from the tail-vein of each animal just before euthanasia and they were analysed for haemochromocytometric values. From each group, 6 rats were sacrificed by CO2 overdose after 7 and 90 days respectively. During necropsy, the presence of fluid collection, infection and erosion or other signs of rejection were noted. Then, almost the entire anterior abdominal wall was resected en bloc, including the initial implant, the interface, a 1-cm border of neighbouring native tissue (the explant), and the left side of abdominal wall, which served as an internal control (for histological analysis only). Explants and control specimens were then cut into one sample measuring 10 × 10 mm to be fixed in 4 % paraformaldehyde and one strip measuring 15 × 5 mm, perpendicular to the longest axis of the animal, to be stored in normal saline solution on ice for mechanical tensile tests, which were performed no later than 10 h after sacrifice (Fig. 1).
Mechanical properties
Tensile mechanical tests were performed using a dynamic mechanical analyser mod. 2980 (TA Instruments, New Castle, DE, USA) at 37 °C, a preload of 0.2 N and a speed of 1 N/min up to the separation of the mesh from the abdominal wall. Strips were prepared for mechanical testing. First, stitches fixing the mesh to the abdominal wall were removed from the animal. Then, the muscular layer of the abdominal wall was dissected for an extension of 5 mm from the cranial border of the mesh. Resulting strips—5 mm wide and 15 mm long—consisted of three parts: 5 mm of mesh and neoformed fibrotic tissue over the mesh, 5 mm of mesh on the abdominal wall and 5 mm of the abdominal wall only. Specimens were clamped into the upper grip with the mesh side and into the lower one with the abdominal wall side. In the case of partial or complete reabsorption of the biological mesh, the fibrotic tissue over the mesh site was clamped into the upper grip. The gauge length was set at 8 mm. Force versus elongation curve was plotted for each tested specimen; maximum force (Fmax) and the corresponding elongation (Δlmax) before the separation of the mesh from the abdominal wall, stiffness (S), and the secant moduli at 30 and 50 % elongation (S30%, S50%) were considered to be the mechanical parameters.
Histology
Paraformaldehyde-fixed explant specimens were embedded in paraffin and cut into slices 5 μm thick in a longitudinal fashion, so that each slice would contain the implant, with the interface and the surrounding native tissue. Sections were stained with H&E and Masson’s trichrome stain. The latter stains keratin and muscle fibres red, collagen and bone blue or green, cytoplasm light red or pink, and cell nuclei dark brown to black. Microscopic evaluation of H&E stains was performed to quantify the presence of foreign body giant cells (FBGC), polymorphonuclear cells (PMN) and vessels. Five non-overlapping fields per slide were counted at a magnification of 400× using an Axioplan 40 microscope (Carl Zeiss, Oberkochen, Germany) and the average cell count calculated. Fields were randomly selected at the interface between the implant (or fascia for control specimens) and surrounding tissue. A scale was used analogous to that described by Badylak et al. [18]. The organisation, composition and amount of collagen were analysed semi-quantitatively on Masson’s stains. The organisation of collagen was scored between totally disorganised to well-organised (0–3); the composition, ranging from absent (0), cellular (1), mixed (2), to a (nearly) acellular (3) collagen scar; and the amount of collagen was assessed as absent (0), minimal (1), moderate (2) to abundant (3), as previously described [19].
Statistics
Results are reported as means and standard error of the mean. The Mann–Whitney U test (non-parametric) was performed. p values < 0.05 were considered statistically significant. All analyses were performed using JMP Statistical Discovery version 7.0.1 (SAS Institute, Cary, NC, USA).
Results
Growth of rMSCs on scaffolds
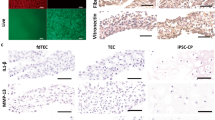
No cell growth was observed on Gynemesh PS at each time point, either with superficial seeding by cell pipetting or with a 25-gauge needle injection. With both seeding procedures, no cell growth was observed inside the collagen-based matrix of Pelvicol, and only a small number of cells could be visualised on the external surface of the graft after 7 days. Conversely, SIS displayed cell growth, not only on the surface of the graft, but also through the collagen layers. After an incubation period of 7 days, cells were spread homogeneously and, by analysing several sections, we observed a high cell density that decreased from the peripheral to the central area of the scaffold. After 14 days cells were distributed in colonies of higher density. At the peripheral part of the colonies, which had contact with the scaffold, cells maintained their typical fibroblastic-like morphology. At the 7-day time point rMSCs were displaced both on Pelvitex fibres (PP and collagen) and pores, where only collagen was present. Cell distribution was considered homogeneous. After 14 and 21 days, rMSCs could be observed only around mesh fibres and interstices: they were no longer present inside pores. Since Pelvitex was analysed on DiI staining, we could only obtain information about the distribution of rMSCs, but not about the cell morphology.
