Abstract
Introduction and hypothesis
The aim of this manuscript was to provide a systematic literature review of clinical trial evidence for a range of electrical stimulation therapies in the treatment of lower urinary tract symptoms (LUTS).
Methods
The databases MEDLINE, BIOSIS Previews, Inside Conferences, and EMBASE were searched. Original clinical studies with greater than 15 subjects were included.
Results
Seventy-three studies were included, representing implanted sacral nerve stimulation (SNS), percutaneous posterior tibial nerve stimulation (PTNS), and transcutaneous electrical stimulation (TENS) therapy modalities.
Conclusions
Median mean reductions in incontinence episodes and voiding frequency were similar for implanted SNS and PTNS. However, long-term follow-up data to validate the sustained benefit of PTNS are lacking. Despite a substantial body of research devoted to SNS validation, it is not possible to definitively define the appropriate role of this therapy owing largely to study design flaws that inhibited rigorous intention to treat analyses for the majority of these studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Symptoms of lower urinary tract dysfunction are often challenging to treat and have a significant adverse effect on quality of life (QOL). A significant proportion of the adult population experiences lower urinary tract symptoms (LUTS) and the prevalence of these symptoms increases with age [1, 2]. A five country, population-based random survey conducted in 2005 found that 64.3% of adults reported at least one LUTS [1]. The prevalence of symptoms of overactive bladder (OAB) in the USA has been estimated at 16.5% with 6.1% experiencing urge urinary incontinence (UUI) [2].
Initial treatment of OAB and other LUTS include lifestyle and behavioral modifications and conservative therapies such as bladder retraining, pelvic muscle exercises, and biofeedback. These interventions are frequently used in combination with antimuscarinic or anticholinergic agents. Despite trying conservative measures and pharmacotherapy, 40% of patients either do not achieve an acceptable level of therapeutic benefit or remain completely refractory to treatment [3].
Until the advent of electrical stimulation techniques, refractory cases were offered long-term catheterization or surgical management (bladder distension, bladder augmentation, detrusor myectomy, or urinary diversion), but high recurrence and complication rates have limited the widespread use of surgery for many patients [4]. Electrical stimulation techniques are available as an alternative that is less invasive, both for treatment of refractory OAB and nonobstructive urinary retention (UR).
The purpose of electrical stimulation (or neuromodulation) for treatment of voiding dysfunction is to target nerves that control the pelvic floor and specifically bladder function. Stimulation can be applied via noninvasive surface electrodes as with transcutaneous electrical stimulation (TENS) devices, through percutaneously placed needles/wires, or through a fully implanted device. TENS and percutaneously applied stimulation therapies are typically delivered during intermittent treatment sessions in a clinic or home setting, and ongoing treatment may be required to sustain benefit. The most widely employed electrical stimulation technique at present is implanted sacral nerve stimulation (SNS). This technique is currently approved by the US Food and Drug Administration for three indications: UUI, urgency-frequency syndrome (UF), and UR [5].
Recent evidence suggests that the mechanism of action (MOA) for electrical stimulation may be related to reorganization of spinal reflexes and regulation of cortical activity [6]. However, a definitive and comprehensive explanation of electrical stimulation MOA remains to be established. Moreover, selection of patients most likely to derive significant long-term benefits from these therapy modalities remains challenging. In light of these uncertainties, this systematic review aims to rigorously examine the available clinical trial evidence for electrical stimulation therapies for the treatment of various LUTS.
Materials and methods
Extensive electronic searches were conducted to identify published studies on electrical stimulation for LUTS. Searches revealed publications from 1970 onwards. Only those published in the English language were selected for review. The databases searched were MEDLINE, BIOSIS Previews, Inside Conferences, and EMBASE.
The literature search identified 456 articles. Three reviewers screened all titles and abstracts independently and excluded reports not meeting the following initial inclusion criteria:
-
English language
-
Available as full-text study
-
An original publication of a clinical study, not a review
Full-text copies of all reports deemed to be potentially relevant were retrieved and a more detailed assessment of the article’s fit with the agreed upon inclusion criteria was conducted independently by each of the three reviewers. Studies sought were those which examined more than 15 subjects and reported outcomes based on voiding diaries/charts, urodynamic measures, QOL assessments, or adverse events. The participants were adults or children with various neurogenic and non-neurogenic LUTS, not including stress incontinence. Investigations of anal/vaginal/genital stimulation and magnetic or maximal electrical stimulation were excluded. Although these modalities may have some utility in LUTS treatment, the authors contend that they have not been widely adopted in the clinical setting and pragmatic barriers to administering these stimulation techniques have largely relegated them to experimental use. In addition, studies of electrical stimulation used in tandem with other therapies were excluded. Final inclusion and exclusion of articles required consensus among the three reviewers.
Data analyses were conducted separately for the three electrical stimulation modalities identified through our review. Pooling of data to obtain mean outcome results was not possible owing to the fact that many articles reported on individual subjects who were also reported on by other articles included in our review. Therefore, our analysis relied on an examination of the median and range of mean values for each reported clinical baseline and outcome data parameter. Median mean values were calculated by patient subgroup (e.g., UUI, UR, UF) for each parameter, provided that adequate data were available.
Results
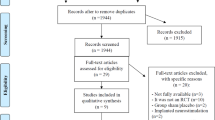
From an initial 456 publications identified by the literature search, 73 articles were included, of which 8 are randomized controlled trials (RCTs) [7–14], 7 are comparative studies [15–21], 46 are prospective [22–67] and 10 are retrospective case series [68–77], and 2 combine prospective and retrospective case series in the same article [78, 79]. The screening process is summarized in Fig. 1.
The included studies were published between the years of 1983 and 2009: 40 studies investigated treatment outcomes related to implanted neurostimulation devices, 20 studies investigated outcomes related to percutaneous stimulation modalities, and 13 studies investigated TENS.
Implanted sacral nerve stimulation
Forty studies provide data on the use of implanted SNS for the treatment of LUTS. These studies were published between the years of 1993 and 2009 and include 5 RCTs [9–11, 13, 14], 23 prospective [24, 25, 27–30, 32, 34, 35, 37, 41, 42, 44, 46, 48, 56, 58, 61–66] and 10 retrospective case series [68–77], and 2 combined prospective/retrospective case series [78, 79]. UUI was the most common indication studied: 34 studies included patients with UUI symptoms and 11 studies investigated this population exclusively.
