Abstract
Pelvic floor dysfunction is a hidden problem with a magnitude unknown to many. Statistics show that one in every ten women will have pelvic floor dysfunction so severe that it will require surgery. Several studies have shown that pelvic floor injuries during a vaginal delivery can be considered a significant factor in the development of urinary incontinence, fecal incontinence, and pelvic organ prolapse. The objective of the present work is to contribute to the clarification of the mechanisms behind pelvic floor disorders related to a vaginal delivery. For this purpose, a numerical simulation based on the finite element method was carried out. The finite element model intends to represent the effects that the passage of a fetal head can induce on the muscles of the pelvic floor, from a mechanical point of view. The model used for the simulation represents the pelvic bones, with the attached pelvic floor muscles and the fetus. In this work, we simulated the movements of the fetus during birth, in vertex position. We simulated the engagement, descent, flexion, internal rotation, and extension of the fetal head. Results for the pelvic floor stretch values obtained during the passage of the fetus head are presented; the deformation field is also shown. The results were obtained using the finite element method and a three-dimensional computer model of the pelvic floor and fetus. The maximum deformation obtained was 0.66 for a vertical displacement of the fetal head of approximately 60 mm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pelvic floor dysfunctions represent an extensive problem with unknown dimensions. A study conducted by Olsen et al. [1], based on a population under one health care system, showed that 11% of women had surgery for urinary incontinence or pelvic organ prolapse during their lifetime. Furthermore, statistics show that 30 to 40% of women suffer from some degree of incontinence in their lifetime [2]. Another study, conducted by Rortveit et al. [3] showed that the prevalence of this problem among nulliparous women ranged from 8 to 32%, increasing with age. They also showed that parity was associated with incontinence, the first delivery being the most significant.
Clinical aspects of pelvic floor disorders have been extensively studied, in particular, the effect of pregnancy and childbirth [4, 5]. However, it is widely recognized that the understanding of the mechanism of damage to the pelvic floor components (muscles, nervous, fascia) is still very limited. In particular, some quantification related with the biomechanics of the pelvic floor, as what are the strain and stress fields for a specific action, is not completely obtained [5].
During delivery, the pelvic floor experiences several changes, which cannot be measured in vivo due to clinical, technical, and ethical reasons. Therefore, with this work, a biomechanical method of modeling a biologic process, in this case delivery, to estimate biomechanical changes (stretch, strain, etc.) on tissues is presented. Knowledge of these biomechanical changes might help to explain known phenomena associated with delivery and pregnancy, like damage to the pelvic floor tissues, including the levator ani muscle.
The construction of a 3D geometric model (such as the pelvic cavity) that can be manipulated, by numerical methods, to simulate the living human is still a challenge, namely, due to the high complexity of human anatomy as recognized by several authors [6, 7]. There is also great lack of understanding of continuum biomechanics of soft biological tissues, as recognized by Humphrey [8].
There have been some recent attempts to model the human body [6], the pelvic floor cavity [9, 10], and its contents [11, 12]. However, at present, there is not enough knowledge about the structural relationships identified by magnetic resonance imaging (MRI) [11] to allow a precise numerical modeling [13, 14] to be developed.
In this work, we make use of the finite element method [15, 16] to conduct a biomechanical study of the pelvic floor muscles. Using a finite element model, which simulates the pelvic bones, pelvic floor muscles and fetus, we measured the stretches and the deformations on the pelvic floor induced by the passage of the fetus during a vaginal delivery.
A similar method as been used before to obtain the levator ani stretch during a vaginal delivery [13, 14], but to our knowledge, the work presented here is the first made using realistic models for the fetus and pelvic floor.
Materials and methods
The finite element model used in this work was constructed using the geometrical point data obtained from cadaver measurements by Janda et al. [9]. All the measurements were performed on one embalmed 72-year-old female cadaver obtained for scientific research. The specimen was selected for having no pathology to the pelvic floor. The cause of death was unknown and presumably not affecting the pelvic floor musculature. The result was a 3D point-set of the pelvic floor.
