Abstract
Purpose
An understanding of the behavior of a new ACL graft in the femoral tunnel during knee motion and external loading can provide information pertinent to graft healing, tunnel enlargement, and graft failure. The purpose of the study was to measure the percentage of the tunnel filled by the graft and determine the amount and location of the graft–tunnel contact with knee motion and under external knee loads.
Methods
Single bundle anatomical ACL reconstruction was performed on six cadaveric knees. Specimens were positioned with a robotic testing system under: (1) passive flexion–extension, (2) 89-N anterior and posterior tibial loads, (3) 5-N m internal and external torques, and (4) 7-N m valgus moment. The knees were then dissected, repositioned by the robot and the geometry of the femoral tunnel and graft were digitized by laser scanning. The percentage of tunnel filled and the contact region between graft and tunnel at the femoral tunnel aperture were calculated.
Results
The graft occupies approximately 70% of the femoral tunnel aperture and anterior tibial loading tended to reduce this value. The graft contacted about 60% of the tunnel circumference and the location of the graft–tunnel contact changed significantly with knee flexion.
Conclusion
This study found that the graft tends to rotate around the tunnel circumference during knee flexion–extension and contract under knee loading. The “windshield–wiper” and “bungee cord” effect may contribute to femoral tunnel enlargement, affect graft healing, and lead to graft failure. There can be a considerable motion of the graft in the tunnel after surgery and appropriate rehabilitation time should be allowed for graft–tunnel healing to occur. To reduce graft motion, consideration should be given to interference screw fixation or a graft with bone blocks, which may allow an earlier return to activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior cruciate ligament (ACL) reconstruction often employs a soft tissue graft with an extra-cortical fixation on the femur. With this type of reconstruction, it has been found that there is frequently a space between the graft and tunnel at the tunnel aperture [13]. Even though a graft is typically sized to the tunnels in ACL reconstruction, due to contraction when tensioning, the graft may not fill the tunnel aperture area [24]. Due to this effect, studies have found that even though the femoral tunnel is positioned in the center of the footprint, the centroid of the graft may be deviated from the tunnel center [23, 27]. Lee et al. evaluated the graft filling area and the graft position within the femoral tunnel apertures in double-bundle ACL reconstruction, and found that grafts did not fill the tunnel aperture area and the centers of the grafts differed slightly from the centers of the tunnel apertures [23]. In another clinical study, imaging one year after double-bundle ACL reconstruction found that the anteromedial (AM) graft was shifted posteriorly and medially from the femoral tunnel center, while the posterolateral (PL) graft was not shifted from center [27]. Fujii et al. measured the semitendinosus tendon graft shift at the tunnel aperture with graft bending angle with a simulated femoral bone tunnel and found that the graft only filled a portion of the tunnel, the center of the graft may be different from the center of the insertion site [13].
With space between the graft and tunnel, the graft may change position in the tunnel with knee motion. Movement of the graft in the tunnel could have adverse effects on the graft, the bone tunnel aperture, and graft healing. Femoral tunnel enlargement after ACL reconstruction has been noticed clinically [15, 25, 26, 39]. And while some reports suggested that bone tunnel enlargement is mainly the result of an immune response to allograft tissue, other studies suggest that other biological, as well as mechanical factors may play a more important role [12, 15, 32]. Mechanical factors contributing to tunnel enlargement may include stress deprivation of bone within the tunnel wall, graft–tunnel relative motion, improper tunnel placement, and aggressive rehabilitation [17, 39]. Moreover, it has been suggested that graft motion in the tunnel could have a deleterious effect on graft healing and perhaps led to graft failure [13, 24, 27, 38].
While there have been imaging studies of the static position of the ACL in the tunnel and deformation of the graft within the tunnel, the ACL graft dynamic behavior in the tunnel has not be studied and has clinical implications. The aim of this study was to evaluate the graft position and movement in the femoral tunnel using precision measurement techniques. Based on previous static imaging studies, it is hypothesized that the graft position will significantly change with knee flexion and loading. If there is considerable motion of the graft, this can have implications for graft healing, return to activity and possible graft and tunnel damage.