It is evident that some reports include patients who also appear in other reports. As such, it is not possible to determine the total number of subjects enrolled in the complete body of 40 studies. Our review identified three series of interlinked studies which each resulted in multiple publications from shared study cohorts; however, the extent of population overlap within the complete body of SNS implantation studies could not be definitively determined. The largest of these series represents results of a multicenter, prospective randomized trial sponsored by Medtronic, referred to as MDT-103. Taken as a whole, this series of studies investigated treatment of a minimum of 103 UUI patients, 29 UF patients, and 60 UR patients and resulted in at least 12 publications [9–11, 13, 25, 34, 35, 37, 65, 68, 71, 75].
Medtronic’s InterStim was the most commonly employed implantable pulse generator (IPG) device, and 15 of the 40 studies specified use of InterStim exclusively while an additional 3 studies (each retrospective) indicated use of both InterStim and the earlier generation devices Itrel I and/or II. In addition, a 2007 study described use of both InterStim and two-channel bilateral stimulation devices, Synergy and Twin [64]. Seven studies specified the use of either Itrel I and/or Itrel II. Twelve studies did not specify the type of IPG implanted.
Over the period in which the articles in this review were published, the standard procedure for implantation evolved. The original implantation technique involved a preliminary test stage referred to as a percutaneous nerve evaluation (PNE). The PNE test involves introduction of a temporary wire electrode to the sacral foramen to establish the integrity of the sacral nerves. Success of this test stimulation is generally defined as improvement of at least one major voiding symptom by more than 50%. Patients who are selected for permanent implantation based on successful test stimulation undergo an open sacral procedure with placement of a permanent electrode and IPG.
In 2003, Spinelli et al. published a description of a two-stage implantation procedure [48], and 13 of the articles dating back to December 2002 [46, 48, 56, 61, 62, 64, 66, 71, 73–77] indicate explicitly that this technique was employed (6 of these investigated both one-stage and two-stage implantation outcomes). Two-stage implantation enables test stimulation to be performed using the permanent quadripolar lead. Because only local anesthesia is applied, the patient remains awake during lead placement and thus sensory responses are accessible. Once in position, the electrode is connected to an external pulse generator by a temporary lead and left in place for an extended period (typically 3–4 weeks) during which the patient is evaluated for improvement in primary voiding symptoms (stage 1). If the voiding symptoms improve by more than 50%, subjects will undergo surgery to implant the IPG (stage 2). The basis of this procedure is a minimally invasive method for stage 1 testing, enabling elimination of an additional surgery for implantation of the permanent lead and increasing the success rate of patients qualifying for IPG implantation.
Patient outcomes for SNS implantation were determined based on various measures including voiding symptom parameters, urodynamic exam measures, QOL assessments, and subjective patient satisfaction ratings. In addition, many articles reported on complications, and a number of reports investigated adverse events exclusively. Because some reports include patients who also appear in other reports, pooling of data was not viable.
Thirty-seven of the studies included long-term follow-up data and median follow-up time was 18.8 months (range 4–84.2 months). (One study investigated two study arms, a one-stage and two-stage implant, and reported distinct mean follow-up periods for each arm [77]. Another study reported distinct mean follow-up periods for each of three clinical indications investigated [64].) Two studies that investigated adverse event outcomes exclusively did not include long-term follow-up data [73, 76].
Of the 40 SNS studies, 34 (85%) reported outcomes based on clinical symptom parameters. These data were typically assessed in comparison to baseline clinical symptom measures and obtained through patient recording of voiding diaries. Table 1 presents outcome data based on clinical symptom parameter measures for UUI, UF, and UR study cohorts. Data related to cohorts defined as OAB, combined UUI/UF, or neurogenic bladder dysfunction were not included in this table. Data were not recorded for outcome measures that were reported on by fewer than two studies. All studies reported improvements in primary voiding symptom parameters for treated subjects based on comparison with baseline assessment. The degree of improvement varied considerably from study to study as evidenced by the wide range in outcome measure data.
Fourteen articles reported on the number of patients experiencing at least a 50% improvement, either with regard to a specific voiding parameter or in their clinical symptoms in general. The percentage of successful treatments indexed in the table is based on the number of subjects who actually received the implantation and does not consider the number who failed the preliminary test stimulation. (An intention to treat analysis, which considers the total number of patients initially evaluated, is presented in Table 2.) One study reported that 100% of its patients experienced greater than 50% improvement in both incontinence episodes and daily pad use [44]. It should be noted that this study followed treatment of only 12 subjects.
Of the 40 SNS studies, 13 (32%) reported outcomes based on urodynamic examination parameters. However, urodynamic parameters documented by these studies varied considerably, and there was almost no agreement from study to study in terms of parameters selected for investigation. As such, there was no basis for comparison of these data.
Of the 40 studies, 26 (65%) reported on the success rate of the preliminary test stimulation period, including both PNEs and first-stage test stimulation modalities. Aside from the 40 articles that investigated implanted SNS, 4 additional studies [17, 26, 43, 61] provided data on percutaneous SNS used either as a test stimulation preliminary to selection for implantation or in a temporary stimulation capacity. These studies, which were published between 1994 and 2006, each included patients with various LUTS. Three of these studies focused on the identification of predictors for success of neuromodulation: two using PNE stimulation and one using first-stage stimulation. The fourth study investigated bilateral versus unilateral SNS in a randomized crossover study design [17]. This study did not find a significant difference between bilateral and unilateral stimulation and presented outcome versus baseline voiding parameter data only in graphic form and thus was not included in our analysis.
Test stimulation success was uniformly defined as at least 50% improvement in primary voiding symptoms. Overall, the success rate of the test stimulation procedures ranged from 28% for a cohort of 154 patients with various LUTS [68] to 100% for a cohort of 51 UF patients [11], with a median success rate of 54%. Nine articles presented results of a first-stage test stimulation procedure. The success rate for the first-stage test stimulation procedure ranged from 66% for a cohort of 76 patients with various LUTS [76] to 93% for a cohort of 15 patients with various LUTS [43], with a median success rate of 79%.
