Abstract
Introduction and hypothesis
This study is about evaluating safety and potential risk factors for complications following pelvic organ prolapse repair with the GYNECARE PROLIFT system.
Methods
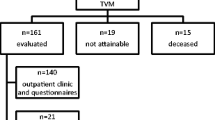
This is a prospective observational study (Canadian Task Force II-1) performed in a tertiary referral center. A total of 114 women with pelvic organ prolapse, POP-Q stage ≥2, underwent a pelvic floor repair that included Prolift.
Results
During a mean follow-up time of 7 months there were six procedure failures (5.3%) and 14 mesh exposures (12.3%), more commonly on the anterior vaginal wall. Age was inversely related to the risk of having late mesh exposure (p = 0.02) with an odds ratio of 1.99 (95% confidence interval 1.10–3.59) for each decrease of 10 years in age. Late mesh exposure was significantly more common in sexually active patients (p = 0.016).
Conclusions
Prolift repair has a high anatomical success rate. Young age and sexual activity are risk factors for mesh exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical management is considered the definitive treatment of choice for advanced stages of symptomatic pelvic organ prolapse (POP). The estimated lifetime risk of having a surgical procedure for POP is 11.1%. Of these, one in three may require additional surgery for recurrent prolapse [1]. High failure rates and the need for reoperation with native-tissue POP repair have brought about the use of mesh for these procedures. Based on the surgical principles and improved outcomes of abdominal mesh hernia repairs, pelvic surgeons have recently introduced synthetic and biological meshes into pelvic floor repair [2]. While there is good quality of evidence in favor of mesh in abdominal pelvic reconstructive surgery and vaginal continence surgery [3], high quality evidence is still lacking when it comes to vaginal use of mesh in POP repair [2]. Data to date on anterior and posterior repairs does not demonstrate a benefit for using mesh or at best shows conflicting results [2].
The most frequently used non-absorbable synthetic mesh in pelvic reconstructive surgery, type I macroporous polypropylene mesh, is associated with a low incidence of infection and vaginal mesh exposure [4]. Soft polypropylene mesh has been used for tension-free vaginal pelvic floor repair and is marketed under the brand name GYNECARE PROLIFT Pelvic Floor Repair System (Johnson & Johnson, Somerville, NJ) with Food and Drugs Administration approval. The PROLIFT repair system is available for anterior or posterior repair or for total repair in women with vaginal or uterine prolapse, using the tension-free vaginal mesh (TVM) technique [5, 6]. TVM procedural kits have been developed in an attempt to improve outcomes of POP repair in a more reproducible and simplified approach. Mesh kits act like hammocks and do not rely on the weak native tissue of the patient for support. Therefore, they should potentially pose less risk of recurrent POP. The kits have been rapidly accepted worldwide despite paucity of data regarding long-term success and complications. Some health systems like the British National Health Service (NHS) have advocated the use of mesh for symptomatic anterior vaginal wall prolapse with warning about the risk of complications and paucity of long-term data [7]. The NHS recommends that mesh repair be performed only by gynecologists with expertise in pelvic floor repair.
Short-term results following TVM kit procedures show a high anatomical success rate following Prolift repair [8] as well as with other mesh kits [9]. Data on medium and long-term outcomes as well as potential complications following the use of these kits is still lacking.
We performed a prospective analysis of our experience to date with the GYNECARE PROLIFT Pelvic Floor Repair System. The objective of this study is to assess Prolift procedures for safety and to identify potential risk factors for the development of postoperative complications.
Materials and methods
This prospective observational study included 114 consecutive patients who underwent TVM repair with the Prolift kit between February 2006 and October 2007. Patients were fully informed of the nature of the surgical procedure and provided with current information on the risks and benefits associated with the procedure and with mesh repair.
The inclusion criteria included symptomatic POP-Q stage 3 or greater prolapse undergoing primary repair and POP-Q stage 2 or greater for patients undergoing repeat prolapse repair. The exclusion criteria included patients who were unwilling to accept potential mesh related complications, patients with shortened vaginas with a TVL <5 cm and patients with vaginal stenosis from previous repair. The patients were referred to our tertiary level referral center and later operated on by the principal surgeon (AL)
Data collection was institutionally approved and included a thorough history, pre- and postoperative questionnaires which included the Australian pelvic floor questionnaire [10], prolapse quality of life questionnaire [11] and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire [12]. Prolapse classification was done according to the ICS POP-Q classification system [13]. Urodynamics were conducted for patients with severe prolapse and/or bladder symptoms.
