Abstract
Introduction and hypothesis
Extracorporeal biofeedback was developed to reduce patient discomfort when performing strengthening exercises. The efficacy and safety of extracorporeal biofeedback combined with pelvic floor muscle training (PFMT) for the treatment of female stress urinary incontinence (SUI) were evaluated.
Methods
One hundred and six participants with SUI were enrolled in a 12-week PFMT program using extracorporeal biofeedback intervention. A standard pad test was performed, and pelvic floor muscle strength was assessed using the Oxford scale. Measurements were taken with a perineometer at baseline and at a 12-week follow-up visit. An objective cure was defined as less than 2 g of urine leakage by the standard pad test. The long-term effects of extracorporeal biofeedback and PFMT were investigated by interviewing the participants 12 months after treatment.
Results
Seventy-one participants completed the 12-week extracorporeal biofeedback intervention. The objective cure rate was 52.1 %, and there was a significant reduction in pad weight over the time period. The incontinence visual analogue scale, the Sandvik severity index, and the incontinence quality-of-life questionnaire domains were significantly improved after treatment (p < 0.001). The strength of the PFM was significantly increased after the 12-week treatment. After PFMT, 64.3 % of 56 participants reported good treatment compliance, and 24 participants (42.9 %) had continued PFMT at home 12 months after treatment. Age and baseline pad weight were negative predictive factors for an objective cure of SUI.
Conclusions
Pelvic floor muscle training using extracorporeal biofeedback can be an effective and safe conservative treatment option for female SUI without the discomfort caused by vaginal sensors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic floor muscle training (PFMT) as a treatment for stress urinary incontinence (SUI) became widespread during the mid-1990s when Arnold Kegel published his successful treatment of female SUI [1]. The goal of pelvic floor muscle exercises is to strengthen and gain control over the pelvic muscles [2]. PFMT has been used in an effort to prevent postpartum urinary incontinence [3]. PFMT is therefore a widely used and well-established form of SUI treatment, with success rates varying from 21 % to 84 %, although it has been found to have better subjective than objective outcomes [4–7]. A long-term prospective controlled study of 162 women found that after 1 year of follow-up, significantly more women in the control group reported SUI than in the group of women who had been taught to perform PFMT [8]. The effect of pelvic floor muscle exercises alone, without any other form of biofeedback, is a 56 % to 95 % reduction in incontinence episodes [9, 10].
However, the insertion of a vaginal sensor can result in discomfort or pain in conventional pelvic floor biofeedback methods [11]. The extracorporeal biofeedback device described here is chair-shaped with a sensor on the center of the chair. With this instrument, there is no need to insert a probe into the vagina, which is more convenient and reduces the possibility of embarrassment as it can be used while clothes are worn. We investigated the efficacy and safety of extracorporeal biofeedback combined with PFMT for the management of female SUI.
Materials and methods
Study design
This was a 12-week prospective, single-arm study conducted at two university hospitals from June 2008 to November 2009. Women with symptoms of SUI and more than 2 g of urine leakage on a standard pad test with a full bladder were enrolled in this study. The exclusion criteria were as follows:
-
1.
Urge-predominant mixed incontinence
-
2.
True or overflow incontinence
-
3.
Stage II or higher pelvic organ prolapse
-
4.
Urinary tract infection
-
5.
Neurological or psychiatric disease
-
6.
Pregnancy
To exclude these criteria, pelvic examination, including pelvic organ prolapse quantification (POP-Q) staging, urinalysis, uroflowmetry and post voiding residual, and neurological examination were performed before enrollment. All participants provided written informed consent, and the Institutional Review Boards (IRB) of the participating sites approved this study (IRB number: 2007-06-075). This study is registered at http://www.clinicaltrials.gov (registration number: NCT00910338).