On the basis of the in vitro data, SIS and Pelvitex were selected for the in vivo part of the experiment.
Macroscopic evaluation
All animals had an uneventful recovery with no clinical signs of wound dehiscence or infection. During necroscopy no herniations or erosions were observed in any of the groups at each time point. Seroma formation was observed around the mesh in three groups after 7 days (PN: 67 %; PS: 17 %; SS: 50 %). In 1 animal from the PS group and in 2 from the SN group a small haematoma was observed at the 7-day time point. Regarding the biological graft SIS, it was still visible in all animals after 7 days. After 90 days a partial or complete reabsorption was observed and the mesh site was replaced by a layer of fibrotic collagen. In detail, in the group implanted with native graft (SN), 67 % of animals had a partial graft reabsorption and in 33 % the implant material was completely dissolved. In the group seeded with rMSCs (SS), 67 % of animals showed a poorly represented graft, while in 33 % the mesh was considered not to have been reabsorbed at all.
Blood haemochromocytometric test
We analysed the blood haemochromocytometric values of animals implanted either with or without rMSCs at 7 and 90 days (Table 1). In animals implanted with Pelvitex no difference was noted after 7 days when comparing the groups. Conversely, after 90 days in the PS group we noticed a lower count of neutrophil cells (p = 0.0131). The SS group had a higher count of monocytes at 7 (p = 0.0225) and 90 days (p = 0.0131), of neutrophil cells after 7 days (p = 0.0358) and platelets after 90 days (p = 0.02).
Histology
We compared the optical semi-quantitative histological findings of animals implanted either with or without rMSCs at 7 and 90 days (Table 2). Seven days after surgery, explants of the PS group showed less mature collagen characterised by a more cellular rather than fibrous pattern compared with explants of the PN group (p = 0.0351). After 90 days no difference was visible. SIS explants showed no significant differences at 7 days. After 90 days, SS explants showed a higher collagen amount (p = 0.0042), which had a more organised pattern (p = 0.0052) and a more advanced maturation level (p = 0.0059) compared with the SN group (Fig. 2).
Furthermore, we analysed the histological features of the contra-lateral (left side) abdominal walls, which served as internal controls. After 7 days, the control tissues of animals in which a rMSC-seeded mesh was implanted, showed a larger amount (p = 0.0002) of more organised (p = 0.0263) and more mature (p = 0.0009) collagen. After 90 days these differences were no longer significant. At the same time point, an increase in neovascularisation (p = 0.0278) was observed in animals that received rMSCs. The amounts of foreign body giant cells and polymorphonuclear cells of the groups were not different.
Mechanical tensile tests
A mechanical comparative study 7 and 90 days after implantation was performed. Seven days after implantation, S30%, S50%, and Fmax were significantly different (p < 0.05) compared with SN 7 (Table 3). Ninety days after implantation, the only significant difference was observed for Fmax comparing SN90 with SS90 (Table 3). Maximum elongation values were not significantly different (Table 3). Differences in the mechanical behaviour, and hence in the bond between the mesh/neotissue and the abdominal wall tissue, are well represented by the F/Δl curves. In fact, the F/Δl curves related to SS90 exhibited a higher slope, probably because of a strong bond between mesh and abdominal tissue.
All F/Δl curves, except PS90, had an initial region of low stiffness. After that two different behaviours could be detected: a linear increase in stiffness for PN7 and PS7, and an increase in stiffness for PN90 and PS90 (Table 4). At 7 days, in PN explants, the mesh separated from the abdominal wall before 30 % deformation and had the lowest adhesion force.
Discussion
Clinical trials have already demonstrated the potential benefits of MSCs in the treatment of skeletal system diseases [20] and myocardium infarction [21]. In pelvic floor dysfunctions, MSCs have been successfully used in the treatment of stress urinary incontinence [22]. In this study we confirmed the feasibility of incorporating rMSCs on both synthetic and biological prosthetic materials commonly used in pelvic reconstructive surgery. However, the adherence and growth of rMSCs on the selected biomaterials varied according to the chemical and textile characteristics of the mesh.