Materials and methods
With institutional approval (Committee for Oversight of Research and Clinical Training Involving Decedents #453), six intact fresh-frozen cadaver knees were used in this study. Samples were frozen at − 20 °C and thawed overnight at room temperature prior to testing and all knees were assessed arthroscopically before the test for any ligament abnormality or injury. The tibia and femur were sectioned approximately 15 cm from the joint line and the ends of femur and tibia were potted in epoxy putty [30].
Anatomic single-bundle ACL reconstruction was performed via arthroscopy by the same surgeon [3, 9, 28]. The intact ACL was first excised and removed with the use of a punch, a shaver and an ablator and the ACL insertion sites were marked with an awl and ablator. Previously harvested human cadaver hamstring grafts were used [11, 36]. The grafts were passed through the loop of an extra-cortical button, folded and whip stitched and trimmed to 8 mm diameter. A guidewire was inserted into the centers of ACL footprints, tunnels were made with a 7.5 mm drill and dilated to 8 mm. The graft was passed and tensioned at 30° of knee flexion under 40 N force using a tension-meter (Meira Corp., Nagoya, Aichi, Japan), fixed with a metal interference screw in the tibial tunnel [34]. Additionally, post-tie fixation was applied to augment tibial fixation [7].
The knees were kept moist with physiologic saline solution during testing. A six-joint robotic system with repeatability of motion within ± 0.02 mm at each joint, with a force-moment load cell that has an accuracy of ± 0.2 N and ± 0.1 N m according to the manufacturer, was used for testing the knee specimens [36]. The potted tibia and femur were placed in the robotic system. The passive path of the intact knee flexion–extension was determined from full extension (FE) of the knee to 90° of knee flexion in 0.5° increments by minimizing forces (< 0.5 N) and moments (< 0.25 N m) in all remaining degrees of freedom [40, 41].
The specimens were subjected to the following loading conditions: (1) an 89.0 N anterior tibial (AT) load (simulated KT1000 test [10]) to test anterior tibial translation (ATT), (2) an 89.0 N posterior tibial (PT) load to test posterior tibial translation (PTT) [29], (3) a 5.0 N m internal tibial torque to test internal rotation (IR), (4) a 5.0 N m external tibial torque to test external rotation (ER) [33] and (5) a 7.0 N m lateral bending moment to test valgus rotation [21, 23]. The ATT and PTT were measured at FE, 15°, 30°,45°, 60° and 90° of knee flexion, while internal/external and valgus rotations were measured at FE, 15° and 30° of knee flexion [20].
After robotic testing, soft tissues, including muscles, the ligaments and the menisci, were removed, and a portion of the condyles were removed to allow visualization of the ACL graft. The dissected femur, tibia with ACL graft, were mounted once again to the robotic testing system. The previous passive path and motion under loading were repeated by the robotic system so that the dissected knee’s position was the same as that of the intact knee.
Geometric analysis
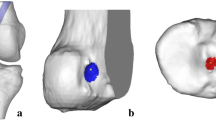
The three-dimensional surface geometry of the bone, including lateral femoral condyle, the ACL graft, and the femoral inter-articular tunnel aperture were scanned and the femoral inter-articular tunnel aperture edge was also measured with a 1 mm probe using a digitizer (FARO Technologies, Inc., Lake Mary, FL) [14]. The single point reliability of the digitizing system is 0.0043 mm [2] After scanning, the graft was removed from the tunnel, and the femoral tunnel was re-scanned, and the tunnel edge was digitized with a 1 mm probe. The scanned data were processed using graphics software (Geomagic, Inc., Research Triangle, NC) (Fig. 1a, b).
a dissected knee with single-bundle ACL reconstruction, b laser scan image with ACL graft outlined in red, c laser scan image of the lateral femoral condyle without ACL graft and an 8 mm diameter cylinder fitted to the femoral tunnel, and d yellow outline representing the graft cross-section inside the tunnel cylinder at the femoral intra-articular aperture plane
The femoral tunnel was represented as the best fit of an 8 mm cylinder to the scanned geometry of the tunnel inner wall of the bone with the graft removed (Fig. 1c). Due to individual variation of the femoral bony insertion geometry, the femoral intra-articular aperture shape will be different between samples. To normalize the femoral intra-articular aperture, a plane perpendicular to the femoral tunnel longitudinal axis was placed at the location which was defined by the centroid of the probing points digitized around the femoral intra-articular aperture (Fig. 1c). Once defined, the cross-sectional area of the graft at this plane was determined by first positioning the fitted femoral tunnel cylinder in the scanned geometries with the graft by overlapping two scanned femoral condyle geometries (with and without graft), and then finding the interception area of the scanned graft tissue geometry inside the cylinder at this plane (Fig. 1d).
