Abstract
The purpose of this study was to compare smooth muscle content of anterior vaginal wall in women with pelvic organ prolapse (POP) and control subjects. Specimens were taken in the midline from the apex of anterior vaginal cuff from eleven women with POP and eight control subjects operated for hysterectomy without prolapse. Masson’s trichrome stain was used to determine the distribution of collagen in the extracellular matrix of the vaginal muscularis and to quantify the collagen in area of interest. Slides of alpha smooth muscle actin were detected using antibodies. Morphometric analysis was used to compare and to quantify the smooth muscle content of the vaginal muscularis. Fractional area of nonvascular vaginal smooth muscle of women with POP was significantly decreased in comparison to control subjects (41.9 vs 61.9%, p = 0.005). Fractional area of connective tissue was significantly increased (56.8 vs 35%, p = 0.004). Fractional area of blood vessels was similar (2.2 vs 3.4%, p = 0.20).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is postulated that pelvic organ support is maintained by complex interactions between levator ani muscles of the pelvic floor and connective tissues of the bladder, vaginal wall and rectum [1]. Failure of normal levator ani functions is an important hallmark of pelvic organ prolapse (POP). However, the mechanisms by which the levator ani support vaginal wall and how these mechanisms fail during the development of prolapse are still not well understood. Pelvic floor neuropathy and site-specific fascial tears have been identified as causes of POP [1]. There is also evidence that suggests abnormalities of connective tissue repair may predispose women to prolapse [2]. The fascial and connective tissues of the pelvic floor may also lose strength as the results of aging and loss of neuroendocrine signaling in pelvic tissues [3]. Smooth muscle fibers that arise from the vaginal wall are attached to the levator ani muscle complex [4]. Expression of smooth muscle proteins in the anterior vaginal wall was altered significantly in women with prolapse [5]. These data suggested that abnormalities in the anatomy, physiologic features and cellular biologic features of alpha smooth muscle actin in the vaginal wall may contribute to the pathophysiologic factors of POP. Thus, the goal of the current study was to evaluate the histologic features of the anterior vaginal wall and to show differences between women with POP and control subjects to test the hypothesis that smooth muscle content is decreased in the vagina of women with prolapse of the anterior vaginal wall. A secondary study objective was the comparison of the anterior vaginal wall content in primary vs recurrent organ prolapse.
Materials and methods
Vaginal tissues were obtained from eleven women who underwent surgical procedure for POP and eight women who underwent hysterectomy for benign gynaecologic disease (control group without POP). Among women operated for POP, six underwent surgical procedure for primary POP and five for recurrent POP. Written informed consents were obtained. Ethical Committee approval was not required in France for such study.
In women with POP or recurrent POP, a full-thickness biopsy specimen was taken from the anterior colpectomy on each side of the midline incision. In control subjects’ specimen were taken from the apex of the anterior vaginal wall after total hysterectomy.
Masson’s trichrome stain was used to determine the distribution of collagen in the extracellular matrix of the vaginal muscularis and to quantify the collagen in area of interest. The vaginal section tissue, formalin-fixed, paraffin-embedded were sectioned at 5 µm and mounted on slide. Thereafter, each slide was deparaffinized in xylene and graded alcohols to water and was stained Masson’s trichrome stain as described by Nezelof [6]. The smooth muscle was stained in pink included vascular wall smooth muscle and the collagen in blue.
For immunochemistry, formalin-fixed and paraffin-embedded tissues were sectioned at 5 µm and mounted on a slide. The slides were deparaffinized in xylene and graded from alcohols to water. Epitope retrieval was then performed in a double boiler by using 0.01 mmol/l citrate buffer (pH = 6.0) as an epitope retrieval solution. After, the slides were placed in hydrogen peroxide for 10 min to quench endogenous peroxidase activity and rinsed into water.
Thereafter, each slide was incubated in primary antibody (Smooth muscle actin, 1/100, 1A4 clone, DAKO) for 30 min at room temperature. After a similar incubation with biotinylated secondary antibody (antibody anti-mousse, DAKO) for 15 min, the slides were rinsed into water. Then, they were incubated with peroxidase-conjugated streptavidin (DAKO) for 10 min. Reaction product was developed by immersing the slide in diaminobenzidine solution at room temperature for 5 min. Slides were rinsed in water and counterstained in hematoxylin for 3 min. The slides were dehydrated in graded alcohols and xylene, and finally protected with a coverslip. The immunochemistry was realised with automate Autostainer plus (DAKO) to better reliability and reproducibility.