To detect an improvement rate of 69 % with 97.5 % confidence, the necessary sample size was determined to be a total of 100 women. The expected drop-out rate was 17 %. These figures were computed conservatively based on findings from previous studies [12, 13]. The primary endpoint was an SUI cure rate of 12 weeks after PFMT with extracorporeal biofeedback. An objective cure was defined as less than 2 g of urine leakage on a standard pad test. We considered SUI to be cured when participants obtained an objective cure. A standard pad test and PFM strength measurements using a perineometer and the Oxford scale [14] were assessed at baseline and after 12 weeks of treatment. For the standard pad test, participants’ empty bladders were filled with 300 ml of saline; then, the weight of the pad was measured after 20 jumps and three coughs [15]. Perineometry, which is an instrument for measuring the strength of voluntary contractions of the pelvic floor muscles, was used to assess intravaginal pressure with a vaginal probe [16, 17]. This perineometry was built into the extracorporeal biofeedback device. After inserting the vaginal probe, the participant contracted her pelvic floor muscles with maximal strength and duration. After taking three different measurements with a 30-min interval, the maximal pressure was recorded. The Oxford scale was used for digital muscle testing. The investigator used one digit (the index finger) for palpation if there was PFM contact with the examining finger or both the index and middle fingers if there was incomplete contact. One investigator per institute carried out the Oxford grading after training in a center for standardizing the methodology. Following confirmation of correct contraction without the contraction of the abdominal or leg muscles, the strength of the PFM was then graded using the six-point modified Oxford scale (0 = nil, 1 = flicker, 2 = weak, 3 = moderate, 4 = good, and 5 = strong).
Assessments with the incontinence visual analogue scale (VAS), the Sandvik severity index [18], and the incontinence quality-of-life questionnaire were performed at baseline and after 4 and 12 weeks of treatment. The Stamey grading system was used for evaluation of the baseline SUI symptom grade [19]. Treatment compliance was evaluated at 4 weeks and 12 weeks. “Good” compliance was defined as a participant who performed PFMT with biofeedback more than 30 sessions a day for more than two thirds of all days. “Poor” compliance was defined as a participant who underwent PFMT with biofeedback more than 30 sessions each day for less than one third of all days. Compliance in the remaining participants was considered “Reasonable.” The participants’ perceptions of treatment benefits and satisfaction were evaluated at 12 weeks by using a benefit, satisfaction, and willingness to recommend (BSW) questionnaire. The long-term effects of extracorporeal biofeedback and PFMT were analyzed by interviewing all participants 12 months after treatment over the telephone. We inquired as to whether or not the participants had used PFMT at home. We also assessed the current status of the participants’ SUI based on the Sandvik questionnaire.
Extracorporeal biofeedback device mechanism
The extracorporeal biofeedback machine initially generates a force in the center of the chair to help the user accurately recognize the pelvic muscles. In the second step, the user recognizes gentle pressure on the pelvic floor muscle. Through a sensor located on the center of the chair, an approximately 10-kg weight is pressed on the user’s pelvic muscle. For the third step, the pelvic muscle is to be contracted against the physical pressure of the sensor. With the physical pressure, the user recognizes the pelvic floor muscle as it is pressed and contracts the pelvic floor muscle intentionally. When the user contracts the pelvic floor muscle, the muscle becomes tight and thick between the bilateral pelvic bones. Then, the power between the bilateral pelvic bones pushes the sensor downward. With the downward force, the sensor recognizes how much contractile force the user is exerting. The machine has a variety of built-in PFMT programs and after the user chooses a program, the desired pressure and the user’s actual exerted pelvic floor muscle pressure are displayed in real time on the monitor as a graph. In this way, the user can learn how to exercise by looking at the monitor. An analogous situation is if a person tries to strengthen his/her upper arm with no addition weight, he/she cannot control the power exerted. However, by using a 10-kg dumbbell, he/she can control the amount of exertion desired. The same principle is applied with the extracorporeal biofeedback device. With this instrument, there is no need to insert a probe into the vagina, which reduces possible embarrassment as well as the risk of introducing pathogens into the vaginal cavity (Fig. 1).