In Fig. 1, the process used for the creation of the model is shown. Using the available points, we started by creating lines, which allowed the definition of surfaces that were then used for the creation of the finite element mesh. Initially, we obtained a 3D model made with four nodes shell elements, which we then extruded to obtain the final 3D mesh, using eight nodes hexahedral elements (volumetric elements).
All the nodes connected to the pelvic bone, ligament, and coccyx were fixed in the finite element simulation, and all the others were left free.
After the creation of the finite element model for the pelvic floor, a bony model was joined to the pelvic floor model. To join the two models, the initial pelvic floor model had to be improved.
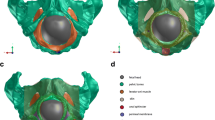
To correctly support the pelvic floor muscles, additional meshes (Mesh 1 and Mesh 2), representing the structures that give support to the pelvic floor, had to be introduced, as can be seen in Fig. 2.
With the Mesh 1, we intend to simulate the different connections between muscles of the pelvic floor and the coccyx. With the Mesh 2 in the same figure, we simulate the behavior of the arcus tendineus, obturator fascia, and the obturator internus, which have a very important role in supporting the pelvic floor muscles.
Figure 2 also shows the principal dimensions of the pelvic floor model, which are in accordance with other models of the pelvic floor [13].
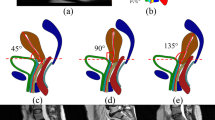
The principal obstetric dimensions for the fetal head are the following: suboccipito-bregmatic diameter, 10 cm; suboccipito-frontal diameter, 10.5 cm; occipito-frontal diameter, 12.0 cm; mento-vertical diameter, 13.0 cm; and submento-bregmatic diameter, 11.5 cm. The dimensions of the fetal head are in accordance with the literature [17]. The simulation was performed using the implicit version of the ABAQUS software. The pelvic floor was modeled using hexahedral, elements. The constitutive model adopted in this work for the 3D behavior of the pelvic floor muscles [18] is a modified form of the model proposed by Humphrey and Yin [19]. The fetus was modeled using tetrahedral elements and was considered as a deformable body, but with a very high stiffness. Summarily, in our model, we used the hexahedral elements to model the pelvic floor muscles and tetrahedral elements to model the fetus and the bones. The reason for this procedure is related with the higher efficiency that it is, in general, associated with hexahedral elements in comparison to the tetrahedral. The movement of the fetus was imposed by controlling the movement of several points belonging to the fetal model.
In this work, the movements of the fetus during birth in the vertex position were simulated, namely, the engagement, descent, flexion, internal rotation, and extension of the fetal head.
To evaluate the maximum muscular stretch, several levels along the pelvic floor muscles were defined, as shown in Fig. 3. The initial lengths are also shown in Fig. 3. Measuring the length of the levels during the simulation and knowing their initial value allowed us to determine the evolution of the stretch values for each path. The stretch ratio is defined as the ratio between the current tissue length to the original tissue length.
Results
Figure 3 shows the values of the initial lengths and the lengths of each level for a vertical displacement of the fetal head of 60 mm. For this position of the head, the stretch on each level can be carried out as the ratio between the values on the right column and the middle column.
Figure 4 shows the evolution of the stretch values obtained for the pelvic floor on the referenced levels during fetal descent. The maximum value obtained for the stretch was 1.63, obtained on level 1, for a vertical displacement of the fetal head of approximately 60 mm. This maximum stretch value occurs during the extension of the fetal head.
To present a better comparison of the obtained results, for all levels, only one scale varying between 0 and 1 was considered, called normalized length. For example, 0 represents one of the extremities, 0.5 represents the middle position, and 1 represents the position in the opposite extremity (Fig. 3).