An intention to treat analysis was possible for only 8 (20%) [27, 32, 44, 48, 52, 64, 71, 74] of the 40 studies of implantable SNS. Since RCTs randomized only patients with successful test stimulation results, these studies were not amenable to analysis. Table 2 presents intention to treat success rate data for eight studies (six prospective and two retrospective). The intention to treat success rate considers the number of patients who ultimately experienced treatment success (i.e., greater than 50% improvement in their clinical symptoms) against the total number of patients initially evaluated via test stimulation. Subjects who declined implantation despite successful test stimulation were omitted from our analysis.
Surgical revision by patient was the most commonly documented measurement of adverse outcomes among the 40 implanted SNS studies and was reported in 14 (35%) of the articles. Three of these studies reported pooled data on the same cohort of 219 patients [11, 34, 37]. Therefore, these data were only included once in our analysis. The surgical revision rate by case ranged from 9.7% for a cohort of 103 patients at 18-month follow-up [67] to 48.3% for a cohort of 149 patients with a mean follow-up of 64.2 months [61]. The median surgical revision rate was 28.7%. A small number of studies documented surgical revision by numbers of procedures, and it is clear that a significant number of patients required two or more reoperations to correct complications. The most frequently reported adverse events were pain at IPG or lead site, lead migrations, infections, and electric shock.
Percutaneous posterior tibial nerve stimulation
Sixteen articles, published between the years of 2000 and 2010, provide data on the use of percutaneous posterior tibial nerve stimulation (PTNS) for treatment of LUTS. These include 13 prospective case series [33, 36, 38, 42, 45, 47, 49, 50, 53, 54, 57, 59, 67] and 3 randomized comparative studies [18, 19, 21]. Eight of the studies included adults with OAB and seven of these investigated this population exclusively. Aside from the studies that targeted OAB specifically, three additional studies investigated urgency-frequency syndrome and two studies investigated treatment of UUI. Treatment of UR was addressed in three articles, but two of these were separate studies of the same cohort. Two studies investigated treatment of children with various LUTS and one study investigated treatment of LUTS secondary to Parkinson’s disease.
Some articles included patients who also appear in other PTNS articles. In particular, our review identified a series of six interrelated articles, which share common authors and common study elements [19, 38, 47, 49, 50, 53]. As a result, it was not possible to definitively determine the total number of subjects enrolled in the complete body of 16 PTNS articles included in our review.
PTNS involves insertion of a 34-gauge stainless steel needle approximately 3–4 cm cephalad to the medial malleolus of the left or right ankle. A surface electrode is applied on the same leg near the arch of the foot. The needle and electrode are connected to a low-voltage electrical stimulator. The stimulation current is increased to elicit curling of the big toe or fanning of all toes.
The majority of the studies [11 of the 16 articles (69%)] applied weekly outpatient sessions over a 10- to 12-week period and defined a “session” of PTNS as a 30-min period. Two other studies, including one arm of a comparative trial, investigated more frequently applied stimulation [19, 33], and one comparative study applied the stimulation therapy for 60 min in weekly sessions over 8 weeks [18]. One long-term follow-up study allowed patients under supervision of the investigator to select their own symptom maintenance treatment intervals, which resulted in a mean treatment interval of 21 days [67]. In the study on Parkinson’s disease patients, only acute effects of PTNS were evaluated [57].
Patient outcomes for PTNS were determined based on various measures including voiding symptom parameters, urodynamic exam measures, QOL assessments, and subjective patient satisfaction. In general, outcomes were evaluated at the end of a prespecified treatment period; however, one of the studies evaluated outcomes 1 year after cessation of treatment and another long-term follow-up study reported on outcomes following 6 and 12 months of maintenance therapy [59]. The study of Parkinson’s disease patients only evaluated acute effects during one session of PTNS.
Of the 16 PTNS studies, 15 (94%) reported outcomes based on clinical symptom parameters. Only the acute study conducted on Parkinson’s disease patients did not report on symptomatic improvements.
Table 3 presents outcome data based on clinical symptom parameter measures. Data were not recorded for outcome measures that were reported on by fewer than two studies. The two Vandoninck et al. studies investigating treatment of UR patients presented identical data on the same cohort of 39 patients, and thus these data were only included once in our analysis [49, 53]. Both pediatric studies documented only the number of patients outside the normal range for each parameter rather than recording actual data for those parameters, and thus these data were also excluded from our analysis [45, 54]. Incontinence episode data reported for OAB or urgency-frequency cohorts were included with the UUI patient subgroup data, as many (not all) of the articles reporting on OAB/urgency-frequency syndrome identified UUI subgroups within the study population and attributed incontinence data only to those subgroups.
Of the 16 PTNS studies, 7 (44%) reported outcomes based on urodynamic examination measures. Each of these studies reported significant changes in one or more variables. However, urodynamic parameters documented by these studies varied considerably and there was very little agreement from study to study in terms of the parameters selected for investigation. As such, there is no basis for pooled analysis of these data. Of the 16 PTNS studies, 8 (50%) reported outcomes based on QOL assessments. Each of these studies reported that QOL measures improved significantly.
Table 4 presents intention to treat success rate data for 14 of the 16 PTNS studies included in our review. The measure of success column of the table underscores the broad heterogeneity in the definition of treatment success among this group of studies. The table does not include data from Kabay et al. [57], which investigated treatment of patients with Parkinson’s disease, since this was not an interventional study. In addition, the Congregado Ruiz et al. [52] paper did not provide data on the percentage of patients for whom treatment was deemed successful.
The MacDiarmid et al. [67] paper reported on 12-month follow-up results for a cohort of patients preselected as “responders” after the original 12-week OrBIT trial. A true intention to treat analysis for this study must consider the originally enrolled “nonresponder” patients as well. Thus, our analysis tracked the results for this PTNS cohort starting with the originally enrolled study participants to produce an intention to treat analysis for the combined sequence of studies (Peters et al. [21] and MacDiarmid et al. [67]).
Of the 16 PTNS studies, 12 (75%) reported on adverse events. Uniformly, no serious side effects were observed. Minor complications noted include transient pain at the stimulation site, minor bleeding, diarrhea, headaches, calf cramps, and low back pain.
