Abstract
Introduction and hypothesis
Electrical nerve stimulation is a widely used treatment for overactive bladder but there is no consensus regarding the best placement of electrodes or protocols. We hypothesised that some non-implanted neurostimulation protocols would be more effective compared to others for treating urinary symptoms and improving quality of life among adults diagnosed with non-neurogenic overactive bladder.
Methods
A systematic review and meta-analyses of randomized clinical trials were performed in five electronic databases: PubMed/MEDLINE, Lilacs, CINAHL, Web of Science, and PEDro. The main outcome was urinary symptoms—frequency, nocturia, and urgency—and the secondary outcome quality of life. Some protocol characteristics were extracted, e.g., frequency, pulse width, intensity, intervention time, and electrode placement.
Results
Nine randomized controlled trials were included. Tibial neurostimulation showed better results than sacral neurostimulation for urge incontinence (mean difference = 1.25 episodes, 95% CI, 0.12–2.38, n = 73). On the pooled analysis, the different neurostimulation protocols—intravaginal, percutaneous tibial, and transcutaneous tibial nerve stimulation—demonstrated similar results for urinary frequency, nocturia, and urgency as well as quality of life. In general, effect sizes from meta-analyses were low to moderate. The best reported parameters for percutaneous tibial nerve stimulation were 20-Hz frequency and 200-μs width, once a week.
Conclusions
There was evidence that tibial neurostimulation is more effective than sacral neurostimulation for urge incontinence symptoms among patients with non-neurogenic overactive bladder. Overall, there was no superiority of an electrical nerve stimulation electrode placement and protocol over others considering urinary symptoms and quality of life. Further studies with three-arm trials are necessary. This study was registered at PROSPERO: CRD4201810071.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overactive bladder (OAB), according to the International Continence Society, is a complex dysfunction encompassing symptoms of urgency, augmented urinary frequency, and nocturia, which may also be associated with urinary incontinence [1]. Approximately two-thirds of women and one-third of men develop urinary incontinence associated with OAB, affecting quality of life (QoL) [2, 3]. The first-line treatment for OAB is cognitive behavioural therapy, and the second-line treatment is pharmacological management with anti-muscarinic or β3-adrenoreceptor agonist drugs [3], which lead to various negative side effects in several systems such as the gastrointestinal, cardiac, neurological, urogynecological, and nasopharyngeal systems [4, 5]. Hence, due to their low tolerance and adherence rates, the use of these drugs is limited among older individuals.
Current practice recommends the use of neurostimulation as a non-pharmacological and alternative treatment option for OAB, particularly if conventional treatment fails or if the medications are not tolerated [3, 6]. The detrusor overactivity can be supressed by two different mechanisms; the first occurs by direct inhibition of bladder preganglionic neurons and the second by inhibition of interneuronal transmission in the afferent limb micturition reflex [7]. Neurostimulation aims to inhibit the reflex activity of the detrusor muscle by stimulating somatic afferent pathways capable of blocking the processing of visceral afferent signals; therefore, electric stimulation of the nerve or dermatome blocks the afferent inputs from the bladder. The tibial nerve (L4–S3) divides the sacral roots with the somatic afferent pudendal nerve (S2–S4), providing inhibition of spinal cord sensory processing [3, 5,6,7,8]. The mechanism explaining why neurostimulation is effective for treating OAB is not fully understood. Despite voiding control being mostly voluntary, various somatic and visceral afferent nerve stimulations, including electrical stimulation, may awaken the primitive mechanism of inhibitory modulation of the micturition reflex in the spinal cord [7]. Electrical superficial electrodes and vaginal or anal probes stimulate motor efferent fibres of the pudendal nerve, causing pelvic floor muscle contractions that inhibit detrusor contractions by activating the A3 Mahoney reflex, which postpones the micturition desire [9].