Morphometric analysis was used to compare and to quantify the smooth content of the vaginal muscularis in control women and women with genital prolapse. Immunochemistry of tissue cross-sections were stained with alpha smooth muscle actin antibody, and histology of tissue slides were stained Masson’s trichrome as described previously. Two pictures of each were analysed with Leica microscope (Leica microsystem®, Wetzlar, Germany). The pathologist examiner (G.G.) was blinded to the subject’s clinical history. The muscle (excluding vascular smooth muscle), the vascular wall and collagen fibrosis were outlined manually with the aid of a computerized image analysis system (Image ProPlus, Springfield, MD, USA), on each cross-section [1, 5]. Thereafter, the area of three components was quantified by computer software. The fraction of smooth muscle, vascular walls and collagen fibrosis in the area of interest was brought back to the total muscularis area assessed with a computerized image analysis system (mean determination from two cross-sections).
Statistical analysis
Results are expressed as mean ± SD. Quantitative data were reported in median and 25–75 percentiles. Comparison was done using a non-parametric test (Wilcoxon Mann and Whitney test). Comparison for qualitative data used the Fischer’s exact test due to the low number of subjects included in the present study. A probability value of <0.05 was considered significant.
Results
Clinical characteristics of women from whom vaginal samples were obtained are listed in Table 1. Staging was assigned using the POP-Q system. Classification in primary POP and recurrent POP are listed in Table 2. Patient’s characteristics, pre-operative diagnosis and operative procedures in primary and recurrent POP are listed in Table 3.
The structural anatomy of the anterior vaginal wall consisted of squamous epithelium, lamina propria, muscularis and adventitia. Immunochemistry with anti-bodies to smooth muscle alpha actin was used to identify smooth muscle cells in anterior vaginal wall. Vaginal mucosa, comprised of squamous epithelium and lamina propria, contained few smooth muscle cells, except those of small arterioles and venules that perforated the lamina propria. Numerous dilated venules in the lamina propria were observed in samples from women with anterior wall prolapse. This network of dilated venules was not observed in the lamina propria of vaginal tissues from control subjects.
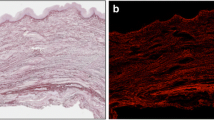
The vaginal muscularis consisted of interdigitating smooth muscle bundles and vascular complexes. In women without genital prolapse (Fig. 1a), vaginal smooth muscle cells were organised in discrete, tightly-packed bundles that were oriented in circular and longitudinal directions. In muscularis from women with prolapse (Fig. 1b), smooth muscle bundles were smaller, fewer in number and poorly organized. Masson’s trichrome stain was used to illustrate the distribution of collagen staining surrounded discreet muscular bundles of the muscularis. In normal vaginal tissues, dense compact collagen staining surrounded discreet muscular bundles of the muscularis (Fig. 1c). In muscularis from women with prolapse (Fig. 1d), smooth collagen fibbers were loosely dispersed between the poorly organised muscle bundles. Vaginal adventitia consisted of collagen, elastin, vaginal smooth muscle and neurovascular bundles.
Histologic appearance of vaginal wall in POP vs control group. a In women without prolapse, smooth muscle bundles were tightly packed and oriented in circular and longitudinal directions. b In women with POP, smooth muscle bundles were smaller, fewer in number, and poorly organised. c In normal vaginal tissues, dense compact collagen staining surrounded discreet muscular bundles of the muscularis. d In muscularis from women with prolapse, collagen fibers were loosely dispersed in the extra-cellular space between the poorly organised muscle bundles
Morphometric analysis was used to compare and quantify the smooth muscle content of the vaginal muscularis in control women, women with POP and women with recurrent POP. The fraction of cells that were immunoreactive with alpha actin relative to the entire area of the muscularis was quantified. Fractional area of nonvascular vaginal smooth muscle of women with POP was significantly decreased compared to control subjects Fractional area of connective tissue was significantly increased. Fractional area of blood vessels was decreased. Results are shown in Table 4.