Transcutaneous electrical stimulation (TENS)
Thirteen articles reported on the use of TENS for treatment of LUTS. These studies were published between the years of 1983 and 2006 and include three RCTs [7, 8, 12], three comparative studies [15, 16, 20] and seven prospective case series [22, 23, 31, 39, 40, 51, 55]. Four of the studies investigated treatment of pediatric populations and one of these focused on children with spina bifida. Three studies investigated treatment of detrusor instability, and two investigated OAB. The remaining studies investigated treatment of various LUTS (two studies) and irritative voiding dysfunction (one study). Each of the 13 TENS studies included in the review were conducted independently of other studies included and the cumulative number of subjects treated (TENS only) is 377.
Two of the RCTs compared TENS treatment to placebo stimulation treatment and one compared TENS treatment to respective arms of medical therapy and no treatment. The three comparative trials compared TENS treatment to medical therapy, percutaneous neuromodulation, and biofeedback training, respectively.
The majority, 8 of 13 (62%), of the studies applied TENS therapy intermittently in either an outpatient or home/long-term care setting. In general, patients were instructed to complete therapy sessions of a specific duration at predetermined intervals. Session durations varied from 15 min to 2 h and one study instructed patients to use stimulation for “up to 6 h daily” (this study did not specify a particular number of sessions per day). The frequency of sessions varied from 3 days per week to twice daily.
Two of the studies (15%) investigated outcomes based on continuous application of TENS therapy. One of these studies [22] applied TENS continuously over a 3-week period; the other [31] specified 12 h of ambulatory TENS each day for a period of 1 week. Acute TENS therapy was investigated by four of the studies (31%). For intermittent and continuous studies (ten studies), follow-up generally occurred after cessation of therapy. However, in a number of studies, patients were given the option to continue therapy. Follow-up (or length of time over which therapy was applied) was not specified by one study [20] and ranged from 1 week to 6 months in the remaining studies. Mean follow-up could not be determined owing to the lack of precision in the documentation of follow-up time in many of the articles.
Table 5 summarizes the various anatomical stimulation sites and therapy delivery modes investigated by the body of TENS studies included in this review. Three of the studies investigated two stimulation sites, either on a case-by-case, patient preference basis (two studies) or as part of a randomized trial design (one study). We were only able to include two of these three studies in the tally, as the earliest study in the TENS series [22] investigated tibial or perineal nerve stimulation applied on a case-by-case basis, but did not specify either the number of subjects treated with each modality or whether stimulation was applied continuously or intermittently. One article reported on three separate TENS studies (one intermittent and two acute) conducted on a single cohort of 36 patients [15].
Patient outcomes for TENS studies were determined based on various measures including clinical symptom parameters, urodynamic exam measures, QOL assessments, and urinary symptom scores. Primary outcomes for the acute studies were generally assessed via urodynamic parameters.
Of the 13 TENS articles, 7 (54%) documented outcomes based on clinical symptom parameters. Among these studies there was very little agreement in terms of parameters selected for investigation. Table 6 presents outcome data based on clinical symptom parameter measures for two detrusor instability studies and three pediatric studies. Data were not recorded for outcome measures that were reported on by fewer than two studies.
Of the 13 TENS articles, 8 (62%) reported outcomes based on urodynamic examination parameters, including 2 RCTs, 3 comparative, and 4 prospective case series. Each of the six acute studies, reported on in five articles, investigated outcomes in terms of urodynamics. As outlined previously in Table 5, the acute studies investigated perineal regional stimulation (three), posterior tibial nerve stimulation (two), and sacral nerve stimulation (one). Of the three perineal acute stimulation studies, one reported only on the number of patients improved, but did not note whether changes were significant, a second reported that there were no significant changes in urodynamic parameters, and the third noted significant changes relative to three urodynamic parameters. Of the two acute tibial nerve stimulation studies, one noted no significant changes in urodynamic parameters and the second noted significant changes in two parameters. One article reported on acute stimulation of the sacral nerve and noted significant changes in two urodynamic parameters.
Five articles reported on urodynamic examination parameters for intermittent or continuous TENS therapy. One of these studies reported only on the number of patients improved based on urodynamic testing [22], and the aforementioned RCT of children with spina bifida reported no significant difference between active and placebo groups [7]. The remaining three studies documented between one and five significant changes in urodynamic parameters; however, there was no agreement among these studies in terms of parameters selected for investigation.
Of the 13 TENS articles, 2 (15%) reported on QOL assessments, including 1 RCT and 1 comparative trial. The comparative trial found no significant differences from baseline assessments [16]. The RCT reported an 89% improvement [12]. Only 8 of the 13 (62%) TENS studies provided a definitive definition of successful treatment outcome [12, 20–22, 31, 39, 40, 55], as presented in Table 7.
Very few [3 of 13 (23%)] of the TENS therapy studies reported on complications. No serious complications were documented but local skin irritation was noted.
Discussion
We have conducted a systematic literature review to assess the efficacy of electrical stimulation therapies in current clinical use for treatment of various LUTS. This review, which includes 73 articles, represents the most comprehensive systematic analysis of electrical stimulation therapies for this indication to date. However, two systematic reviews of implanted SNS therapy with more limited inclusion criteria than ours have been recently published [80, 81]. Siddiqui et al. conducted a review that included 16 studies of SNS therapy for treatment of female patients with OAB. This review found that almost half the subjects reported no daily incontinence episodes after treatment [80]. Kessler et al. limited their review to subjects with neurogenic lower urinary tract dysfunction and conducted a meta-analysis of 26 studies that concluded the pooled success was 68% for the test phase and 92% for permanent SNS in this population [81]. Each of these reviews examined treatment of only a small subset of the broad LUTS population examined by the present review. Our review is also unique in its inclusion of all modalities of electrical stimulation and thus offers an important basis for comparison of these differing approaches.
Patient outcomes reported in the clinical trials we examined were documented with various outcome measures, including voiding symptom parameters, urodynamic exam measures, and QOL assessments. Our review attempted to examine therapy efficacy based on each of these categories of data. However, pooled analyses of urodynamic and QOL data proved to be inconclusive given the broad heterogeneity of parameters investigated and instruments applied. As a result, our analyses of efficacy relied primarily on clinical voiding symptom outcomes. These voiding symptom parameters provide an objective and clinically meaningful measure of therapy success and were recorded by 81% of the studies included in the review.