Neurostimulation protocols for OAB can be transcutaneous, covering three possible placement regions for the surface electrodes: over the sacrum in the region of the sacral nerve roots [8], over the tibial nerve at the ankle [3, 8], or intra-vaginal. It can also be percutaneous, with needle electrodes inserted near the tibial nerve [8, 10]. Preliminary evidence shows neurostimulation to be a safe and cost-effective intervention to reduce urinary symptoms and improve long-term QoL [3, 6, 11, 12]. Studies comparing the use of neurostimulation to placebo/sham treatment or with pharmacological treatment show beneficial results for OAB [3, 5, 6, 8].
This notwithstanding, the findings are inconclusive regarding the most appropriate neurostimulation protocol considering different parameter settings such as frequency, pulse width, and intensity, highlighting the absence of intervention parameters consensus [6, 10, 11]. The nonexistence of standardized protocols might compromise the effectiveness of OAB treatment. The guideline from the American Urological Association classifies the use of peripheral tibial nerve stimulation for non-neurogenic OAB as a third-line treatment with grade C evidence strength [3]. Also, sacral implanted neuromodulation is recommended as a third-line treatment for patients with severe refractory OAB symptoms with grade C evidence strength. This is an invasive treatment as an implantable device is placed on the iliac crest and the electrodes are directly connected into levels S3–S4 [3]. Thus, it is not the focus of the present study.
In addition, systematic reviews [6, 10, 11] and a guideline [3] suggest forthcoming studies with higher levels of evidence, aiming at standardization of neurostimulation protocols for OAB to improve health outcomes [3, 6, 10, 11]. Therefore, considering the great usability and wide variability of neurostimulation protocols for non-neurogenic OAB treatment, the aim of the present study was to analyse current literature on the effectiveness of different neurostimulation protocols for treating urinary symptoms and improving quality of life among adults diagnosed with non-neurogenic overactive bladder.
Materials and methods
Design
This systematic review report is based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [13] and was registered at the International Prospective Register of Systematic Reviews-PROSPERO (CRD4201810071).
Search strategy
Systematic searches were performed in five databases on 19 April 2020: PubMed/MEDLINE (via National Library of Medicine), Lilacs, CINAHL with full text (EBSCO), Web of Science (Thomson Reuters Scientific), and PEDro. A further manual search of the included references was also conducted. The full search strategy is available as supplementary material.
Inclusion criteria
The inclusion criteria were: (1) adults aged > 18 years with a diagnosis of non-neurogenic OAB; (2) randomized clinical trials or quasi-randomized clinical trials comparing different neurostimulation protocols (e.g., frequency, pulse width, intensity, intervention time, and electrode placement); (3) related to the primary outcomes nocturia, urinary frequency, urgency, and urge incontinence, with the secondary outcome QoL. The included studies were articles published in English, Spanish, French, and Portuguese. Articles on sacral neuromodulation implants were excluded.
Data extraction and quality assessment
After the database searches, titles were screened to identify duplicate publications, which were removed. KZ, IK, and BBHB screened titles to find potential studies for full reading and, in sequence, the three authors extensively read the available articles to select those that met the eligibility criteria. Any disagreements were resolved by consensus. The extracted information included authorship, year of publication, sample characteristics (age and clinical diagnosis), instruments, neurostimulation protocol, results, and study limitations.
The methodological quality of the included studies was assessed by the Risk of Bias tool from the Cochrane Collaboration [14]. Studies were classified as good quality (low risk in all items); fair quality (high risk of bias for one domain or two criteria unclear); poor quality (two or more criteria listed as a high or unclear risk of bias).
Data analysis
Data were analysed as continuous variables and presented as standardized mean differences (SMD) and 95% confidence intervals to pool primary and secondary outcomes. Meta-analyses were performed on Review Manager (RevMan®) (computer programme), version 5.3 (Copenhagen, The Cochrane Collaboration, 2014), comparing the different neurostimulation protocols, which were visually displayed in the forest plot. The data heterogeneity was assessed through I2 statistics. If significant heterogeneity was identified (I2 > 50%), studies were pooled for meta-analysis using a random-effects model; if not, a fixed-effects model was chosen. The value of effect sizes was interpreted as follows, in accordance with Cohen: effect size < 0.5 = small; effect size 0.5–0.8 = moderate; effect size > 0.8 = large [14].