To determine whether the decreased content of smooth muscle in the anterior vaginal wall in women with POP was related to recurrence of prolapse, biopsy specimens from women with anterior vaginal prolapse were compared with those from women with recurrent POP. Results of the second analysis showed no significant difference of fractional area of nonvascular vaginal smooth muscle, of connective tissue and blood vessels between primary prolapse cases and recurrent POP. Results are shown in Table 5.
Discussion
The pathogenesis of pelvic organ prolapse (POP) is incompletely understood. The site-specific defect theory is based on the premise that tears in the endopelvic fascia that surround the vaginal wall allow herniation of pelvic organs [1]. The association of POP with vaginal delivery is consistent with this theory [7]. The major finding of our investigation is that morphometry of anterior vaginal wall tissue from women with prolapse of the anterior compartment is significantly altered compared with the normal anterior vaginal wall. The fraction of smooth muscle in the muscularis of the anterior vaginal wall is significantly decreased in women with POP compared with normal control subjects independently of age or race as described previously in literature [1]. Biomechanical properties of vaginal tissue are significantly lower in POP than in control group, independently of age or menopausal status [8]. The concern of our study is that age and menopausal status were different between the control subjects and the primary POP group. This obvious selection bias might have affected our results and probably explain the large differences of smooth muscle fraction area between the two groups. Also, the racial mix was dramatically different between control group and POP group.
The smooth muscle bundles appeared disorganised, and the area of alpha actin staining that was relative to the total cross-sectional area was decreased significantly [9]. This process is associated with increased cell migration and decreased contractility [10]. The loss of smooth muscle in prolapsed vagina may result from smooth muscle cell apoptosis. It is intriguing to speculate that smooth muscle cell apoptosis (consequent to hypoxia, denervation or trauma) is followed by migration and proliferation of surrounding fibroblasts. These fibroblasts may contribute to the synthesis of abnormal collagen and the consequent altered viscoelastic properties of the vaginal wall [11]. Clearly, further studies are necessary to test this hypothesis. No histological study was undertaken until now to compare fraction of smooth muscle in the muscularis of the anterior vaginal wall in primary and recurrent POP.
Other investigations found that caldesmon expression is increased substantially in vaginal smooth muscle of women with POP [5]. Caldesmon inhibits actin-activated myosin magnesium adenosine triphosphatase activity and inhibits the maintenance of contractile force [12]. Thus, this disproportionate increase in caldesmon, relative to myosin, may result in inhibition of vaginal smooth muscle contractility and force maintenance [13].
For the first time, it has been found that younger women with genital prolapse have a lower collagen concentration than age-matched controls, which indicates a constitutional tissue weakness contributing to genital prolapse development [14]. It suggests a different organisation of the endopelvic connective tissue extracellular matrix.
Other study demonstrates that weakened pelvic floor of the lower genito-urinary tract in women with stress urinary incontinence is due, in part, to decreased collagen synthesis and secretion and/or altered ratio of collagen 3/1 synthesis by the fibroblasts of the endopelvic fascia and skin compared to that of women without evidence of pelvic floor weakening [15]. These data suggest that the lower collagen content in the endopelvic fascia and skin of women with stress urinary incontinence is not due to reduced collagen synthesis or selective reduction in synthesis of either type 1 or 3 collagen. The mean of collagen 3/1 synthesized in fibroblasts was not significantly different between fibroblasts obtained from women with or without stress urinary incontinence in this study [15].
Different organisation of collagen fibrils in stress-incontinent women of fertile age were noticed [16]. The collagen concentration was almost 30% higher and the diameters of collagen fibrils were 30% larger in the incontinent group of women. The organisation of the collagen fibrils also differed, with considerably higher cross-linking. These alterations should result in a more rigid form of extracellular matrix, suggesting a connective tissue with impaired mechanical function.