Our findings indicate that median mean reductions in incontinence episodes and voiding frequency were similar for implanted SNS and PTNS, with median mean values of 72 and 66% for incontinence episodes and median mean values of 40 and 32.5% for voiding frequency for SNS and PTNS, respectively. It was not possible to make any meaningful generalizations related to outcomes for the TENS studies due to the significant heterogeneity among this relatively small number of studies in terms of mode of therapy delivery, definition of patient subgroups, and outcome measures.
There is a substantial body of clinical research investigating implanted SNS for treatment of LUTS. Our review uncovered 5 RCTs and 25 prospective case series related to this therapy, which were published over a period of 17 years (1993–2009). In total, however, it is unclear how many unique studies exist, as many of these reports list common authors and overlapping dates of recruitment.
One shortcoming of the currently available PTNS data relates to the lack of long-term follow-up data. The large majority of the PTNS studies evaluated patients only at the end of the treatment period (typically 12 weeks). Only two PTNS studies included longer-term follow-up. One article evaluated outcomes 1 year after cessation of treatment and another report looked at outcomes after 6 and 12 months of maintenance therapy. Additional long-term follow-up studies are needed to validate the ability of this therapy to produce sustained benefit.
An intention to treat analysis was conducted for each of the intervention categories. This analysis was especially revealing in the case of implanted SNS, owing to the fact that the number of implanted subjects for which outcomes were assessed was frequently much lower than the number of subjects initially evaluated. For implanted SNS, treatment success was typically defined as greater than 50% improvement in clinical symptoms. However, for the two other intervention categories, there was considerable variability in the definition of treatment success.
Unfortunately, relatively few of the SNS studies were amenable to intention to treat analysis. First, we were not able to include SNS RCTs, as these studies randomized only patients with successful test stimulation results. In addition, a full 35% of the SNS studies did not document the number of patients initially evaluated for treatment but rather enrolled patients only after successful test stimulation. Finally, many other studies pooled mean reduction data rather than isolating the number of patients who realized treatment success.
As a result, only eight SNS studies qualified for intention to treat analysis. Thus, any generalizations about these data must be treated with caution. With that caveat, it appears that investigations of the two-stage implantation procedures reported slightly higher success rates. Whereas intention to treat analyses of the six studies that employed one-stage implantation with PNE documented yielded success rates uniformly below 50%, analyses of the two studies that employed two-stage implantation each yielded success rates well above 50%. This increase in success may be attributed to a number of factors including enhanced targeting of the sacral nerves afforded by the two-stage technique, the introduction of the tined lead, which may have decreased the rate of lead migration, and overall improvement in surgical technique and patient selection after years of experience with the InterStim device. Clearly, however, more data demonstrating definitive patient success are needed to fully validate the potential of this intervention.
In contrast to the implanted SNS studies, wherein success rates were almost uniformly defined (if defined at all) as greater than 50% improvement in primary voiding symptoms, the definition of success for the PTNS and TENS studies varied widely. The measure of success in these studies ranged from subjective outcomes such as “request for continuation of treatment” to unquantified “improvement of symptoms” to quantified improvement (e.g., “greater than 50% reduction in incontinence episodes”). Owing to this broad heterogeneity in the definition of success among these studies, it is not possible to make any generalizations about the relative efficacy of these therapies.
Our review included eight RCTs, five of which evaluated implanted SNS and three of which evaluated TENS. Of the five RCTs investigating SNS, three reported on clinical symptom parameters as outcomes, and these studies each observed significant benefits in terms of mean reduction from baseline. Four of the SNS RCTs also found significant improvement in QOL outcomes in favor of the stimulation groups. In contrast, results of the TENS RCTs were equivocal. Notably, one of these studies, which employed a sham stimulation strategy as placebo arm, reported that, in fact, a significant difference in nighttime urinary incontinence was observed for the placebo group but not for the treatment arm. Only one of the TENS RCTs reported a significant success rate for the treatment arm as compared to a watchful waiting control arm.
Our findings indicate that implanted SNS studies enrolled patients with higher disease severity at baseline as compared to the studies focused on the less invasive therapy modalities. For example, the rate of UUI patient enrollment for the SNS studies (85%) was significantly higher than the other two intervention categories, PTNS and TENS, which investigated UUI patients at a rate of 62.5 and 8%, respectively. Baseline values for both incontinence episodes in the UUI population and voiding frequency in the UF/OAB populations were significantly higher in the implanted SNS studies (median mean values of 7.5/day and 17.3/day, respectively) as compared to the other two therapy modalities.
As risk and potential severity of adverse events increase with increasing invasiveness, the safety profiles of the three intervention strategies examined by this review differ dramatically. For SNS, adverse outcomes were measured most commonly in terms of surgical revision rates, and these rates were significant: a median rate of 28.7% based on reports by 14 studies. Other significant adverse events associated with SNS include pain at the IPG site, infections, and electric shock. In contrast few adverse outcomes were observed during the PTNS and TENS investigations and these were limited to minor, transient complications (e.g., diarrhea, minor bleeding, headaches, muscle cramps, and irritation at the stimulation site). Clearly, these adverse event data must be taken into account as practitioners assess the respective risk-benefit profiles of each of these interventions.
This review focused broadly on the treatment of various LUTS and excluded studies of stress urinary incontinence, pelvic pain, and interstitial cystitis. Categorizing patient subgroups presented some challenges. The preferred designations, and those applied most commonly in the reports, were UUI, UF, and UR. These designations were used whenever possible. However, the descriptors “OAB” and “urgency-frequency syndrome” are also important patient subgroup designations. As these subgroups include both patients with and without UUI, they present challenges for recording voiding parameter data due to the fact that incontinence data are only relevant to a subset of the overall population. Most of the articles reporting on OAB/urgency-frequency syndrome identified UUI subsets within the study population and attributed incontinence data only to those subsets. Thus, incontinence episode data reported for OAB or urgency-frequency syndrome cohorts were analyzed with the UUI subgroup data. A number of studies relied exclusively on urodynamic definitions, such as detrusor instability or detrusor hyperreflexia, to describe study cohorts. These designations were retained and data were not integrated with clinically designated patient subgroups.