Pelvic floor muscle training (PFMT) with an extracorporeal biofeedback device. a First step—resting state. The user sits on the chair, and the sensor of the device is not yet elevated. Typically, the loosened pelvic floor muscle drooped because of the weight of the pelvic organs. b Second step—pressure. The sensor is elevated, and the user recognizes the pelvic floor muscle being gently pressed by up to approximately 10 kg of pressure. The pelvic floor muscle is pushed upward by the sensor. c Third step—contraction exercise. The user’s pelvic floor muscle is displayed on the monitor via the sensor. When the user contracts the pelvic floor muscle, the muscle becomes tight and thick between the bilateral pelvic bones. Then the power between the bilateral pelvic bones pushes the sensor downward. As the strength of the pelvic floor muscle contraction increases, the downward force to the sensor also increases
PFMT with extracorporeal biofeedback
All participants visited the same physiotherapist twice a week for the first 4 weeks and then once a week for the next 8 weeks. PFM activity was measured at baseline and 4 and 12 weeks after beginning the treatment. The Hue & Joy extracorporeal biofeedback device (HnJ-5000; Furon Medical, Korea) was used in the study at an outpatient clinic (Fig. 2). To our knowledge, this paper is the first publication to discuss the use of this device. It is a chair-shaped device with a sensor. Any pad worn by participants was removed, and patients wore thin clothes in order to be able to feel the force more effectively. At first, the patients sat on the center of the chair. After entering the patients’ height and weight measurements into the software (HNJ version 2.0; Kangwondo, Korea), the sensor rose to a height based on each patient’s characteristics to press approximately 10 kg of force on the pelvic muscle. Because the sensor was longitudinally located in the middle of the chair, and the sensitivity of the sensor was measured effectively in the range of 400 mm × 400 mm, the sensor was able to accurately press against the patient’s perineum and measure the contractile force of the pelvic muscle. There were several PFMT programs in the software included with the machine. The physiotherapist chose the level of the program according to the strength of the pelvic floor muscle. If the patient quickly adapted to the program, the physical therapist increased the difficulty to a higher level. If the patient was not able to perform the exercises, the physical therapist decreased the difficulty to a lower level. Patients were treated over 28 to 35 physiotherapeutic sessions. Each session consisted of two 15-s contractions followed by a relaxation program. The two contraction–relaxation programs were composed of endurance contractions and five to seven quick flicks. Participants were taught to gradually extend the period of each contraction from 1.5 s to 5 s in the endurance contraction part of the programs. All participants were given verbal and written instructions for home practice. Participants were advised to perform 30 sessions of PFMT (100 contractions) at home daily.
Extracorporeal biofeedback device. The sensor on the center of the chair puts as much as 10 kg of pressure on the user’s pelvic muscle. Responding to the physical pressure, the user recognizes the pelvic floor muscle and intentionally contracts the muscle. The contractile force of the user’s pelvic muscle is displayed on the monitor via the sensor
Statistical analysis
We used a paired t-test or Wilcoxon’s signed rank test with Bonferroni correction to compare objective and subjective variables before and after treatment. The generalized estimation equation (GEE) was used to evaluate changes on the Sandvik questionnaire and Oxford scales. We assessed differences in treatment compliance according to muscle strength using Spearman’s correlation analysis. Factors predictive of SUI treatment success, which was defined by the results of a standard pad test, were identified using logistic regression analysis. Multivariate analysis was used to analyze the effects of the measured variables, including age, body mass index (BMI), parity, menopausal status, symptom duration and grade, baseline pad test, and perineometry. p < 0.05 was considered statistically significant for all tests.
Terminology
Methods, definitions, and units conformed to the standards jointly recommended by the International Urogynecological Association (IUGA) and the International Urogynecological Society (ICS), except where specifically noted [20].
Results
Participants’ baseline characteristics
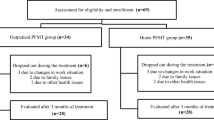
We enrolled a total of 106 participants in our study. Thirty-five women (34 %) did not complete the study. Twenty-nine participants withdrew their consent for personal reasons. Five participants did not attend follow-up, and one participant was excluded because of vaginal bleeding caused by a uterine myoma. Seventy-one participants were ultimately included in our dataset. At the 12-month follow-up, 56 participants were interviewed by telephone (Fig. 3). The average age of the participants was 52.2 (range: 34–73) years. The mean number of vaginal deliveries was 2.2, and the mean symptom duration was 61.8 months. Sixty-three percent of participants had Stamey grade 1 symptoms of SUI; 28.6 % had grade 2, and 8.6 % had grade 3. Participants’ demographic data are listed in Table 1.