The uterosacral ligaments are an important part of the pelvic support system and connective tissue alterations are thought to contribute to the development of POP. A new finding strongly indicates that increased expression of matrix metalloproteinase 2 in uterosacral ligaments is associated with POP [17]. Metalloproteinase, a family of zinc dependent proteinases, are secreted as inactive zymogens [18] and are capable of degrading extracellular matrix macromolecules essential in normal and pathologic tissue remodelling processes [19–21]. A recent study suggests that changes in the connective tissue composition are at least partially involved in the pathophysiology of POP. Authors focused on uterosacral ligaments, as they are an important part of the pelvic support system and constitute a reproducible anatomical landmark [22–24]. Metalloproteinase 2 degrades type 4 collagen, which is a major component of the basement membrane [25, 26]. It is tempting to speculate that elevated metalloproteinase 2 expression leads to enhanced matrix dissolution and reduced connective tissue strength, which in turn may facilitate the progression of POP.
In our study, the fraction of smooth muscle in the muscularis of the anterior vaginal wall is significantly decreased in women with POP compared with normal control subjects. There is no significant difference between primary and recurrent cases of POP in this fractional area. Reversibility of these changes may influence the failure rates of surgical treatment of POP. The prospect of reversing hepatic fibrosis has generated great interest now that basic science advances are being translated into promising new anti-fibrotic therapies [27]. Realistic expectations for successful anti-fibrotic therapies reflect solid evidence of fibrosis regression in patients treated effectively for viral liver disease, as well as growing quality in the understanding mechanisms of extracellular matrix production and degradation. Fibrosis is a progressive pathological process in which wound-healing myofibroblasts of the liver respond to injury by promotion replacement of the normal hepatic tissue with a scare-like matrix composed of cross-linked collagen. Until recently, it was believed that this process was irreversible [28]. However, emerging experimental and clinical evidence is starting to show that even cirrhosis is potentially reversible [28]. Effects of interferon-gamma on hepatic fibrosis in chronic hepatitis B virus (HBV) infection were proved [29]. Interferon gamma improves fibrosis scores in patient’s chronic HBV infection most likely by antagonizing profibrogenic transforming growth factor-beta effects, thereby providing a molecular explanation for its antifibrotic effects [29].
Reversal of hepatic fibrosis lead us to continue progress and find applications in POP to identify the determinants and dynamic of fibrosis reversibility, to discover additional targets for anti-fibrotic therapy and to develop customized multi-drug regimens. Advances on reversal fibrosis of connective tissue in POP may have a big incidence on the surgical treatment and profoundly impact the vaginal repair of anterior vaginal wall prolapse with absorbable meshes. A growing number of aging women are presenting as candidates for surgical repair of genital prolapse. The scientific understanding of tissue healing, both in hernia and POP, as well as the fate of implant materials used to reinforce repairs, is therefore a clinical need. The perfect implant material certainly is not yet available. Thus, reversibility of these histopathological findings may influence success rates of surgical treatment using biomaterials and could be in favour of bioabsorbable meshes.
In summary, vaginal wall morphologic features were altered significantly in women with POP. The smooth muscle content of the vaginal muscularis was decreased in women with prolapse of the anterior vaginal wall compared with vaginal tissues from normal control subjects. There is no significant difference between primary and recurrent cases of POP in this fractional area. Progress in identifying genetic determinants of fibrosis could further refine patient selection for clinical trials and shorten their duration, as well as unearthing new directions of scientific inquiry in POP. Reversibility of fibrosis may influence the choice of synthetic or bioabsorbable meshes in POP repair in the future.