In conclusion, although there is a substantial body of research devoted to implanted SNS validation, defining the appropriate role of this therapy for LUTS patients remains challenging, especially given the therapy’s invasiveness and considerable risk profile. The two main factors inhibiting a more definitive assessment of SNS relate to the significant study population overlap identified by our review and study design flaws that inhibited rigorous intention to treat analysis based on numbers of patients initially evaluated for therapy. Although early data on PTNS are promising, long-term follow-up studies are needed to verify the ability of this therapy to confer sustained benefit. Our review revealed a general lack of concerted and focused research devoted to TENS therapy for treatment of LUTS.
References
Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S et al (2006) Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 50:1306–1315
Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R et al (2003) Prevalence and burden of overactive bladder in the United States. World J Urol 20:327–336
Oerlemans DJAJ, van Kerrebroeck PEV (2008) Sacral nerve stimulation for neuromodulation of the lower urinary tract. Neurourol Urodyn 27:28–33
Sherman ND, Amundsen CL (2007) Current and future techniques of neuromodulation for bladder dysfunction. Curr Urol Rep 8:448–454
Abrams P, Blaivas JG, Fowler CJ, Fourcroy JL, Macdiarmid SA, Siegel SW, van Kerrebroeck P (2003) The role of neuromodulation in the management of urinary urge incontinence. BJU Int 91:355–359
Apostolidis A (2011) Neuromodulation for intractable OAB. Neurourol Urodyn 30:766–770
Marshall DF, Boston VE (1997) Altered bladder and bowel function following cutaneous electrical field stimulation in children with spina bifida–interim results of a randomized double-blind placebo-controlled trial. Eur J Pediatr Surg 7(Suppl 1):41–43
Bower WF, Moore KH, Adams RD, Shepherd R (1998) A urodynamic study of surface neuromodulation versus sham in detrusor instability and sensory urgency. J Urol 160:2133–2136
Schmidt RA, Jonas U, Oleson KA, Janknegt RA, Hassouna MM, Siegel SW, van Kerrebroeck PE (1999) Sacral nerve stimulation for treatment of refractory urinary urge incontinence. Sacral Nerve Stimulation Study Group. J Urol 162:352–357
Weil EH, Ruiz-Cerdá JL, Eerdmans PH, Janknegt RA, Bemelmans BL, van Kerrebroeck PE (2000) Sacral root neuromodulation in the treatment of refractory urinary urge incontinence: a prospective randomized clinical trial. Eur Urol 37:161–171
Hassouna MM, Siegel SW, Nyeholt AA, Elhilali MM, van Kerrebroeck PE, Das AK et al (2000) Sacral neuromodulation in the treatment of urgency-frequency symptoms: a multicenter study on efficacy and safety. J Urol 163:1849–1854
Svihra J, Kurca E, Luptak J, Kliment J (2002) Neuromodulative treatment of overactive bladder–noninvasive tibial nerve stimulation. Bratisl Lek Listy 103:480–483
Das AK, Carlson AM, Michael Hull M, U.S. MDT-103 Study Group (2004) Improvement in depression and health-related quality of life after sacral nerve stimulation therapy for treatment of voiding dysfunction. Urology 64:62–68
Guys JM, Haddad M, Planche D, Torre M, Louis-Borrione C, Breaud J (2004) Sacral neuromodulation for neurogenic bladder dysfunction in children. J Urol 172:1673–1676
Hasan ST, Robson WA, Pridie AK, Neal DE (1996) Transcutaneous electrical nerve stimulation and temporary S3 neuromodulation in idiopathic detrusor instability. J Urol 155:2005–2011
Soomro NA, Khadra MH, Robson W, Neal DE (2001) A crossover randomized trial of transcutaneous electrical nerve stimulation and oxybutynin in patients with detrusor instability. J Urol 166:146–149
Scheepens WA, de Bie RA, Weil EHJ, van Kerrebroeck PEV (2002) Unilateral versus bilateral sacral neuromodulation in patients with chronic voiding dysfunction. J Urol 168:2046–2050
Karademir K, Baykal K, Sen B, Senkul T, Iseri C, Erden D (2005) A peripheric neuromodulation technique for curing detrusor overactivity: Stoller afferent neurostimulation. Scand J Urol Nephrol 39:230–233
Finazzi Agrò E, Campagna A, Sciobica F, Petta F, Germani S, Zuccalà A, Miano R (2005) Posterior tibial nerve stimulation: is the once-a-week protocol the best option? Minerva Urol Nefrol 57:119–123
Barroso U Jr, Lordêlo P, Lopes AA, Andrade J, Macedo A Jr, Ortiz V, Barroso U Jr (2006) Nonpharmacological treatment of lower urinary tract dysfunction using biofeedback and transcutaneous electrical stimulation: a pilot study. BJU Int 98:166–171
Peters KM, MacDiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES et al (2009) Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 182:1055–1061
McGuire EJ, Zhang SC, Horwinski ER, Lytton B (1983) Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol 129:78–79
Nakamura M, Sakurai T, Tsujimoto Y, Tada Y (1986) Bladder inhibition by electrical stimulation of the perianal skin. Urol Int 41:62–63
Dijkema HE, Weil EHL, Mijs PT, Janknegt RA (1993) Neuromodulation of sacral nerves for incontinence and voiding dysfunctions. Clinical results and complications. Eur Urol 24:72–76
Elabbady AA, Hassouna MM, Elhilali MM (1994) Neural stimulation for chronic voiding dysfunctions. J Urol 152:2076–2080
Koldewijn EL, Rosier PF, Meuleman EJ, Koster AM, Debruyne FM, van Kerrebroeck PE (1994) Predictors of success with neuromodulation in lower urinary tract dysfunction: results of trial stimulation in 100 patients. J Urol 152:2071–2075
Bosch JL, Groen J (1995) Sacral (S3) segmental nerve stimulation as a treatment for urge incontinence in patients with detrusor instability: results of chronic electrical stimulation using an implantable neural prosthesis. J Urol 154:504–507