SUI cure rate after 12 weeks of PFMT with extracorporeal biofeedback
The mean urinary leakage on a standard pad test was significantly decreased from 20.6 ± 20.0 g to 7.3 ± 13.6 g after PFMT with extracorporeal biofeedback (p < 0.001). Thirty-seven women (52.9 %) were objectively cured on the standard pad test, and a cure was defined as 2 g or less of leakage with a standardized bladder volume (Table 2). Twenty-three participants (32.9 %) had 0 g of urine leakage on the pad test at 12 weeks. The SUI cure rates were 77.4 %, 41.7 %, 50 %, and 17.6 % for women with baseline pad weights of 2 to 9 g, 10 to 19 g, 20 to 29 g, and greater than 30 g respectively.
Change in PFM strength and subjective symptoms
The strength of the pelvic floor muscles as measured by a perineometer was significantly increased after 12 weeks of PFMT with extracorporeal biofeedback (p < 0.001) (Table 2). There was also significant improvement in muscle strength as measured by the Oxford scale after 12 weeks (p < 0.001). Incontinence VAS was significantly improved after 12 weeks of treatment compared with baseline (6.5 ± 2.2 at baseline to 3.6 ± 2.4 at 12-week follow-up, p < 0.001), as were the Sandvik severity index and the incontinence quality of life (I-QoL) questionnaire domains (p < 0.001; Table 2).
Treatment compliance, perception of treatment benefit and satisfaction, and long-term outcomes
The distribution of treatment compliance after 4 weeks was as follows: 74.3 % had good adherence, 8.6 % had reasonable adherence, and 17.1 % had poor adherence. After 12 weeks, 64.3 % of all participants reported good treatment compliance, and 18.6 % of participants showed poor compliance.
Changes in PFM strength as measured by the Oxford scale were different according to treatment compliance at 12 weeks (p = 0.001). The PFM in participants with good treatment compliance was stronger than that in those with poor treatment compliance (p = 0.009).
According to the BSW questionnaire, 58.6 % of participants reported that they experienced a marked benefit. Ninety-four percent said that they were either satisfied or very satisfied with the treatment, with 35.7 % being very satisfied. Furthermore, 65.7 % of participants reported that they were willing to undergo an additional round of treatment, and 94.3 % of participants reported that they would recommend the treatment to others. Additional subjective reports showed that 5.7 % of participants found the treatment unsatisfactory. However, these patients adhered to the training protocol despite their dissatisfaction.
We were able to evaluate the long-term effects of the extracorporeal biofeedback device in 56 participants via telephone interview 12 months after treatment. Fifteen participants (26.8 %) answered that they currently experienced no incontinence, while 41 participants (73.2 %) experienced some type of incontinence. Of these, 7 experienced incontinence less than once per month, 16 experienced incontinence more than once per week, and 8 experienced incontinence every day. Of the 56 participants who completed the telephone interview, 24 (42.9 %) answered that they had done PFMT at home, while 32 (57.1 %) had not performed PFMT at all since the study.
Predictive factors for an SUI cure
Age and baseline pad weight were factors predictive of successful SUI treatment on univariate analysis (p < 0.05), and these variables remained significant in the multivariate analysis of all measured variables, including BMI, number of vaginal deliveries, menopausal status, symptom duration and grade, and baseline perineometry (age: OR 12.27, 95 % CI 1.72 to 87.43, p = 0.012; baseline pad weight: OR 0.02, 95 % CI 0.00 to 0.25, p = 0.001; Table 3). In participants younger than 60, the odds ratio of being cured was 12.27 when compared with participants older than 60 years. The odds ratio of an objective cure was 0.02 when comparing participants with baseline pad weights greater than 30 g with those with baseline pad weights between 2 and 9 g.
Adverse effects
No adverse events related to the treatment were reported. One participant discontinued the treatment owing to vaginal bleeding caused by a uterine myoma.