On Fig. 5 the evolution of the strain along the different levels (normalized lengths), for different vertical displacements of the fetal head is shown. The strain-E1 is defined as the ratio between the variation in length and the original tissue length. The strain values are calculated for each finite element cell, along the different levels. Proceeding in this fashion, we can show the location along the different curves where the strains are higher. For example, the point A (Fig. 3), located on level 5 on the left side (normalized length = 0), is subjected to a deformation of 0.3, for a descent of the fetus head of 90 mm (Fig. 5e).
As one would expect, the evolution of the strains is closely related with the evolution of the stretches; therefore, the maximum values for the strain appear for roughly the same vertical displacement of the fetus head (60 mm). As it is possible to observe in Fig. 5c, a maximum value of 0.66 for the strain E1 on level 1 is obtained, for a vertical displacement of 60 mm.
A close observation of the evolution of the strains along level 1 shows that we obtain high values of strain on the edges of level 1, which correspond to the points of attachment of levator ani muscle and the pubococcygeus muscle to the pelvic bones. The maximum values for the strain appear in an area that corresponds to the middle length of the levator ani muscle and the pubococcygeus muscle.
Discussion
Initial investigations on the mechanisms responsible for contraction-induced injury, using nongravid, passive striated appendicular muscles, obtained a stretch value of 1.5 for the maximum noninjurious stretch [13, 20]. The maximum stretch ratio of 1.63 found in this study exceeds this largest noninjurious stretch (1.5 stretch ratio). If injury can be caused by fiber stretch exceeding a maximum permissible value, we may conclude that a risk exists for injury of the muscles of the pelvic floor during the second stage of labor.
The problem studied here is very complex, and any methodology used will be prone to have limitations and to criticism. To properly interpret our findings, we need to consider the limitations involved.
In relation with the data (namely, geometry and mechanical properties) of the pelvic floor used, some doubts can be formulated because of the absence of information about any vaginal delivery.
It was assumed that the stretch was uniform along the levels considered, which is not true because it can vary locally along and across a muscle band, especially if thickness varies, leading to a conservative estimate of muscles strains. Time-dependent material property effects on tissue stretch were not considered. Although these may affect the tissue stresses [13], they will not affect our estimates of the maximum average tissue stretch because they do not affect the inherent geometric difference between the sizes of the prelabor urogenital hiatus and fetal head.
During the last weeks of pregnancy, the pelvic floor experiences several changes to facilitate the delivery, which might lessen the maximal stretches. These modifications were not considered on this study [21].
During delivery, the fetal head configuration changes to reduce the volume of the skull and facilitate its passage through the birth canal [17]. The occipital bone is displaced under the two parietal bones during childbirth, reducing the size of the posterior fontanelle, which is called molding. During molding, the parietal bones may also slip under each other. This effect was not considered on this study.
A multitude of variables such as variations in maternal pelvic shape, fetal head shape, the degree of molding during delivery, symphyseal diastasis, types of episiotomies, and presenting orientation may affect the maximum muscle stretch ratios, thus affecting the final results.
As investigation progresses, clinicians will eventually shift from a condition-based approach to an injury-based approach, radically transforming both clinical research and patient care. An enhanced precision in defining pelvic floor disorders will revolutionize our ability to define and implement appropriate treatment, as well as to conduct focused clinical research.
The computer model presented in this work is a first step in understanding how obstetrical factors and interventions might influence levator ani injury risk, since experimental measurements of levator stretch in laboring women are not currently feasible for many clinical, technical, and ethical reasons. This model could also be used to study the effect of assisted delivery, as well as the effect of malposition, such as occipitoposterior position, on the degree of muscle stretch during delivery, which will constitute future research work.
The present numerical simulation shows that the muscles of the pelvic floor are submitted to high deformations during the passage of the fetus head.
During a vaginal delivery, the levator ani muscle and the pubococcygeus muscle are the muscles that are subjected to the largest values of stretch and strain. These muscles are the ones at greater risk for a stretch related injury.