Results
Flow of studies through the review
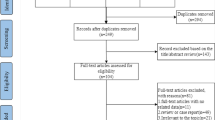
The first literature search of electronic bibliographic databases retrieved 3024 titles: 757 were from Lilacs, 41 from PEDro, 388 from Web of Science, 1070 from PubMed/MEDLINE, and 768 from CINAHL. After removing duplicates, 1944 studies were screened by titles and abstracts and 29 were considered potentially relevant. After full reading of the available articles, nine studies met the inclusion criteria; details from the search are available in supplementary file 1. Four studies were added and, after manual screening of the references from included studies, no further studies were selected. Thus, nine studies were analysed in this systematic review. The flow chart of the selected studies is available in Fig. 1.
Characteristics of the included studies
The countries that published the included articles were: Brazil [16,17,18,19] India [20], Turkey [21], Italy [22], the UK [23], and Spain [24]. The publication years ranged from 2011 to 2020, demonstrating ongoing interest in this treatment option. The studies included from 15 to 101 subjects (464 subjects in total). The average age of participants in all studies ranged from 41.8 to 69.57 years. In addition to the diagnosis of OAB, only one study included subjects with mixed urinary incontinence [16], which means a diagnosis of non-neurogenic OAB associated with stress-related urinary incontinence. Seven studies had a two-arm design [16, 17, 19, 21,22,23,24], while two had a three-arm design [18, 20].
Methodological quality of the included studies
Five studies were considered of poor quality [16, 19, 21, 22, 24] and four of fair quality [17, 18, 20, 23] (Fig. 6). Approximately 44.4% of the studies presented an unclear risk of selection bias, 66.6% an unclear risk of performance bias, 55.5% an unclear risk of detection bias, and 77.7% an unclear risk of reporting bias.
Effect of intervention
There was great variability in the adopted neurostimulation parameter settings, such as frequency, pulse width, intensity, application time, and electrode placement. Neurostimulation of the tibial nerve (transcutaneous or percutaneous) was investigated in all included studies. Percutaneous stimulation was applied in four studies [21,22,23,24], intra-vaginal transcutaneous neurostimulation was performed in three [16, 21, 22], and stimulation in the sacral region in three [17, 19, 20]. The treatment protocols are given in Table 1.
Assessment instruments
Several instruments were used to evaluate the outcomes of the studies (Table 1). The most used instruments were a voiding diary [16, 17, 19, 21,22,23,24] and the International Consultation on Incontinence Questionnaire Overactive Bladder (ICIQ-OAB) [17, 18]. Regarding QoL, the most common instruments were the Incontinence Quality of Life Instrument (I-QOL) [16, 24], Overactive Bladder Questionnaire Short Form (OAB-q SF) [22, 23], and International Consultation on Incontinence Questionnaire Overactive Bladder (ICIQ-OAB) [17,18,19]. Other instruments were used to identify the symptoms of urgency in subjects and the perception of improvement (Table 2).
Intravaginal versus tibial neurostimulation
Three studies compared intra-vaginal with tibial neurostimulation regarding urinary frequency and nocturia (Fig. 2A and B). Results for urgency and urge incontinence could not be pooled as quality assessment showed high risk of reporting, detection, and performance bias [21, 22], and one study reported unclear risk also for selection bias [17].
Forest plot of intra-vaginal versus transcutaneous tibial nerve stimulation for urinary symptoms (urinary frequency and nocturia). (A) Urinary frequency (how many times urinate per day). (B) Nocturia (how many times subject urinates during sleeping hours). IVN = intravaginal neuromodulation; TTNS = transcutaneous tibial nerve stimulation
On pooled results, no significant differences were found between intravaginal and tibial neurostimulation for urinary frequency [mean difference (MD) –0.24 times a day, 95% CI –1.45 to 0.96, n = 69] and for nocturia (MD 0.07 times to urinate during sleeping hours, 95% CI –0.22 to 0.37, n = 69).