Discussion
The results of the present study indicated that PFM exercises in combination with an extracorporeal biofeedback device are efficacious in decreasing urine leakage and increasing muscle strength. Objective cure (2 g or less of leakage) was observed in 52.1 % of women who performed PFMT with biofeedback. This finding was consistent with the conclusions of randomized, controlled trials and meta-analyses comparing the effects of PFMT with and without biofeedback [21, 22]. Several studies have proved the effectiveness of strengthening the pelvic floor muscles in treating SUI, and others have shown that these exercises in association with biofeedback are safe and effective [23]. Hirsch et al. found that home-based biofeedback was efficient in 85 % of patients with stress and mixed incontinence [24], and Morkved et al. reported an objective cure rate of 58 % in women training with biofeedback and of 46 % in women training without it [25]. Bo et al. were the first to compare the efficacy of PFMT, electrical stimulation, and vaginal cones for the treatment of SUI [6]. In that study, the PFMT group was found after 6 months to be superior to all of the groups with regard to treatment compliance and exhibited significant improvements in pelvic floor muscle strength, leakage on pad testing, number of leakage episodes over 3 days, and leakage and social indices compared with the control group. Overall, that study demonstrated the superiority of PFMT over electrical stimulation and vaginal cones in the treatment of female SUI. Jundt et al. studied 78 women with SUI or mixed urinary incontinence. The patients were instructed on how to use an electromyogram-controlled biofeedback machine with PFMT for 3 to 6 months. Only 36 women were available for review after a mean of 26 months, and they had a cure/improvement rate of 47.2 % [26].
As in previous studies, our results showed decreases in pad weight in the standard pad test and increases in PFM strength. However, we used an extracorporeal biofeedback device to assist PFMT. In performing PFMT with conventional biofeedback, a vaginal pressure probe is placed inside the vagina to measure vaginal squeeze pressure. This is often embarrassing or uncomfortable for patients, and the results may be incorrect or invalid since the size of the vaginal opening influences the measurement of PFM contraction [27]. The measurement of squeezing pressure can also be inaccurate because an increase in abdominal pressure will increase the measured pressure [28]. The PFM is one of the walls of the abdominal cavity, and pressure originating from the abdominal cavity increases the pressure in the urethra, vagina, and rectum. Both Bo et al. and Bump et al. [29] have shown that straining is a common error when women attempt to contract their PFM, and straining can result in erroneous measurements. Because we used an extracorporeal biofeedback device rather than a traditional device, we were able to minimize the chance of error and increase patient comfort.
In clinical practice, PFMT programs still lack consistency in the recommended number of contractions per day and training sessions per week [30]. Bo et al. reported significant improvement in an “intensive” treatment group compared with a “standard” treatment group [31]. However, another study found no significant difference between intensive and standard treatment groups [32]. One of the causes of this inconsistency is thought to be embarrassment and discomfort due to the use of vaginal probes or digital examination by a physician. Liao et al. suggested that women perform at least 90 to 120 contractions per day (30 min) [30]. They found that only 27 % of the women with urinary incontinence performed 90 to 120 contractions per day (30 to 40 min per day), while 33 % and 27 % of participants reported that they performed PFMT for 1 to 2 h per week and per month respectively. In the current study, the rate of good treatment compliance at 4 and 12 weeks after treatment with PFMT and biofeedback was 64.3 % overall. We used a chair-type biofeedback device instead of a vaginal probe or digital examination to increase compliance. The benefit of the extracorporeal biofeedback device used in this study was less discomfort for participants. This extracorporeal device may be useful in clinical practice as the treatment is less invasive, and the device can even be used by patients recovering from surgery. When we evaluated factors that could affect the cure rate, age and pad weight were found to be significant predictors, implying that patients older than 60 and those with a large amount of leakage on standard pad tests had a lower chance of symptom improvement when treated with PFMT combined with extracorporeal biofeedback. A study that evaluated the predictors of outcome in a multicomponent behavioral treatment of urinary incontinence (stress, urge, and mixed) reported that fewer incontinent episodes at baseline, previous surgery for incontinence, and a lower education level were associated with pad-free status 8 weeks after treatment [33]. Although that study included urge and mixed incontinence as well as stress incontinence and used multicomponent behavioral treatment, the finding that the degree of baseline incontinence could predict symptom improvement was similar to the results of our study.