The center point of the graft cross-sectional area was determined by finding the centroid of the outline of the graft cross-section area defined in the previous step. The percentage of femoral tunnel aperture that was filled by the graft was determined as the ratio of the cross-sectional area of graft to the total cross-sectional area of the 8 mm diameter tunnel. The contact region between the graft and the tunnel circumference was calculated by finding the portion of the graft’s outline that overlapped with the femoral intra-articular aperture’s outline and the mid-point of the contact region was also calculated. The angular position (α) of the contact region was defined as the angle between the long axis of the femur and the tunnel center to mid-point line and evaluated before and after knee motion or external load. Shown in Fig. 2 is an example of the graft centroid location, the region of the tunnel aperture filled by the graft, the region of graft–tunnel contacts and the angle between the mid-point of contact and the femoral axis.
Definition of the graft centroid location (orange +), the area of the femoral tunnel aperture filled by the graft (green area), the graft–tunnel contact region (red), the center of the graft–tunnel contact (yellow star) and angle between the center of the graft–tunnel contact region and femoral axis (α) at full knee extension. The contact region percentage is the ratio of red line to the entire tunnel circumference, and the percentage of the femoral tunnel aperture filled by the graft is the ratio of the green area to the entire tunnel circular area
Statistical analysis
The statistical analysis was performed using the software package Minitab (Minitab; Minitab Inc., State College, PA, USA). Differences in graft contact angular position, graft–tunnel aperture contact percentage and the percentage of the femoral tunnel aperture filled by graft were analyzed using one-factor repeated-measures analysis of variance (ANOVA) with knee load as the factor with a post hoc analysis using a Bonferroni correction with statistical significance was set at p < 0.05. An a priori power analysis (G*Power 3.1.9.4) was done to estimate the sample size with the primary variable being graft–tunnel contact angle with knee flexion angle. An ANOVA repeated-measures test with α = 0.05 and a power of 0.8 was used. It was estimated that the difference between means would be 15° (difference in flexion angles) and the standard deviation would be 5°. Based on an effect size of 1.0, a sample size of 5 was determined.
Results
After ACL reconstruction, knee kinematic under external loads were tested before dissection. There was no significant difference in the ATT between the ACL reconstructed knees (3.3 ± 2.0 mm, 5.6 ± 3.7 mm, 6.9 ± 3.4 mm, 7.3 ± 2.8 mm, 6.5 ± 2.6 mm, 5.3 ± 1.8 mm) at full extension, 15°, 30°, 45°, 60° and 90° knee flexion and intact ACL knee (5.5 ± 1.7 mm, 7.3 ± 1.9 mm, 8.1 ± 1.7 mm, 7.9 ± 1.9 mm 6.8 ± 2.0 mm, 5.4 ± 1.7 mm). There were also no significant differences in the kinematics between the intact ACL and ACL reconstructed knees under posterior, internal rotational, external rotational, and valgus loadings at any flexion angle, indicating intact knee kinematics were not significantly different from those of the ACL reconstructed knees before dissection.
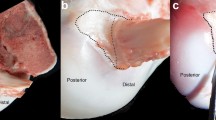
Figure 3 gives an example of the graft–tunnel contact location at the tunnel aperture and the percentage of the graft–tunnel exit occupied by the graft without external loading at different knee flexion angles. The percentage of the tunnel aperture filled by the graft during knee flexion–extension and under knee external loads is given in Fig. 4. During flexion–extension and with knee loading, the percentage varied from a minimum of 52.8% to a maximum of 90.4%. However, no statistical difference was found in the percentage of tunnel filled with knee load (Fig. 4).