Recorded surgery duration included the Prolift repair as well as the concomitant surgery. The duration of the Prolift repair by itself was not recorded. Follow-up after the surgical repair included a detailed interview, pelvic examination, and repeating the questionnaires. Patients were advised to abstain from intercourse until satisfactory healing is confirmed at the 6-week check. Where there was incomplete healing, abstinence was advised until confirmation of healing. Procedure failure was defined as a postoperative ≥POP-Q stage 2. Early mesh exposure was defined as mesh exposure ≤6 weeks from surgery and late mesh exposure was defined as exposure beyond this time period. Patients with mesh exposure ≤1 × 1cm were treated conservatively first for a minimum of 2 months with topical estrogen. Surgical treatment was reserved for patients with persistent mesh exposure where the size of exposed mesh was ≥1 × 1cm.
Surgical technique
The repair procedure was done as previously described by the French TVM group [5, 6] and based on recommended training materials by Gynecare International (Johnson & Johnson, Somerville, NJ). All patients received a standard protocol of perioperative antibiotic prophylaxis which included second generation cephalosporin and thromboprophylaxis with compression stockings and low molecular weight heparin.
The technique included vaginal infiltration with 60 ml of 0.5% Marcaine with 1:200,000 adrenaline diluted 1:3 with saline and a full-thickness vertical incision through the vaginal wall leaving the fascia attached to the vaginal epithelium. Following dissection of the vesicovaginal and rectovaginal spaces the cannula/retrieval devices were inserted through the skin incisions and through the obturator foramen and paravesical spaces for the anterior mesh or ischiorectal fossa and pararectal spaces for the posterior mesh. The mesh implants were placed in the anterior, posterior, or both compartments depending on the patient’s clinical findings. For the total Prolift, a single incision was made along the vaginal wall from front to back. The mesh was divided into separate anterior and posterior segments when the uterus was preserved. A cystoscopy and a rectal examination were performed, accordingly, following placement of the anterior or posterior cannula/retrieval devices before pulling the implant into a tension-free position. The incision was closed with a 0-Vicryl non-locking suture without removal of any vaginal tissue.
Concomitant surgery included urinary continence procedures, hysterectomy when required, anterior or posterior native-tissue repair in patients with ≤stage 2 prolapse with no prior pelvic floor repair. A vaginal pack with estrogen cream and urinary catheter were removed after 24 h.
Statistical analysis
Comparisons between the three Prolift surgery groups (Total, Anterior, and Posterior) on normally distributed continuous variables such as duration of surgery were conducted using analysis of variance, with post hoc Bonferroni pairwise comparisons. Where the data were skewed such as for blood loss, the Kruskal–Wallis H test was used to compare the surgery types.
Associations between single potential predictor variables and study endpoints (e.g., procedure failure, early and late mesh exposure) were assessed by Student’s t tests for normally distributed continuous variables (e.g., age) and Mann–Whitney U Z tests for significantly skewed continuous variables (e.g., BMI) and chi-square (Fisher’s exact test) for categorical variables.
Forward stepwise logistic regressions were conducted to investigate exploratory predictive models for the three study endpoints: procedure failure (POP-Q staging 2–4); early mesh exposure (up to 6 weeks), and late mesh exposure. Independent variables considered included age, BMI, parity, menopausal status, preoperative POP-Q stage >2, hypertension, diabetes, uterus intact, postoperative sexual activity, recurrent prolapse, postoperative dyspareunia, total Prolift, anterior Prolift, and posterior Prolift. Variables achieving statistical significance of p ≤ 0.10 in the univariate analyses were considered in the regression models. Associations between potential predictor variables were explored prior to inclusion in the regression analysis to avoid problems with multicollinearity.
Age, parity, and POP-Q staging were also divided into categorical variables defined as follows: age class (≤61 years, age >61 years), parity class (parity ≤2, parity >2), and POP-Q stage class (stage ≤2, stage >2). All tests were two-sided and significance set at 0.05 unless stated otherwise.
Results
Information was available for 114 patients, with an average age 61.2 years (SD, 11.1; range, 31 to 84 years). Table 1 presents patients’ background, including demographics, previous gynecological surgery, and preoperative urinary and bowel symptoms associated with pelvic floor disorders. Preoperative pelvic assessment (Table 2) showed 22 of the patients (19.3%) had stage 2, 72 (63.2%) had stage 3, and 20 (17.5%) had stage 4 POP-Q prolapse.