References
Boreham MK, Wai CY, Miller RT et al (2002) Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am J Obstet Gynecol 187:56–63
Norton PA, Boyd C, Deak S (1992) Collagen synthesis in women with genital prolapse or stress urinary incontinence. Neururol Urodyn 11:300–301
Smith ARB, Hosker GL, Warrel DW (1989) The role of partial denervation of the pelvic floor in the aetiology of genitourinary prolapse and stress incontinence of urine: a neurophysiological study. Br J Obstet Gynecol 96:24–28
DeLancey JOL, Starr RA (1990) Histology of the connection between the vagina and levator ani muscles. J Reprod Med 35:765–771
Boreham MK, Miller RT, Schaffer JI et al (2001) Smooth muscle myosin heavy chain and caldesmon expression in the anterior vaginal wall of women with or without pelvic organ prolapse. Am J Obstet Gynecol 185:944–952
Nezelof C (1972) Masson’s trichrome stain. In: Techniques microscopiques. Flammarion, Paris, France: 86–89
Weber AM, Walter MD (1997) Anterior vaginal prolapse: review of anatomy and techniques of surgical repair. Obstet Gynecol 89:311–318
Lei L, Song Y, Chen R (2007) Biomechanical properties of prolapsed vaginal tissue in pre- postmenopausal women. Int Urogynecol J Pelvic Floor Dysfunct 18:603–607
Ford LE, Seow CY, Pratusevich VR (1994) Plasticity in smooth muscle: a hypothesis. Can J Physiol Pharmacol 72:1320–1324
Ueki N, Sobue K, Kanda K et al (1987) Expression of high and low molecular weight caldesmon during phenotypic modulation of smooth muscle cells. Proc Natl Acad Sci USA 84:9049–53
Payne AM, Yue P, Pritchard K et al (1995) Caldesmon mRNA splicing and isoform expression in mammalian smooth muscle and no muscle tissues. Biochem J 305:445–450
Hemric ME, Chalovich JM (1988) Effects of caldesmon on the ATPase activity binding of smooth and skeletal myosin subfragments to actin. J Biol Chem 263:1878–1885
Lash JA, Seller JR, Hathaway DR (1986) The effects of caldesmon on smooth muscle heavy actomeromyosin ATPase activity and binding of heavy meromyosin to actin. J Biol Chem 261:16155–16160
Westergren Soderberg M, Falconer C et al (2004) Young women with genital prolapse have a low collagen concentration. Acta Obstet Gynecol Scand 83:1193–1198
Chen Y, Desautel M, Anderson A et al (2004) Collagen synthesis is not altered in women with stress urinary incontinence. Neurourol Urodyn 23:367–373
Falconer C, Blomgren B, Johanson O et al (1998) Different organisation of collagen fibrils in stress-incontinent women of fertile age. Acta Obstet Gynecol Scand 77:87–94
Boris G, Watermann D, Hancke K et al (2006) Increased expression of matrix metalloproteinase 2 in uterosacral ligaments is associated with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 17:478–482
Nagase H (1997) Activation mechanisms of matrix metalloproteinases. J Biol Chem 378:151–160
Woessner FJ (1991) Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 75:2145–2154
Steller-Stevenson WG (1996) Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol 147:1345–1350
Tokuraku M, Sato H, Murakami S et al (1995) Activation of the precursor of gelatinase A/72 kDa type 4 collagenase/MMP-2 in lung carcinomas correlates with the expression of membrane-type matrix metalloproteinase (MT1-MMP) and with lymph node metastasis. Int J Cancer 64:355–359
DeLancey JOL (1992) Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 166:1717–1724
Amundsen CL, Flynn BJ, Webster GD (2003) Anatomical correction of vaginal vault prolapse by uterosacral ligament fixation in women who also require a pubovaginal sling. J Urol 169:1770–1774
Buller JL, Thompson JR, Cundiff GW et al (2001) Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol 97:873–879
Roebuck MM, Helliwell TR, Chaudhry IH et al (2005) Matrix metalloproteinase expression is related to angiogenesis and histologic grade in spindle cell soft tissue neoplasms of the extremities. Am J Clin Pathol 123(3):405–414
Creemers LB, Jansen IDC, Docherty AJP et al (1998) Gelatinase A (MMP2) and cystein proteinases are essential for the degradation of collagen in soft connective tissue. Matrix Biol 17:35–46
Friedman SL, Bansal MB (2006) Reversal of hepatic fibrosis-Fact or fantasy. Hepatology 43(2 Suppl 1):S82–S88
Elsharkawy AM, Oakley F, Mann DA (2005) The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis 10(5):927–930
Weng HL, Wang BE, Jia JD et al (2005) Effect of interferon-gamma on hepatic fibrosis in chronic hepatitis B infection: a randomised controlled study. Clin Gastroenterol Hepatol 3:819–828
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badiou, W., Granier, G., Bousquet, PJ. et al. Comparative histological analysis of anterior vaginal wall in women with pelvic organ prolapse or control subjects. A pilot study. Int Urogynecol J 19, 723–729 (2008). https://doi.org/10.1007/s00192-007-0516-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-007-0516-4