The contact region between the graft portion and bone tunnel wall varied with flexion angle as well as external loads (Fig. 5) and the percentage of contact varied from 55.4 to 66.7%. While in general, knee loading reduces the amount of contact, no statistically significant difference was found in the graft–tunnel contact amount with knee loading.
During passive flexion–extension, the angular position of the graft–tunnel contact region (α) rotates with knee flexion from full extension to 90° knee flexion (Fig. 6). The angular position of the contact region tended to change from anterior distal (158.1° ± 11.5°) to posterior distal (280.9° ± 32.3°) during flexion. Statistical differences were found in the angular position of the contact region at different knee flexion angles (Fig. 6).
The changes in the angular position of contact with external knee loads are given in Fig. 7. With a posterior tibial load, graft–tunnel contact position rotates counterclockwise direction in viewing the tunnel entrance in a right knee while an anterior tibial load causes the position to move clockwise. The contact point also tends to move counterclockwise with internal, external, and valgus loads. Knee load did cause significant changes in the graft–tunnel contact position (Fig. 7).
Discussion
The most important finding of the current study was that the ACL graft position in the femoral tunnel changes with passive flexion and external knee loading. The results showed that the ACL graft position shifts in the tunnel as described by previous studies [19, 39]. It was also found that when placed in a tunnel of the same diameter and tensioned, the soft tissue graft occupies approximately 70% of the tunnel area and contacts about 60% of the tunnel circumference.
The motion of an ACL soft tissue graft in the graft–tunnel has been suggested as a cause of graft failure and tunnel enlargement [4, 6, 8, 16]. Jagodzinski et al. calculated force components of the graft at the femoral tunnel in the sagittal and coronal plane and found force components corresponded to the amount of tunnel enlargement in the radiographic planes, which indicated that that graft–tunnel contact forces play a role in postoperative tunnel expansion [18]. In our study, the movement of the graft around the circumference of the femoral tunnel inter-articular edge with knee flexion and external loading was measured after anatomical ACL reconstruction which successfully restored knee anterior laxity. Results showed that the graft–tunnel contact at the inter-articular aperture moved with knee passive flexion and external knee loading.
Another finding of this study was that when placed in a tunnel of the same diameter and tensioned, a soft tissue graft occupies approximately 70% of the tunnel area and tends to contact under anterior loading [15]. Here, the amount of the tunnel aperture occupied by the graft was reduced, although not significantly under anterior tibial load. The reason that the graft does not fill the tunnel is that it must be sized to be passed through the tunnels under low tension and then the diameter contracts under the fixation tension. Because the graft only partially fills the tunnel, the region of contact between the graft and tunnel edge can change with knee flexion. The change in angular position can be larger than the knee flexion angle which may be due to the irregular contour of the tunnel edge causing addition graft movement and that the tunnel edge has been approximated as being in a plane.
Although the cause of tunnel enlargement remains unknown, one of the postulated mechanical causes is the graft motion within the tunnel [5]. Previous studies have documented the “bungee effect” and “windshield–wiper effect” of an ACL graft which are the longitudinal and transverse movement of the graft at the tunnel exit which may be related to tunnel enlargement [15, 21, 37]. Transverse motions in terms of changing the contact position of the graft around the tunnel circumference were found in this study. Although longitudinal graft deformation was not directly measured, contraction or dilatation in the graft area (the amount of tunnel area occupied by the graft) is an indication of a change in longitudinal graft strain. Motion along the edge of the tunnel aperture could contribute to tunnel wear and enlargement as well as result in damage to the graft. Clinically, movement of the ACL graft in the bone tunnel may offer a possible explanation for the lack of graft–tunnel healing [31].
This study evaluated graft behavior in ACL reconstruction using a soft tissue graft with an extra-cortical fixation on the femur. In this construct, the graft is not anchored to the tunnel, and with graft contraction due to tensioning there is space in the tunnel for graft movement. With knee motion and external loading, the graft changes position in the tunnel and contracts which may be detrimental to graft–tunnel healing. Because the ACL soft tissue graft does not entirely fill the femoral tunnel, the graft center is not at the tunnel center and it has been suggested that tunnel position be altered to account for this [22]. This offset is relatively small, so it was not thought to have a large effect on knee biomechanics and the precise tunnel positioning necessary to account for this is probably beyond current surgical techniques. However, no matter the tunnel placement, there will probably be graft motion in the tunnel prior to healing. Perhaps, the most important clinical implication of these findings is that appropriate rehabilitation time should be allowed for graft–tunnel incorporation.