Nineteen of the patients had an Anterior Prolift repair (16.7%), 14 a Posterior Prolift repair (12.3%), and 81 had a Total Prolift repair (71.1%) (Table 3). Concomitant surgery included 18 incontinence vaginal tape procedures (15.8%), one hysterectomy (0.9%), six anterior native-tissue repairs (5.3%), 11 perineoraphies (9.7%), one laparoscopic colposuspension (0.9%).
Intraoperative complications included two bladder perforations which were repaired with a 2-0 vicryl one-layered closure. One of the patients had an Anterior Prolift (5.3%) and the other a Total Prolift (1.2%). Surgery duration, including concomitant surgery was 106 min on average (SD, 24.7) for all the Prolift procedures. Anterior Prolift with concomitant surgery took 91.3 on average (SD, 33.6), Posterior Prolift took 103.7 min (SD, 24.9), and Total Prolift 110.7 min (SD, 20.8).
Overall average blood loss was 192.5 cc (SD 136.5) with no significant difference between the three types of Prolift repairs. The mean hospital stay was around 3.5 days (SD, 1.4) for all patients and did not differ significantly by type of repair.
Follow-up time and complications are presented in Table 4. Average length of follow-up was 7.4 months (range, 6.3 to 8.4).
Around 30% of the patients had an immediate or early postoperative complication. The most common complications were fever (9.6%), urinary tract infection (9.6%), and vaginal pain (7.9%).
Almost 40% of the patients had late complications (Table 4). Fifty-eight patients were sexually active before surgery. Of the 18 patients (15.8%) who complained of dyspareunia before the surgery, nine (7.9%) reported resolution of the symptom following surgery. Fifty-two patients were sexually active following surgery. Fourteen of these patients (12.3%) developed de novo dyspareunia. Overall, 23 patients (20.2%) complained of postoperative dyspareunia. Stress incontinence resolved following surgery for 28 of the 48 patients. Seven patients (6.1%) developed new onset stress incontinence. Urge incontinence had resolved following surgery for 23 of the 38 patients. Five patients (4.4%) developed de novo urge incontinence following surgery. Four patients (3.5%) had early mesh exposure, 10 (8.8%) experienced late mesh exposure and there were six (5.3%) repair procedure failures (POP-Q stages 2–4).
Ten of the 14 exposures (early and late combined) occurred with the Total Prolift, two with the Anterior Prolift, and two with the Posterior Prolift. There were ten mesh exposures on the anterior vaginal wall, two on the posterior and two on both anterior and posterior vaginal walls.
The cell sizes were too small for valid comparison of the Prolift groups in rates of early complications and specific late complications.
Potential predictor variables and study endpoints
A univariate analysis of potential risk factors for repair procedure failures showed parity and BMI to be significantly different between the women who did or did not experience procedure failure (p < 0.10). Both variables were independent of each other (p = 0.258).
A regression analysis with a forward stepwise method of entry was conducted, and neither BMI nor parity (considered as either a continuous or a categorical variable) emerged as significant predictors of procedure failure.
A univariate analysis of potential risk factors for early mesh exposure (Table 5) showed preoperative POP-Q stage >2, parity, and Total Prolift to be significantly different between the exposure groups (p < 0.10) with POP-Q stage 2 and increased parity being associated with early exposure. Preoperative POP-Q stage was significantly associated with having a Total Prolift procedure, so two separate regression analyses were conducted. When parity (as a continuous variable) and preoperative POP-Q stage were entered into the regression, they each emerged as significant predictors of early mesh exposure (odds ratio (OR) 2.56; 95% confidence interval (CI), 1.07 to 6.09; and OR, 0.02; 95% CI 0.001 to 0.55, respectively). When parity and Total Prolift were entered, neither was a significant predictor.
A univariate analysis of potential risk factors for late mesh exposure (Table 6) showed age, hypertension and postoperative sexual activity to be significant factors (p < 0.10).
An analysis of the relationship between potential predictive variables for late mesh erosion showed that each of these three potential predictors was significantly related to each other (p < 0.05). Older patients were more likely to be hypertensive and less likely to be sexually active than younger patients. In order to avoid problems with multicollinearity, three separate, univariate logistic regressions were run. Hypertension did not emerge as a significant predictor probably due to the fact that the odds ratio for hypertension was extremely low since none of the women with late mesh erosion experienced hypertension. Age and sexual activity were found to be significant independent predictors of late mesh exposure. With regard to age, each decade increase in age decreases the risk for late mesh exposure by 1.99 (95% CI, 1.10 to 3.59, p = 0.022). The mean age of patients with late mesh exposure was 52.3 years, whereas the mean age of the patients with no exposure was 62.0 (p = 0.007, Table 6).