Transcutaneous versus percutaneous tibial neurostimulation
Two studies compared transcutaneous with percutaneous tibial neurostimulation. One of them reported no differences regarding urinary frequency, nocturia, urgency, urge incontinence, and voided volume [24]. On pooled results, no significant differences were found for urgency (MD = 0.70 episodes per day, 95% CI –1.06 to 2.45, n = 92), urinary frequency found (MD = −0.66 times a day, 95% CI –1.50 to 0.17, n = 92), and urge incontinence (MD = 0.25 episodes per day, 95% CI –0.50 to 0.99, n = 92) (Fig. 3A and B). Considering the risk of bias, both studies showed fair quality. Unclear bias risk was reported for performance [23, 24], detection [24], and reporting [23, 24].
Forest plot of transcutaneous tibial nerve stimulation versus percutaneous tibial nerve stimulation for urinary symptoms (urgency and urinary frequency). (A) Urgency (episodes per day). (B) Urinary frequency (how many times subject urinates per day). TTNS = transcutaneous tibial nerve stimulation. PTNS = percutaneous tibial nerve stimulation
Sacral versus tibial neurostimulation
Three studies compared the neurostimulation of the sacral nerve with the posterior tibial nerve. One of them performed a three-arm trial, although it could not be pooled on meta-analysis because it provided only the full score of OABSS and did not report specific symptoms [20]. This study, which had fair methodological quality and reported an unclear risk of selection bias, found that simultaneous stimulation of the sacral and tibial nerve was more effective in relieving OAB symptoms compared to sacral or tibial nerve stimulation alone [20]. The other two studies were pooled (Fig. 4 A and 4B) [17, 19]. Regarding the risk of bias, two were of fair quality and reported an unclear risk of selection [17, 19], performance, detection [19], reporting [19, 20], and other biases [17].
One low risk of bias RCT [17] and one high risk of bias RCT [19] assessed urge incontinence after sacral or tibial nerve stimulation and found better results for tibial than sacral nerve stimulation (MD 1.25, 95% CI 0.12 to 2.38, n = 73). Regarding urinary frequency (MD 0.03, 95% CI –1.26 to 1.32, n = 73), no differences were found.
Quality of life
Patient QoL was reported in all included studies. Regarding the different protocol effects on QoL, none of the pooled results showed difference to favour any of the protocols (Fig. 5). When analysing Table 2, which compared intra-vaginal and tibial neurostimulation, one study demonstrated improvement of QoL on OAB-q SF 6 (p = 0.017) and OAB-q SF13 scores (p = 0.019) after tibial neurostimulation [22]. Another study that showed significant results regarding QoL compared sacral nerve stimulation with tibial neurostimulation, and both UDI-6 (p = 0.048) and IIQ-7 (p = 0.038) scores were improved [20].
Forest plot comparing neurostimulation protocols for quality-of-life score. (A) Intra-vaginal versus transcutaneous tibial nerve stimulation. (B) Transcutaneous versus percutaneous tibial nerve stimulation. (C) Sacral versus transcutaneous tibial nerve stimulation. IVN = intravaginal neuromodulation; TTNS = transcutaneous tibial nerve stimulation. PTNS = percutaneous tibial nerve stimulation
Discussion
This systematic review and meta-analyses aimed to analyse current literature on the effectiveness of different neurostimulation protocols for treating urinary symptoms and improving quality of life among adults diagnosed with non-neurogenic OAB. Our results showed no difference between protocols for urinary frequency, nocturia, and quality of life. However, there was evidence supporting the use of posterior tibial neurostimulation to improve urge incontinence compared to sacral superficial nerve stimulation. This result could be explained as the tibial nerve is more superficial than sacral nerve roots, the transcutaneous electrodes placed on the tibial posterior muscle awake the inhibitory primitive reflex in the spinal cord, arousing the inhibitory reflex on the detrusor and normalizing bladder functioning [7].
Tibial neurostimulation was performed in all the studies included in the present systematic review. The percutaneous modality was applied in four studies [21,22,23,24], while the other studies used transcutaneous application [13,14,15,16, 18,19,20]. No differences were found in the between-group analysis regarding percutaneous or transcutaneous tibial neurostimulation application [19, 20]. These results are consistent with previous reviews, which highlighted the effectiveness of tibial neurostimulation compared to sham groups [6]. Similarly, guidelines consider tibial neurostimulation the best treatment optio for OAB in clinical practice [3], as it presents analogous results to pharmacological treatment, without the reported systemic side effects. In addition, previous authors reported that tibial neurostimulation is a more comfortable, safer, and cost-effective treatment option [25]. Regarding urinary symptoms and QoL, tibial neurostimulation presented more positive effects than intra-vaginal application [18].