The main limitation of this study was the absence of a control group. However, we conducted this study with a reasonable sample size and obtained significant results compared with conclusions from previous randomized, controlled trials and meta-analyses. From our data, we concluded that extracorporeal, biofeedback-assisted PFMT was an effective therapy for women with SUI. However, we cannot determine whether PFMT with conventional biofeedback, PFMT alone, or PFMT with extracorporeal biofeedback is superior. Additionally, we calculated the sample size based on a 17 % drop-out expectation. However, 34 % of participants did not complete our 12-week protocol. In contrast to clinical trials that involve medication, studies that use a device tend to be taken less seriously by Korean participants. Furthermore, the study locations were large centers that included patients who traveled long distances to obtain medical care. Enrollment of patients who were required to travel a long distance could have influenced the dropout rate. Regardless of the reason, the unexpectedly high dropout rate may have affected the results of this paper. A second limitation is the use of the Oxford scale, which allows physicians to assess pelvic floor muscle strength using only their finger and thus is very subjective in nature. However, we used perineometry as an objective evaluation, and our results allowed us to understand the correlation between pelvic floor muscle strength and SUI cure rate. Additionally, while biofeedback is usually reserved for patients with low Oxford scores, improvements in those with baseline Oxford scores of 4 and 5 were shown after biofeedback in this study. Patients with Oxford scores of 4 and 5 may have good short time contraction force, but little endurance contraction force. Therefore, these patients could show improvement after extracorporeal biofeedback exercises.
In conclusion, PFMT using extracorporeal biofeedback can be an effective and safe conservative treatment option for female SUI without the discomfort caused by the use of vaginal sensors. Women older than 60 years of age and those with a large amount of urine leakage had a lower chance of symptom improvement with this treatment.
References
Kegel AH (1948) Progressive resistance exercise in the functional restoration of the perineal muscles. Am J Obstet Gynecol 56:238–248
Burgio KL (1994) Behavioral therapy: practical approach to urinary incontinence. Contemp Urol 6:24, 29–36, 41
Gormley EA (2002) Biofeedback and behavioral therapy for the management of female urinary incontinence. Urol Clin N Am 29:551–557
Burgio KL, Robinson JC, Engel BT (1986) The role of biofeedback in Kegel exercise training for stress urinary incontinence. Am J Obstet Gynecol 154:58–64
Burns PA, Pranikoff K, Nochajski TH, Hadley EC, Levy KJ, Ory MG (1993) A comparison of effectiveness of biofeedback and pelvic muscle exercise treatment of stress incontinence in older community-dwelling women. J Gerontol 48:M167–M174
Bo K, Talseth T, Holme I (1999) Single blind, randomised controlled trial of pelvic floor exercises, electrical stimulation, vaginal cones, and no treatment in management of genuine stress incontinence in women. BMJ 318:487–493
Yun JM, Kim SJ, Lee KS (2000) The effect of pelvic floor muscle training with biofeedback and functional electrical stimulation for genuine stress urinary incontinence. Korean J Urol 41:627–632
Morkved S, Bo K (2000) Effect of postpartum pelvic floor muscle training in prevention and treatment of urinary incontinence: a one-year follow up. BJOG 107:1022–1028
Benvenuti F, Caputo GM, Bandinelli S, Mayer F, Biagini C, Sommavilla A (1987) Reeducative treatment of female genuine stress incontinence. Am J Phys Med 66:155–168
Ferguson KL, McKey PL, Bishop KR, Kloen P, Verheul JB, Dougherty MC (1990) Stress urinary incontinence: effect of pelvic muscle exercise. Obstet Gynecol 75:671–675
Kondo A, Yamada Y, Niijima R (1995) Treatment of stress incontinence by vaginal cones: short- and long-term results and predictive parameters. Br J Urol 76:464–466
Dwyer NT, Kreder KJ (2005) Conservative strategies for the treatment of stress urinary incontinence. Curr Urol Rep 6:371–375