Being sexually active postoperatively increases the odds of late mesh exposure by 10.47 (95% CI, 1.27 to 85.96, p = 0.029). Following the POP repair, 52 of the patients were sexually active, 51 were not, and 11 women (9.6%) did not declare if they were sexually active or data were not complete. Late mesh exposure occurred in nine of the 52 sexually active patients (17.3%) and in only one of the 51 sexually non-active patients (2%).
Discussion
Our results show that while the TVM repair using the GYNECARE PROLIFT Pelvic Floor Repair System has a low procedure failure rate, it is associated with significant postoperative morbidity, including mesh exposure, dyspareunia, and de novo urinary symptoms. The prolapse recurrence rate was only 4–5%, substantially less than the 30–45% reported with native-tissue repair without mesh [1, 14, 15]. Prolapse, although mesh exposure after vaginal mesh repair has been reduced from 5–30% to 5–15% with the use of Prolene [6], still remains a significant postoperative complication. Our results show a mesh exposure rate of 12.3%, requiring surgical repair in most cases. Patients who developed late mesh exposure were more than 9 years younger on average and had a twofold risk of developing mesh exposure per 10-year decrease in patients’ age.
Although the exact etiology of mesh exposure is unknown, it is believed that early and late mesh exposures are two different processes with similar outcomes [16]. Early exposure is likely to be extrusion of the mesh as a result of the procedure itself from either damage to the vaginal tissue, infection, or improper closure of the mucosa. Late exposure is likely to result from chronic erosion of the tissue due to mechanical stress and the long-term interaction of the mesh with the surrounding tissue. Age has previously been described as a risk factor for mesh erosion with conflicting results. Deffieux and colleagues found age over 70 to be a risk factor [17] whereas Achtari et al. found the risk of erosion to be lower with increasing age [18]. Achtari mentions that the finding was a surprise since, intuitively, older postmenopausal patients have a thinner atrophic vaginal epithelium which is more likely to impair healing. They do not provide an explanation and suggest that a larger cohort study clarify that.
We were also initially surprised with our finding of younger age as a risk factor for erosion. When analyzing for confounding variables we found that younger patients were more sexually active and that sexual activity was also an independent risk factor for late mesh exposure occurring most commonly on the anterior vaginal wall. The exact etiology is unknown but may be as a result of increased mechanical stress on the tissue due to friction. Sexual activity early after vaginal surgery has been linked to a higher rate of early mesh exposure due to tissue friction [16], but there are no reports linking sexual activity to late mesh exposure. We expected to find a higher rate of dyspareunia amongst patients developing mesh exposure, but dyspareunia was not found to be a risk factor. Hypothetically, dyspareunia might even have a protective impact since pain might cause patients to avoid sexual intercourse.
There are conflicting results on the connection between vaginal mesh repair and dyspareunia. Milani and colleagues showed that, postoperatively, the dyspareunia rate increased by 20% for an anterior repair and 63% for a posterior repair [19]. In contrast, Dwyer and colleagues [20] showed a decrease in dyspareunia rate from 25.8% to 9.1% following surgery with only three cases of de novo dyspareunia. According to our results, dyspareunia is relatively common both before and after mesh repair. We found a 20.2% postoperative dyspareunia rate. This is higher than the reported rate of prolonged severe dyspareunia in the general female population between 50 and 60 years of age which is 6.5% [21]. The rate of postoperative de novo dyspareunia was 12.3%, but there was also a 7.9% rate of dyspareunia resolution following surgery, so the overall increase in dyspareunia was 4.4% as a result of the Prolift repair.
The GYNECARE PROLIFT Pelvic Floor Repair System is a relatively novel procedure which has not undergone rigorous evaluation and comparison to standard treatments. As in any surgical procedure, the risks and benefits for these procedures require close attention. Our results for an average follow-up of more than 7 months show a low procedure failure rate with a substantial mesh exposure rate, especially for young and sexually active patients who require a repair for the anterior vaginal wall. When discussing vaginal mesh repair, especially with sexually active patients, the high success rate should be mentioned along with possible complications such as mesh exposure. These patients should also be aware of other repair procedures such as abdominal or laparoscopic sacrocolpopexy and native-tissue repair, especially for the posterior vaginal wall. There is a dire need for a long-term follow-up study and randomized controlled trials for the TVM kits in order to provide better patient counseling and to promote technological alterations that may make these kits successful and safer.