Three previous systematic reviews analysed the effects of neurostimulation protocols for non-neurogenic OAB with sham/placebo groups but, unlike our study, did not compare different neurostimulation protocols. A previous review involved findings with a moderate-to-high risk of bias, showing that neurostimulation improved the non-neurogenic OAB in children [26]. The second review found moderate quality evidence supporting the use of percutaneous tibial neurostimulation; however, it included both trials and observational studies [12]. The third review concluded that electrical stimulation appeared more effective than no treatment and drug treatment for OAB [9]. The findings of our systematic review are specific to non-neurogenic OAB in adults treated with neurostimulation and, in contrast to the previous reviews, we focused on analysis of the most effective neurostimulation protocol.
Despite the lack of consensus regarding neurostimulation parameter settings, the data presented herein are in accordance with the American Urology Association [29], which suggests neurostimulation should be performed twice a week for 30 min for 12 weeks [3]. One trial with patients with neurogenic overactive bladder due to spinal cord injury also suggests that transcutaneous tibial nerve stimulation improved urodynamic parameters, generating similar results as those obtained with anticholinergics [30].
The results from this systematic review should be interpreted with caution, as most of studies had flaws in their methodologies, especially the lack in blinding of participants and personnel and selective reporting, since most of studies did not publish their protocols. There was a wide range of different protocols among studies, hindering the comparison between different protocols.
As with any study of this nature, there is a potential bias in study selection. Five studies were not fully available on the internet and, despite our efforts to contact the authors to request these studies, we did not succeed. Another limitation was the absence of a clear description of the neurostimulation protocols proposed by the authors. The strengths were the inclusion of many languages, searches in five databases, no filters added in the searches, and the investigation of intervention programmes with distinct neurostimulation parameter settings and protocols. Moreover, all included studies used reliable and reproducible assessment methods for urinary symptoms and QoL. All the included studies presented low dropout rates, which minimized the bias for observed effects and, consequently, provided more accurate data regarding the effectiveness of the proposed treatments [27].
Thus, given the absence of standardized intervention protocols, we strongly suggest further studies with a more rigorous methodological plan, with major sample sizes and a clearer description of electrical stimulation parameters; preferably trials with a three-arm design are necessary to investigate the optimisation of electrical neurostimulation parameters for treating non-neurogenic OAB and to avoid loop inconsistencies [28]. In addition, we recommend forthcoming studies that assess the comfort of neurostimulation modalities. Multimodal studies are welcome especially if further studies explore the benefits and effectiveness of the combination of neurostimulation with behavioural therapy for OAB.
In conclusion, the present study shows evidence for the use of tibial posterior neurostimulation within a frequency of 20 Hz and 200 μs width once a week to treat urge incontinence in non-neurogenic OAB patients.
Abbreviations
- OAB:
-
Overactive bladder
- QoL:
-
Quality of life
- ICIQ-OAB:
-
International Consultation on Incontinence Questionnaire Overactive Bladder
- I-QOL:
-
Incontinence Quality of Life Instrument
- OAB-q SF:
-
Overactive Bladder Questionnaire Short Form
- MD:
-
Mean difference
References
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187(1):116–26. https://doi.org/10.1067/mob.2002.125704.
Soler R, Gomes CM, Averbeck MA, Koyama M. The prevalence of lower urinary tract symptoms (LUTS) in Brazil: results from the epidemiology of LUTS (Brazil LUTS) study. Neurourol Urodyn. 2018;37(4):1356–64. https://doi.org/10.1002/nau.23446.
Gormley EA, Lightner DJ, Faraday M, Vasavada SP. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015;193(5):1572–80. https://doi.org/10.1016/j.juro.2015.01.087.