The present work showed a noninvasive procedure, which can be used in the future to estimate the damage that a vaginal delivery can induce on a specific pelvic floor.
References
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL (1997) Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89:501–506
Kenton K, Mueller ER (2006) The global burden of female pelvic floor disorders. Br J Urol 98:1–5
Rortveit G, Hannestad Y, Daltveit AK, Hunskaar S (2001) Age- and type-dependent effects of parity on urinary incontinence. The Norwegian EPINCONT study. Obstet Gynecol 98:1004–1010
Dimpfl Th, Jaeger Ch, Mueller-Felber W, Anthuber C, Hirsch A, Brandmaier R et al (1998) Myogenic changes of the levator ani muscle in premenopausal women: the impact of vaginal delivery and age. Neurourol Urodyn 17:197–205
Gregory WT, Nygaard I (2004) Childbirth and pelvic floor disorders. Clin Obstet Gynecol 47:394–403
DeLancey John OL (1999) Structural anatomy of the posterior pelvic compartment as it relates to rectocele. Am J Obstet Gynecol 180:815–823
Papa Petros PE (2004) The female pelvic floor, function, dysfunction and management according to the integral theory. Springer, Berlin Heidelberg New York
Humphrey JD (2003) Continuum biomechanics of soft biological tissues. Proc R Soc Lond A 459:3–46
Janda S, Van der Helm FCT, Blok SB (2003) Measuring morphological parameters of the pelvic floor for finite elements modelling purposes. J Biomech 36:749–757
Aulignac D, Martins JAC, Pires EB, Mascarenhas T, Natal Jorge RM (2005) A shell finite element model of the pelvic floor muscles. Comput Methods Biomech Biomed Eng 8:339–347
Hoyte L, Jakab M, Warfield SK, Shott S, Flesh G, Fielding JR (2004) Levator ani thickness variations in symptomatic and asymptomatic women using magnetic resonance-based 3-dimensional color mapping. Am J Obstet Gynecol 191:856–861
Boukerrou M, Lambaudie E, Dubois P, Cosson M (2004) Etude préliminaire d’un modèle mécanique de cavité vaginale, ITBM-RBM 25:3–14
Lien KC, Mooney B, DeLancey John OL, Ashton-Miller JA (2004) Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol 103:31–40
Lien KC, Morgan DM, DeLancey John OL, Ashton-Miller JA (2005) Pudendal nerve stretch during vaginal birth: a 3D computer simulation. Am J Obstet Gynecol 192:1669–1676
Fung YC, Tong P (2001) Classic and computational solid mechanics. World Scientific, Singapore
Zienkiewcz OC, Taylor RL (2005) The finite element method for solid and structural mechanics, 6th edn. Elsevier, Amsterdam
Llewellyn-Jones D (2004) Fundamentals of obstetrics and gynaecology. Elsevier, Amsterdam
Martins JAC, Pires EB, Salvado R, Dinis PB (1998) A numerical model of passive and active behaviour of skeletal muscles. Comput Methods Appl Mech Eng 151:419–433
Humphrey JD, Yin FCP (1987) On constitutive relations and finite deformations of passive cardiac tissue: a pseudostrain-energy function. J Biomech Eng 109:298–304
Brooks SV, Zerba E, Faulkner JÁ (1995) Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. J Physiol 488:459–469
DeCherney AH, Nathan L (2003) Current obstetric and gynecologic diagnosis and treatment. Lange medical books, 9th edn. McGraw-Hill, New York
Acknowledgment
Funding by Ministério da Ciência, Inovação e do Ensino Superior, FCT, Portugal, under grants POSI/SFRH/BD/13013/2003, as well as the funding by FEDER under grants POCTI/ESP/46835/2002 are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parente, M.P.L., Jorge, R.M.N., Mascarenhas, T. et al. Deformation of the pelvic floor muscles during a vaginal delivery. Int Urogynecol J 19, 65–71 (2008). https://doi.org/10.1007/s00192-007-0388-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-007-0388-7