We tested four different implant materials that were either synthetic or biological. As expected, no cell growth was observed on the polypropylene-derived mesh (Gynemesh) owing to the non-adherent characteristic of this polymer. rMSCs showed the best adherence and proliferation rate on a non-cross-linked porcine small intestine submucosa (Surgisis). This finding is in agreement with the results published by Ahn et al. [23] who described the capability of SIS to act as a scaffold for human bone marrow stem cell proliferation. On the contrary, the quantity of rMSCs attached to the fibres of the hybrid material tested (Pelvitex) displayed a decreasing trend over time. This phenomenon is probably due to the partial reabsorption of the collagen film, which reduces the potential of cell attachment to the mesh. In the literature there is some evidence suggesting that the cross-linking treatment increases cell adhesion to the graft compared with non-cross-linked material [12]. Conversely, in our experience, rMSCs were poorly attached to the surface of the cross-linked porcine dermal collagen (Pelvicol) at the earlier time point and no cells were present in the inner structure of the material. Probably, the cross-linked, non-porous and dense Pelvicol structure inhibited growth factor and nutrient intake from the culture medium. Therefore, our in vitro study shows that a biological matrix and an open textile structure enhance cell adhesion and proliferation. Both SIS and Pelvitex, as long as the collagen film is present, have these features and represent suitable scaffolds for MSCs.
The aim of the in vivo experiment was to evaluate whether the presence of rMSCs on mesh could have any consequence for the biocompatibility and mechanical behaviour of implant materials.
In Pelvitex, the collagenic pattern at the operation site was not influenced by the presence of stem cells. However, tensile mechanical tests revealed that at late time points Pelvitex explants with rMSCs had lower stiffness values than the original material. This finding is interesting considering that stiffness is clinically related to postoperative pain occurring with synthetic mesh surgery [5].
Furthermore, the role of rMSCs in the systemic inflammatory response was evident in Pelvitex, with a lower neutrophil count at 90 days. Since Pelvitex is a hybrid material, the synthetic PP component plays an important role in the inflammatory response [24]. We assume that the milder systemic inflammatory response mediated by rMSCs could be achieved through two mechanisms: a recognition of rMSCs as self by the host immune system and through the well-known immune-modulation role of these cells [11]. These findings are in accordance with data from Dolce and collaborators describing better biocompatibility, less adhesion formation and a milder inflammatory response when stem cells surrounded a synthetic prosthesis in a rat abdominal hernia model [25]. This is particularly relevant considering the impact of inflammation-related adverse effects on the clinical use of synthetic materials. The use of MSCs could be an interesting approach in the effort to reduce mesh complications.
Conversely, the impact of MSCs on the systemic inflammatory response was not significant with Surgisis. The explanation may be that the immuno-modulant effect of stem cells is more marked when inflammation is stronger, as happens with synthetic mesh implantation. On the other hand, the consequences of rMSCs for SIS were evident in a histological analysis. In fact, we observed an increase in the amount, organisation and maturity of collagen at later time points. Furthermore, an increase in the force needed to separate the biological graft Surgisis from the muscular abdominal wall was associated with the presence of rMSCs. This is probably due to the better integration of the graft with native tissues. The mechanism may involve both the fibroblast differentiation of seeded rMSCs and enhanced cellular trophism. This hypothesis is supported by the evidence of a positive effect of rMSCs on non-operated tissues near to the mesh in terms of collagen features and neovascularisation. Co-stimulation and paracrine effects of SCs have already been proposed [26].
This could have some clinical implications. Evidence indicates that biological mesh has unpredictable long-term viability and does not generate new tissue at the implant site that is strong enough [27, 28]. The use of a biological graft as a scaffold for MSCs could provide an additional mechanical support to weakened tissues [29]. In the near future, the use of a biological graft in addition to the patient’s autologous stem cells could be a promising strategy in improving the surgical repair of prolapse.