White N, Iglesia CB. Overactive bladder. Obstet Gynecol Clin N Am. 2016;43(1):59–68. https://doi.org/10.1016/j.ogc.2015.10.002.
Truzzi JC, Gomes CM, Bezerra CA, et al. Overactive bladder - 18 years - part II. Int Braz J Urol. 2016;42(2):199–214. https://doi.org/10.1590/S1677-5538.IBJU.2015.0367.
Wibisono E, Rahardjo HE. Effectiveness of short term percutaneous Tibial nerve stimulation for non-neurogenic overactive bladder syndrome in adults: A Meta-analysis. Acta Med Indones. 2015;47(3):188–200.
Leng WW, Chancellor MB. How sacral nerve stimulation neuromodulation works. Urol Clin North Am. 2005;32(1):11–8. https://doi.org/10.1016/j.ucl.2004.09.004.
NICE. Urinary incontinence in women: urinary incontinence in women: management management clinical guideline. 2013:1–50.
Stewart F, Lf G, ED R, Mo G, Kapoor A, Jl A. Electrical stimulation with non-implanted electrodes for overactive bladder in adults (review). Cochrane Database Syst Rev. 2016;4:CD010098. https://doi.org/10.1002/14651858.CD010098.pub3.www.cochranelibrary.com.
Jerez-Roig J, Souza DLB, Espelt A, Costa-Marín M, Belda-Molina AM. Pelvic floor electrostimulation in women with urinary incontinence and/or overactive bladder syndrome: A systematic review. Actas Urológicas Españolas. 2013;37(7):429–44. https://doi.org/10.1016/j.acuro.2012.08.003.
Booth J, Connelly L, Dickson S, Duncan F, Lawrence M. The effectiveness of transcutaneous tibial nerve stimulation (TTNS) for adults with overactive bladder syndrome: A systematic review. Neurourol Urodyn. 2018;37(2):528–41. https://doi.org/10.1002/nau.23351.
Wang M, Jian Z, Ma Y, Jin X, Li H, Wang K. Percutaneous tibial nerve stimulation for overactive bladder syndrome: a systematic review and meta-analysis. Int Urogynecol J. 2020. https://doi.org/10.1007/s00192-020-04429-8.
Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. https://doi.org/10.1136/bmj.b2535.
Higgins JPT GS. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated march 2011]. The cochrane collaboration; 2011.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York: Routledge; 1988.
de Menezes Franco M, de Oliveira SF, Mateus CL, de Vasconcelos E, et al. Evaluation of quality of life and loss urine of women with overactive bladder treated with intravaginal or tibial nerve electro stimulation. Fisioter e Pesqui. 2011;18(2):145–50. https://doi.org/10.1590/S1809-29502011000200008.
Jacomo RH, Alves AT, Lucio A, Garcia PA, Lorena DCR, de Sousa JB. Transcutaneous tibial nerve stimulation versus parasacral stimulation in the treatment of overactive bladder in elderly people: a triple-blinded randomized controlled trial. Clinics (Sao Paulo). 2020;75:e1477. https://doi.org/10.6061/clinics/2020/e1477.
Teixeira Alve A, Azevedo Garcia P, Henriques Jácomo R, et al. Effectiveness of transcutaneous tibial nerve stimulation at two different thresholds for overactive bladder symptoms in older women: a randomized controlled clinical trial. Maturitas. 2020;135:40–6. https://doi.org/10.1016/j.maturitas.2020.02.008.
dos Santos BR, Gomes JL, Pompermayer RCL, Abreu GKP. Os benefícios da eletroestimulação transcutânea via nervo tibial posterior e parassacral no tratamento de bexiga hiperativa. Fisioter Bras. 2019;20(2):239–48. https://doi.org/10.33233/fb.v20i2.2420.
Surbala L, Ratan Khuman P, Mital V, Devanshi B. Neuromodulation for overactive bladder with transcutaneous electrical nerve stimulation in adults – a randomized clinical study. Int J Pharm Bio Sci. 2014;5(4):671–9.