The limitations of the study should be noted. First, this study uses laser scanning to measure graft position and shape and requires knee dissection and some bone removal to allow line of sight visual access to the graft and femoral tunnel. It is believed that there was no impingement between graft and condyles in the pre-dissection knee and that the partial removal of knee condyles did not affect graft motion or position. The irregular tunnel edge was also approximated as a plane perpendicular to the tunnel axis placed at the centroid of edge points. Second, our study used a soft tissue graft with extra-cortical button fixation on the femur and interference screw fixation in the tibia tunnel and different fixation techniques could influence graft motion [1, 35]. Also, this was a cadaver study that did not include muscle forces or biological graft healing. The intention of the current study was to investigate graft motion in period after ACL reconstruction prior to any graft–bone healing where there is no adhesion between the graft and tunnel or graft remodeling.
The results of this study show that there is substantial movement of the graft in the tunnel with knee flexion. This may have consequences for graft–tunnel healing and tunnel enlargement. Therefore, for suspensory fixation with soft tissue grafts, consideration should be given to appropriate rehabilitation time and return to activity. Screw fixation or grafts with bone blocks may reduce graft motion.
Conclusion
The position and the relative movement of a new ACL graft in the femoral tunnel with normal knee motion and external loading were measured in this study. Significant movement of the graft around the tunnel circumference, the “windshield–wiper” and “bungee cord” effect, were found during knee flexion–extension and contract under knee loading in anatomical ACL reconstructed knees. These effects may contribute to femoral tunnel enlargement, affect graft healing, and lead to graft failure.
References
Amano H, Tanaka Y, Kita K, Uchida R, Tachibana Y, Yonetani Y et al (2019) Significant anterior enlargement of femoral tunnel aperture after hamstring ACL reconstruction, compared to bone-patellar tendon-bone graft. Knee Surg Sports Traumatol Arthrosc 27:461–470
Araki D, Thorhauer E, Tashman S (2018) Three-dimensional isotropic magnetic resonance imaging can provide a reliable estimate of the native anterior cruciate ligament insertion site anatomy. Knee Surg Sports Traumatol Arthrosc 26:1311–1318
Ballmer PM, Jakob RP (1988) The non operative treatment of isolated complete tears of the medial collateral ligament of the knee. A prospective study. Arch Orthop Trauma Surg 107:273–276
Barber FA, Spruill B, Sheluga M (2003) The effect of outlet fixation on tunnel widening. Arthroscopy 19:485–492
Celik H, Kim JH, Lee SH, Lee DH (2021) Femoral tunnel widening via transcondylar cross-pin fixation versus extracortical suspensory fixation after single-bundle ACLR: a systematic review and meta-analysis. Orthop J Sports Med 9:2325967121993811
Cheung P, Chan WL, Yen CH, Cheng SC, Woo SB, Wong TK et al (2010) Femoral tunnel widening after quadrupled hamstring anterior cruciate ligament reconstruction. J Orthop Surg (Hong Kong) 18:198–202
Choi SH, Ha JK, Jun DJ, Seo JG, Park JH (2017) Additional post-tie for unstable femoral suspensory fixation during anterior cruciate ligament reconstruction using TightRope ® RT : clinical reports on 3 cases. Arthrosc Orthop Sports Med 4:34–38
Clatworthy MG, Annear P, Bulow JU, Bartlett RJ (1999) Tunnel widening in anterior cruciate ligament reconstruction: a prospective evaluation of hamstring and patella tendon grafts. Knee Surg Sports Traumatol Arthrosc 7:138–145