Bosch JL, Groen J (1998) Neuromodulation: urodynamic effects of sacral (S3) spinal nerve stimulation in patients with detrusor instability or detrusor hyperreflexia. Behav Brain Res 92:141–150
Shaker HS, Hassouna M (1998) Sacral nerve root neuromodulation: an effective treatment for refractory urge incontinence. J Urol 159:1516–1519
Cappellano F, Ciotti MG, Pizzoccaro M, Catanzaro M, Santambrogio S, Catanzaro F (1998) Sacral root neuromodulation in the treatment of female urge and mixed urinary incontinence. Urogynaecol Int J 12:111–121
Walsh IK, Johnston RS, Keane PF (1999) Transcutaneous sacral neurostimulation for irritative voiding dysfunction. Eur Urol 35:192–196
Bosch JL, Groen J (2000) Sacral nerve neuromodulation in the treatment of patients with refractory motor urge incontinence: long-term results of a prospective longitudinal study. J Urol 163:1219–1222
Klingler HC, Pycha A, Schmidbauer J, Marberger M (2000) Use of peripheral neuromodulation of the S3 region for treatment of detrusor overactivity: a urodynamic-based study. Urology 56:766–771
Siegel SW, Catanzaro F, Dijkema HE, Elhilali MM, Fowler CJ, Gajewski JB et al (2000) Long-term results of a multicenter study on sacral nerve stimulation for treatment of urinary urge incontinence, urgency-frequency, and retention. Urology 56:87–91
Edlund C, Hellström M, Peeker R, Fall M (2000) First Scandinavian experience of electrical sacral nerve stimulation in the treatment of the overactive bladder. Scand J Urol Nephrol 34:366–376
Govier FE, Litwiller S, Nitti V, Kreder KJ, Rosenblatt P (2001) Percutaneous afferent neuromodulation for the refractory overactive bladder: results of a multicenter study. J Urol 165:1193–1196
Janknegt RA, Hassouna MM, Siegel SW, Schmidt RA, Gajewski JB, Rivas DA et al (2001) Long-term effectiveness of sacral nerve stimulation for refractory urge incontinence. Eur Urol 39:101–106
van Balken MR, Vandoninck V, Gisolf KW, Vergunst H, Kiemeney LA, Debruyne FM, Bemelmans BL (2001) Posterior tibial nerve stimulation as neuromodulative treatment of lower urinary tract dysfunction. J Urol 166:914–918
Hoebeke P, Van Laecke E, Everaert K, Renson C, De Paepe H, Raes A, Vande Walle J (2001) Transcutaneous neuromodulation for the urge syndrome in children: a pilot study. J Urol 166:2416–2419
Bower WF, Moore KH, Adams RD (2001) A pilot study of the home application of transcutaneous neuromodulation in children with urgency or urge incontinence. J Urol 166:2420–2422
Hohenfellner M, Humke J, Hampel C, Dahms S, Matzel K, Roth S, Thüroff JW et al (2001) Chronic sacral neuromodulation for treatment of neurogenic bladder dysfunction: long-term results with unilateral implants. Urology 58:887–892
Aboseif S, Tamaddon K, Chalfin S, Freedman S, Kaptein J (2002) Sacral neuromodulation as an effective treatment for refractory pelvic floor dysfunction. Urology 60:52–56
Scheepens WA, Jongen MMGJ, Nieman FHM, de Bie RA, Weil EHJ, van Kerrebroeck PEV (2002) Predictive factors for sacral neuromodulation in chronic lower urinary tract dysfunction. Urology 60:598–602
Amundsen CL, Webster GD (2002) Sacral neuromodulation in an older, urge-incontinent population. Am J Obstet Gynecol 187:1462–1465
Hoebeke P, Renson C, Petillon L, Vande Walle J, De Paepe H (2002) Percutaneous electrical nerve stimulation in children with therapy resistant nonneuropathic bladder sphincter dysfunction: a pilot study. J Urol 168:2605–2607
Scheepens WA, Van Koeveringe GA, De Bie RA, Weil EHJ, Van Kerrebroeck PEV (2002) Long-term efficacy and safety results of the two-stage implantation technique in sacral neuromodulation. BJU Int 90:840–845
Vandoninck V, Van Balken MR, Finazzi Agrò E, Petta F, Caltagirone C, Heesakkers JPFA et al (2003) Posterior tibial nerve stimulation in the treatment of urge incontinence. Neurourol Urodyn 22:17–23
Spinelli M, Giardiello G, Arduini A, van den Hombergh U (2003) New percutaneous technique of sacral nerve stimulation has high initial success rate: preliminary results. Eur Urol 43:70–74
Vandoninck V, van Balken MR, Finazzi Agrò E, Petta F, Micali F, Heesakkers JPFA et al (2003) Posterior tibial nerve stimulation in the treatment of idiopathic nonobstructive voiding dysfunction. Urology 61:567–572
Vandoninck V, van Balken MR, Finazzi Agrò E, Petta F, Micali F, Heesakkers JPFA et al (2003) Percutaneous tibial nerve stimulation in the treatment of overactive bladder: urodynamic data. Neurourol Urodyn 22:227–232
Amarenco G, Ismael SS, Even-Schneider A, Raibaut P, Demaille-Wlodyka S, Parratte B, Kerdraon J (2003) Urodynamic effect of acute transcutaneous posterior tibial nerve stimulation in overactive bladder. J Urol 169:2210–2215
Congregado Ruiz B, Pena Outeiriño XM, Campoy Martínez P, León Dueñas E, Leal López A (2004) Peripheral afferent nerve stimulation for treatment of lower urinary tract irritative symptoms. Eur Urol 45:65–69
Vandoninck V, van Balken MR, Finazzi Agrò E, Heesakkers JPFA, Debruyne FMJ, Kiemeney LALM, Bemelmans BLH (2004) Posterior tibial nerve stimulation in the treatment of voiding dysfunction: urodynamic data. Neurourol Urodyn 23:246–251
De Gennaro M, Capitanucci ML, Mastracci P, Silveri M, Gatti C, Mosiello G (2004) Percutaneous tibial nerve neuromodulation is well tolerated in children and effective for treating refractory vesical dysfunction. J Urol 171:1911–1913
Yokozuka M, Namima T, Nakagawa H, Ichie M, Handa Y (2004) Effects and indications of sacral surface therapeutic electrical stimulation in refractory urinary incontinence. Clin Rehabil 18:899–907