Ugurlucan FG, Onal M, Aslan E, Ayyildiz Erkan H, Kizilkaya Beji N, Yalcin O. Comparison of the effects of electrical stimulation and posterior tibial nerve stimulation in the treatment of overactive bladder syndrome. Gynecol Obstet Investig. 2013;75(1):46–52. https://doi.org/10.1159/000343756.
Vecchioli Scaldazza C, Morosetti C, Giampieretti R, Lorenzetti R, Baroni M. Percutaneous tibial nerve stimulation versus electrical stimulation with pelvic floor muscle training for overactive bladder syndrome in women: results of a randomized controlled study. Int Braz J Urol. 2017;43:121–6. https://doi.org/10.1590/S1677-5538.IBJU.2015.0719.
Martin-Garcia M, Crampton J. A single-blind, randomized controlled trial to evaluate the effectiveness of transcutaneous tibial nerve stimulation (TTNS) in overactive bladder symptoms in women responders to percutaneous tibial nerve stimulation (PTNS). Physiotherapy. 2019;105(4):469–75. https://doi.org/10.1016/j.physio.2018.12.002.
Ramírez-García I, Blanco-Ratto L, Kauffmann S, Carralero-Martínez A, Sánchez E. Efficacy of transcutaneous stimulation of the posterior tibial nerve compared to percutaneous stimulation in idiopathic overactive bladder syndrome: randomized control trial. Neurourol Urodyn. 2019;38(1):261–8. https://doi.org/10.1002/nau.23843.
Amarenco G, Ismael SS, Even-Schneider A, et al. Urodynamic effect of acute transcutaneous posterior Tibial nerve stimulation in overactive bladder. J Urol. 2003;169(6):2210–5. https://doi.org/10.1097/01.ju.0000067446.17576.bd.
Fernandez N, Chua ME, Ming JM, Zu’bi JMSF, Santos J, Lorenzo AJ, Braga LH, Lopes RI. Neurostimulation therapy for nonneurogenic overactive bladder in children: A Meta-analysis. Urol. 2017;110:201–7. https://doi.org/10.1016/j.urology.2017.08.003.
Nüesch E, Trelle S, Reichenbach S, et al. The effects of excluding patients from the analysis in randomised controlled trials: meta-epidemiological study. BMJ. 2009;339:b3244. https://doi.org/10.1136/bmj.b3244.
Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98–110. https://doi.org/10.1002/jrsm.1044.
Lucas MG, Bedretdinova D, Bosch JLHR, et al. Guidelines on urinary incontinence.; European association of. Urology. 2014; https://uroweb.org/wp-content/uploads/20-Urinary-Incontinence_LR.pdf.
Kamboonlert K, Panyasriwanit S, Tantisiriwat N, Kitisomprayoonkul W. Effects of Bilateral Transcutaneous Tibial Nerve Stimulation on Neurogenic Detrusor Overactivity in Spinal Cord Injury: A Urodynamic Study. Arch Phys MedRehabil. 102(6):1165–9. https://doi.org/10.1016/j.apmr.2020.10.130.
Acknowledgements
The authors thank Prof. Marcos de Noronha from LaTrobe University for supporting the meta-analysis process.
Author information
Authors and Affiliations
Contributions
K. Zomkowski: Protocol/project development; Data collection or management; Formal analysis; Methodology; Project administration; Manuscript writing/editing. I. Kammers: Data collection or management; Formal analysis; Methodology; Manuscript writing/editing. B. B. Hugen Back: Data collection or management; Formal analysis; Methodology; Manuscript writing/editing. G. M. Moreira: Validation; Visualization; Manuscript writing/editing. A. Sonza: Protocol/project development; Validation; Manuscript writing/editing. C. Sacomori: Protocol/project development; Methodology; Validation; Manuscript writing/editing. F. F. Sperandio: Protocol/project development; Methodology; Validation; Visualization; Manuscript writing/editing.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Zomkowski, K., Kammers, I., Back, B.B.H. et al. The effectiveness of different electrical nerve stimulation protocols for treating adults with non-neurogenic overactive bladder: a systematic review and meta-analysis. Int Urogynecol J 33, 1045–1058 (2022). https://doi.org/10.1007/s00192-022-05088-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-022-05088-7