Abbreviations
- POP:
-
Pelvic organ prolapse
- TVM:
-
Transvaginal mesh repair
References
Olsen AL, Smith VJ, Bergstrom JO et al (1997) Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89:501–506
Chen CC, Ridgeway B, Paraiso MF (2007) Biologic grafts and synthetic meshes in pelvic reconstructive surgery. Clin Obstet Gynecol 50(2):383–411
Maher C, Baessler K, Glazener CMA, Adams EJ, Hagen S (2007) Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews (3):CD004014
Amid P (1997) Classification of biomaterials and their related complications in abdominal wall surgery. Hernia 1:15–21
Debodinance P, Berrocal J, Clave H et al (2004) Changing attitudes on the surgical treatment of urogenital prolapse: birth of the tension-free vaginal mesh. J Gynécol Obstét Biol Reprod 33(7):577–588
Collinet P, Belot F, Debodinance P et al (2006) Transvaginal mesh technique for pelvic organ prolapse repair: mesh exposure management and risk factors. Int Urogynecol J 17:315–320
National Institute for Health and Clinical Excellence (2008) Surgical repair of vaginal wall prolapse using mesh. Interventional procedure guidance 267. London, UK
Fatton B, Amblard J, Debodinance P, Cosson M, Jacquetin B (2007) Transvaginal repair of genital prolapse: preliminary results of a new tension-free vaginal mesh (Prolift™ technique)—a case series multicentric study. Int Urogynecol J 18:743–752
Gauruder-Burmester A, Koutouzidou P, Rohne J, Gronewold M, Tunn R (2007) Follow-up after polypropylene mesh repair of anterior and posterior compartments in patients with recurrent prolapse. Int Urogynecol J 18:1059–1064
Baessler K, O’Neill SM, Maher CF, Battistutta D (2009) Australian pelvic floor questionnaire: a validated interviewer administered pelvic floor questionnaire for routine clinic and research. Int Urogynecol J Pelvic Floor Dysfunct 20:149–158
Digesu GA, Khullar V, Cardozo L, Robinson D, Salvatore S (2005) P-QOL: a validated questionnaire to assess the symptoms and quality of life of women with urogenital prolapse. Int Urogynecol J 16:176–181
Rogers RG, Coates KW, Kammerer-Doak D, Khalsa S, Qualls C (2003) A short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Int Urogynecol J 14(3):164–168
Bump RC, Mattiasson A, Bo K et al (1996) The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 175:10–11
Carey M, Higgs P, Goh J, Lim J, Leong A, Krause H, Cornish A (2009) Vaginal repair with mesh versus colporrhaphy for prolapse: a randomised controlled trial. BJOG 116(10):1380–1386
Nguyen JN, Burchette RJ (2008) Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol 111(4):891–898
Mistrangelo E, Mancuso S, Nadalini C, Lijoi D, Constantini S (2007) Rising use of synthetic mesh in transvaginal pelvic reconstructive surgery: a review of the risk of vaginal erosion. JMIG 14:564–569
Deffieux X, de Tayrac R, Huel C, Bottero J, Gervaise A et al (2007) Vaginal mesh erosion after transvaginal repair of cystocele using Gynemesh or Gynemesh-Soft in 138 women: a comparative study. Int Urogynecol J 18:73–79
Achtari C, Hiscock R, O’Reilly BA, Schierlitz L, Dwyer PL (2005) Risk factors for mesh erosion after transvaginal surgery using polypropylene (Atrium) or composite polypropylene/polyglactin 910 (Vypro II) mesh. Int Urogynecol J 16:389–394
Milani R, Salvatore S, Soligo M, Pifarotti P, Meschia M, Cortese M (2005) Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with prolene mesh. BJOG 112:107–111
Dwyer PL, O’Reilly BA (2004) Transvaginal repair of anterior and posterior compartment prolapse with Atrium polypropylene mesh. BJOG 111:831–836
Danielsson I, Sjöberg I, Stenlund H, Wikman M (2003) Prevalence and incidence of prolonged and severe dyspareunia in women: results from a population study. Scand J Public Health 31:113–118
Acknowledgments
We wish to acknowledge the help of Dr. Georgina Luscombe who assisted with the statistical analysis.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaufman, Y., Singh, S.S., Alturki, H. et al. Age and sexual activity are risk factors for mesh exposure following transvaginal mesh repair. Int Urogynecol J 22, 307–313 (2011). https://doi.org/10.1007/s00192-010-1270-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-010-1270-6