Spinelli M, Weil E, Ostardo E, Del Popolo G, Ruiz-Cerdá JL, Kiss G, Heesakkers J (2005) New tined lead electrode in sacral neuromodulation: experience from a multicentre European study. World J Urol 23:225–229
Kabay SC, Kabay S, Yucel M, Ozden H (2009) Acute urodynamic effects of percutaneous posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with Parkinson’s disease. Neurourol Urodyn 28:62–67
Amundsen CL, Romero AA, Jamison MG, Webster GD (2005) Sacral neuromodulation for intractable urge incontinence: are there factors associated with cure? Urology 66:746–750
Nuhoğlu B, Fidan V, Ayyildiz A, Ersoy E, Germiyanoglu C (2006) Stoller afferent nerve stimulation in woman with therapy resistant over active bladder; a 1-year follow up. Int Urogynecol J Pelvic Floor Dysfunct 17:204–207
Cohen BL, Tunuguntla HSGR, Gousse A (2006) Predictors of success for first stage neuromodulation: motor versus sensory response. J Urol 175:2178–2181
Humphreys MR, Vandersteen DR, Slezak JM, Hollatz P, Smith CA, Smith JE, Reinberg YE (2006) Preliminary results of sacral neuromodulation in 23 children. J Urol 176:2227–2231
Van Voskuilen AC, Oerlemans DJAJ, Weil EHJ, van den Hombergh U, van Kerrebroeck PEVA (2007) Medium-term experience of sacral neuromodulation by tined lead implantation. BJU Int 99:107–110
Foster RT Sr, Anoia EJ, Webster GD, Amundsen CL (2007) In patients undergoing neuromodulation for intractable urge incontinence a reduction in 24-hr pad weight after the initial test stimulation best predicts long-term patient satisfaction. Neurourol Urodyn 26:213–217
Kessler TM, Buchser E, Meyer S, Engeler DS, Al-Khodairy A, Bersch U et al (2007) Sacral neuromodulation for refractory lower urinary tract dysfunction: results of a nationwide registry in Switzerland. Eur Urol 51:1357–1363
van Kerrebroeck PEV, van Voskuilen AC, Heesakkers JPFA, Lycklama á Nijholt AA, Siegel S, Jonas U et al (2007) Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 178:2029–2034
White WM, Mobley JD III, Doggweiler R, Dobmeyer-Dittrich C, Klein FA (2009) Incidence and predictors of complications with sacral neuromodulation. Urology 73:731–735
MacDiarmid SA, Peters KM, Shobeiri SA, Wooldridge LS, Rovner ES, Leong FC et al (2010) Long-term durability of percutaneous tibial nerve stimulation for the treatment of overactive bladder. J Urol 183:234–240
Grünewald V, Höfner K, Thon WF, Kuczyk MA, Jonas U (1999) Sacral electrical neuromodulation as an alternative treatment option for lower urinary tract dysfunction. Restor Neurol Neurosci 14:189–193
Everaert K, De Ridder D, Baert L, Oosterlinck W, Wyndaele JJ (2000) Patient satisfaction and complications following sacral nerve stimulation for urinary retention, urge incontinence and perineal pain: a multicenter evaluation. Int Urogynecol J Pelvic Floor Dysfunct 11:231–235
Scheepens WA, van Koeveringe GA, de Bie RA, Weil EHJ, van Kerrebroeck PEV (2003) Urodynamic results of sacral neuromodulation correlate with subjective improvement in patients with an overactive bladder. Eur Urol 43:282–287
van Voskuilen AC, Oerlemans DJAJ, Weil EHJ, de Bie RA, van Kerrebroeck PEVA (2006) Long term results of neuromodulation by sacral nerve stimulation for lower urinary tract symptoms: a retrospective single center study. Eur Urol 49:366–372
Groen J, Ruud Bosch JLH, van Mastrigt R (2006) Sacral neuromodulation in women with idiopathic detrusor overactivity incontinence: Decreased overactivity but unchanged bladder contraction strength and urethral resistance during voiding. J Urol 175:1005–1009
Deng DY, Gulati M, Rutman M, Raz S, Rodríguez LV (2006) Failure of sacral nerve stimulation due to migration of tined lead. J Urol 175:2182–2185
Starkman JS, Wolter CE, Scarpero HM, Milam DF, Dmochowski RR (2007) Management of refractory urinary urge incontinence following urogynecological surgery with sacral neuromodulation. Neurourol Urodyn 26:29–35
Sutherland SE, Lavers A, Carlson A, Holtz C, Kesha J, Siegel SW (2007) Sacral nerve stimulation for voiding dysfunction: one institution’s 11-year experience. Neurourol Urodyn 26:19–28
Guralnick ML, Benouni S, O’Connor RC, Edmiston C (2007) Characteristics of infections in patients undergoing staged implantation for sacral nerve stimulation. Urology 69:1073–1076
Datta SN, Chaliha C, Singh A, Gonzales G, Mishra VC, Kavia RBC et al (2008) Sacral neurostimulation for urinary retention: 10-year experience from one UK centre. BJU Int 101:192–196
Carone R, Bertapelle P, Zanollo A, Spinelli M, Del Popolo G, Lombardi G et al (1999) Sacral neuromodulation in neurogenic lower urinary tract dysfunction: results of a multicenter study group. Urodinamica 9:177–182
Spinelli M, Bertapelle P, Cappellano F, Zanollo A, Carone R, Catanzaro F et al (2001) Chronic sacral neuromodulation in patients with lower urinary tract symptoms: results from a national register. J Urol 166:541–545
Siddiqui NY, Wu JM, Amundsen CL (2010) Efficacy and adverse events of sacral nerve stimulation for overactive bladder: a systematic review. Neurourol Urodyn 29:S18–S23
Kessler TM, La Framboise D, Trelle S, Fowler CJ, Kiss G, Pannek J et al (2010) Sacral neuromodulation for neurogenic lower urinary tract dysfunction: systematic review and meta-analysis. Eur Urol 58:865–874
Acknowledgements
The authors would like to thank Kim Fortier-Gruidl (Statistician) for her assistance with data analysis.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monga, A.K., Tracey, M.R. & Subbaroyan, J. A systematic review of clinical studies of electrical stimulation for treatment of lower urinary tract dysfunction. Int Urogynecol J 23, 993–1005 (2012). https://doi.org/10.1007/s00192-012-1691-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-1691-5