This work is a preliminary study aimed at testing the role of MSCs in the integration of biomaterials and native host tissue. To our knowledge this is the first experiment in which stem cell-seeded grafts have been evaluated ex vivo using a biomechanical test. This dynamic evaluation is complementary to histological findings in order to fully understand the integrating phenomenon. The importance of such a study is underlined by recent evidence regarding mesh surgery. In fact, in 2011, the FDA published an updated report on complications related to the use of mesh products in pelvic floor surgery, identifying concerns over the safety of such devices [30]. Eleven months later, a leading brand involved in pelvic floor reconstructive surgery discontinued its sale of mesh-based surgical kits for pelvic organ prolapse repair.
Limitations of our study were the lack of stem cell labelling in vivo and the usage of heterologous stem cells. We think that the use of heterologous stem cells may be acceptable in rats. In fact, rodents are well-established models for allogenic stem cell transplantation. Furthermore, the harvesting and culturing of rat-derived MSCs, as well as abdominal surgery, are standardised in this animal model. The analysis of the long-term persistence and differentiation of autologous stem cells in the host will require further study. One more limitation may be found in the choice of an abdominal animal model for the study of vaginal implants. In fact, in a rabbit model, histological and biomechanical differences between abdominal and vaginal implantations were noted [31]. On the other hand, no biomechanical differences were noted when a synthetic material was implanted on the abdomen and in the vagina of a sheep model [32]. However, this is uncertain in the rat owing to the limitation of the vaginal route in this model.
In conclusion, some implant materials used in pelvic floor surgery can be effectively used as scaffolds for MSC growth. MSCs play a role in the biocompatibility of meshes in vivo by decreasing the systemic inflammatory response provoked by synthetic materials and by improving collagen characteristics around biological grafts. Future studies will focus on the molecular mechanisms that induce this process.
Abbreviations
- Fmax :
-
Maximum force before the separation of the mesh from the abdominal wall
- FBGC:
-
Foreign body giant cells
- H&E:
-
Haematoxylin/eosin
- MSCs:
-
Mesenchymal stem cells
- PMN:
-
Polymorphonuclear cells
- PN:
-
Pelvitex without rMSCs
- POP:
-
Pelvic organ prolapse
- PP:
-
Polypropylene
- PS:
-
Pelvitex with rMSCs
- rMSCs:
-
Rat-derived mesenchymal stem cells
- S:
-
Stiffness
- S30%:
-
Secant modulus at 30 % elongation
- S50%:
-
Secant modulus at 50 % elongation
- SIS:
-
Small intestine submucosa
- SN:
-
Small intestine submucosa without rMSCs
- SS:
-
Small intestine submucosa with rMSCs
- Δlmax :
-
Maximum elongation before the separation of the mesh from the abdominal wall
References
MacLennan AH, Taylor AW, Wilson DH, Wilson D (2000) The prevalence of pelvic floor disorders and their relation to gender, age, parity, and mode of delivery. Br J Obstet Gynaecol 106:1460–1470
Birch C, Fynes MM (2002) The role of synthetic and biological prostheses in reconstructive pelvic floor surgery. Curr Opin Gynecol Obstet 14:527–535
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL (1997) Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89:501–506
Baessler K, Maher CF (2006) Mesh augmentation during pelvic-floor reconstructive surgery: risks and benefits. Curr Opin Obstet Gynecol 18:560–566
Cobb WS, Kercher KW, Heniford BT (2005) The argument for lightweight polypropylene mesh in hernia repair. Surg Innov 12(1):63–69, review
Deprest J, Claerhout F, Zheng F, Kostantinovic M, Spelzini F, Guelinckx I et al (2005) Synthetic and biodegradable prostheses in pelvic floor surgery. Int Congr Ser 1279:387–397
Nazemi TM, Kobashi KC (2007) Complications of grafts used in female pelvic floor reconstruction: mesh erosion and extrusion. Indian J Urol 23:153–160
Fibbe WE. Mesenchymal stem cells. A potential source for skeletal repair. Ann Rheum Dis 2002;61 [Suppl II]:ii29–ii31.
Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213(2):341–347
Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L et al (2004) Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng 32:112–122
Rasmusson I (2006) Immune modulation by mesenchymal stem cells. Exp Cell Res 312:2169–2179
Ochoa I, Peña E, Andreu EJ, Pérez-Ilzarbe M, Robles JE, Alcaine C et al (2011) Mechanical properties of cross-linked collagen meshes after human adipose derived stromal cells seeding. J Biomed Mater Res A 96(2):341–348
Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF (1997) Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem 67:478–491
Gandhi S, Kubba LM, Abramov Y, Botros SM, Goldberg RP, Victor TA et al (2005) Histopathologic changes of porcine dermis xenografts for transvaginal suburethral slings. Am J Obstet Gynecol 192:1643–1648
De Tayrac R, Alves A, Therin M (2007) Collagen-coated vs noncoated low-weight polypropylene meshes in a sheep model for vaginal surgery. A pilot study. Int Urogynecol J 18:513–520
Mutter D, Jamali FR, Moody DL, Rodeheaver GT, Therin M, Marescu J (2000) The concept of protected mesh to minimize adhesion formation in intraperitoneal abdominal wall reinforcement. preclinical evaluation of a new composite mesh. Hernia 4 [Suppl]:S3–S9
Donzelli E, Salvadè A, Mimo P, Viganò M, Morrone M, Papagna R et al (2007) Mesenchymal stem cells cultured on a collagen scaffold: in vitro osteogenic differentiation. Arch Oral Biol 52(1):64–73
Badylak S, Kokini K, Tullius B, Simmons-Byrd A, Morff R (2002) Morphologic study of small intestinal submucosa as a body wall repair device. J Surg Res 103(2):190–202
Konstantinovic ML, Lagae P, Zheng F, Verbeken KE, De Ridder D, Deprest JA (2005) Comparison of host response to polypropylene and non-cross-linked porcine small intestine serosal-derived collagen implants in a rat model. BJOG 112:1554–1560
Morishita T, Honoki K, Ohgushi H, Kotobuki N, Matsushima A, Takakura Y (2006) Tissue engineering approach to the treatment of bone tumors: three cases of cultured bone grafts derived from patients’ mesenchymal stem cells. Artif Organs 30(2):115–118
Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W et al (2006) Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 367:113–121
Yamamoto T, Gotoh M, Kato M, Majima T, Toriyama K, Kamei Y et al (2012) Periurethral injection of autologous adipose-derived regenerative cells for the treatment of male stress urinary incontinence: report of three initial cases. Int J Urol 19(7):652–659
Ahn HH, Kim KS, Lee JH, Lee MS, Song IB, Cho MH et al (2007) Porcine small intestinal submucosa sheets as a scaffold for human bone marrow stem cells. Int J Biol Macromol 41(5):590–596
Klosterhalfen B, Klinge U, Schumpelick V (1998) Functional and morphological evaluation of different polypropylene-mesh modifications for abdominal wall repair. Biomaterials 19:2235–2246
Dolce CJ, Stefanidis D, Keller JE, Walters KC, Newcomb WL, Heath JJ et al (2010) Pushing the envelope in biomaterial research: initial results of prosthetic coating with stem cells in a rat model. Surg Endoscop 24(11):2687–2693
Altman AM, Abdul Khalek FJ, Alt EU, Butler CE (2010) Adipose tissue– derived stem cells enhance Bioprosthetic mesh repair of ventral hernias. Plast Reconstr Surg 126(3):845–854
Mouritsen L, Kronschnabl M, Lose G (2010) Long-term results of vaginal repairs with and without xenograft reinforcement. Int Urogynecol J Pelvic Floor Dysfunct 21:467–473
Ozog Y, Konstantinovic ML, Verschueren S, Spelzini F, De RD, Deprest J (2009) Experimental comparison of abdominal wall repair using different methods of enhancement by small intestinal submucosa graft. Int Urogynecol J Pelvic Floor Dysfunct 20:435–441
Boennelycke M, Gras S, Lose G (2013) Tissue engineering as a potential alternative or adjunct to surgical reconstruction in treating pelvic organ prolapse. Int Urogynecol J 24:741–747
Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. July 2011 http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf
Hilger WS, Walter A, Zobit ME, Leslie KO, Magtibay P, Cornella J (2006) Histological and biomechanical evaluation of implanted graft materials in a rabbit vaginal and abdominal model. Am J Obstet Gynecol 195(6):1826–1831
Manodoro S, Endo M, Uvin P, Albersen M, Vlacil J, Engels A et al (2013) Graft-related complications and biaxial tensiometry following experimental vaginal implantation of flat mesh of variable dimensions. BJOG 120:244–250
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spelzini, F., Manodoro, S., Frigerio, M. et al. Stem cell augmented mesh materials: an in vitro and in vivo study. Int Urogynecol J 26, 675–683 (2015). https://doi.org/10.1007/s00192-014-2570-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-014-2570-z