Aukee P, Immonen P, Laaksonen DE, Laippala P, Penttinen J, Airaksinen O (2004) The effect of home biofeedback training on stress incontinence. Acta Obstet Gynecol Scand 83:973–977
Brink CA, Wells TJ, Sampselle CM, Taillie ER, Mayer R (1994) A digital test for pelvic muscle strength in women with urinary incontinence. Nurs Res 43:352–356
Wu W, Sheu B, Lin H (2006) Comparison of 20-minute pad test versus 1-hour pad test in women with stress urinary incontinence. Urology 68:764–768
Isherwood PJ, Rane A (2000) Comparative assessment of pelvic floor strength using a perineometer and digital examination. BJOG 107:1007–1011
Kegel AH (1948) The nonsurgical treatment of genital relaxation; use of the perineometer as an aid in restoring anatomic and functional structure. Ann West Med Surg 2:213–216
Sandvik H, Seim A, Vanvik A, Hunskaar S (2000) A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn 19:137–145
Stamey TA (1980) Endoscopic suspension of the vesical neck for urinary incontinence in females. Report on 203 consecutive patients. Ann Surg 192:465–471
Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J et al (2010) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J Pelvic Floor Dysfunct 21:5–26
Berghmans LC, Hendriks HJ, Bo K, Hay-Smith EJ, de Bie RA, van Waalwijk van Doorn ES (1998) Conservative treatment of stress urinary incontinence in women: a systematic review of randomized clinical trials. Br J Urol 82:181–191
Hay-Smith EJ, Bo Berghmans LC, Hendriks HJ, de Bie RA, van Waalwijk van Doorn ES (2001) Pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev: CD001407
Pages IH, Jahr S, Schaufele MK, Conradi E (2001) Comparative analysis of biofeedback and physical therapy for treatment of urinary stress incontinence in women. Am J Phys Med Rehabil 80:494–502
Hirsch A, Weirauch G, Steimer B, Bihler K, Peschers U, Bergauer F et al (1999) Treatment of female urinary incontinence with EMG-controlled biofeedback home training. Int Urogynecol J Pelvic Floor Dysfunct 10:7–10
Morkved S, Bo K, Fjortoft T (2002) Effect of adding biofeedback to pelvic floor muscle training to treat urodynamic stress incontinence. Obstet Gynecol 100:730–739
Jundt K, Peschers UM, Dimpfl T (2002) Long-term efficacy of pelvic floor re-education with EMG-controlled biofeedback. Eur J Obstet Gynecol Reprod Biol 105:181–185
Dumoulin C, Gravel D, Bourbonnais D, Lemieux MC, Morin M (2004) Reliability of dynamometric measurements of the pelvic floor musculature. Neurourol Urodyn 23:134–142
Bo K, Raastad R, Finckenhagen HB (2005) Does the size of the vaginal probe affect measurement of pelvic floor muscle strength? Acta Obstet Gynecol Scand 84:129–133
Bump RC, Hurt WG, Fantl JA, Wyman JF (1991) Assessment of Kegel pelvic muscle exercise performance after brief verbal instruction. Am J Obstet Gynecol 165:322–327
Liao YM, Dougherty MC, Liou YS, Tseng IJ (2006) Pelvic floor muscle training effect on urinary incontinence knowledge, attitudes, and severity: an experimental study. Int J Nurs Stud 43:29–37
Bo K, Hagen RH, Kvarstein B, Jorgensen J, Larsen S (1990) Pelvic floor muscle exercise for the treatment of female stress urinary incontinence: III. Effect of two different degrees of pelvic floor muscle exercises. Neurourol Urodyn 9:489–502
Wilson PD, Herbison GP (1998) A randomized controlled trial of pelvic floor muscle exercises to treat postnatal urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 9:257–264
Burgio KL, Goode PS, Locher JL, Richter HE, Roth DL, Wright KC et al (2003) Predictors of outcome in the behavioral treatment of urinary incontinence in women. Obstet Gynecol 102:940–947
Acknowledgements
This study was supported by a grant of the Korea Healthcare Technology Research and Development Project, Ministry of Health and Welfare, Republic of Korea (A084152).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, H.N., Lee, S.Y., Lee, YS. et al. Pelvic floor muscle training using an extracorporeal biofeedback device for female stress urinary incontinence. Int Urogynecol J 24, 831–838 (2013). https://doi.org/10.1007/s00192-012-1943-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-1943-4