Cohen SB, Fu FH (2007) Three-portal technique for anterior cruciate ligament reconstruction: use of a central medial portal. Arthroscopy 23(325):e321-325
Daniel DM, Stone ML, Sachs R, Malcom L (1985) Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med 13:401–407
Desai VS, Anderson GR, Wu IT, Levy BA, Dahm DL, Camp CL et al (2019) Anterior cruciate ligament reconstruction with hamstring autograft: a matched cohort comparison of the all-inside and complete tibial tunnel techniques. Orthop J Sports Med 7:2325967118820297
Fahey M, Indelicato PA (1994) Bone tunnel enlargement after anterior cruciate ligament replacement. Am J Sports Med 22:410–414
Fujii M, Sasaki Y, Araki D, Furumatsu T, Miyazawa S, Ozaki T et al (2016) Evaluation of the semitendinosus tendon graft shift in the bone tunnel: an experiemental study. Knee Surg Sports Traumatol Arthrosc 24:2773–2777
Fujimaki Y, Thorhauer E, Sasaki Y, Smolinski P, Tashman S, Fu FH (2016) Quantitative in situ analysis of the anterior cruciate ligament: length, midsubstance cross-sectional area, and insertion site areas. Am J Sports Med 44:118–125
Hoher J, Moller HD, Fu FH (1998) Bone tunnel enlargement after anterior cruciate ligament reconstruction: fact or fiction? Knee Surg Sports Traumatol Arthrosc 6:231–240
Ioria R, Di Sanzo V, Vadala A, Conteduca J, Mazza D, Redler A et al (2013) ACL reconstructions with hamstrings: how different technique and fixation devices influence bone tunnel enlargement. Eur Rev Med Pharmacol Sci 17:2956–2961
Iorio R, Vadala A, Argento G, Di Sanzo V, Ferretti A (2007) Bone tunnel enlargement after ACL reconstruction using autologous hamstring tendons: a CT study. Int Orthop 31:49–55
Jagodzinski M, Foerstemann T, Mall G, Krettek C, Borsch U, Paessler HH (2005) Analysis of forces of ACL reconstructon at the tunnel entrance; is tunnel enlargement a biomechanical problem? J Biomech 38:23–31
Jorgensen U, Thomsen H (2000) Behavior of the graft within the bone tunnels following anterior cruciate ligament reconstruction, studied by cinematic magentic resonance imaging. Knee Surg Sports Traumatol Arthrosc 8:32–35
Kato Y, Ingham SJM, Kramer S, Smolinski P, Saito A, Fu FH (2009) Effect of tunnel position for anatomical single-bundle ACL reconstruction on knee biomechanics in a porcine model. Knee Surg Sports Traumatol Arthrosc 18:2–10
L’Insalata JC, Klatt B, Fu FH, Harner CD (1997) Tunnel expansion following anterior cruciate ligament reconstruction: a comparison of hamstring and patellar tendon autographs. Knee Surg Sports Traumatol Arthrosc 5:234–238
Lanzetti RM, Lupariello D, De Carli A, Monaco E, Guzzini M, Fabbri M et al (2017) Can the outside-in half-tunnel technique reduce femoral tunnel widening in anterior cruciate ligament reconstruction? A CT study. Eur J Orthop Surg Traumatol 27:659–664
Lee BH, Bansal S, Park SH, Wang JH (2015) Eccentric graft positioning within the femoral tunnel aperature in anatomic double-bundle anterior cruciate ligament reconstruction using the transportal and outside-in techniques. Am J Sports Med 43:1180–1188
Lee BH, Seo DY, Bansal S, Kim JH, Ahn JH, Wang JH (2016) Comparative magnetic resonance imaging study of the cross-sectional area of anatomic double bundle ligament reconstruction grafts and the contralateral uninjured KNN. Arthroscopy 32:321–329
Mutsuzaki H, Kinugasa T, Ikeda K, Sakane M (2019) Morphological changes in the femoral and tibial bone tunnels after anatomic single-bundle anterior cruciate ligament reconstruction using a calcium phosphate-hybridized tendon graft in 2 years of follow-up. Orthop Traumatol Surg Res 105:653–660
Nebelung W, Becker R, Merkel M, Ropke M (1998) Bone tunnel enlargement after anterior cruciate ligament reconstruction with semitendinosus tendon using endobutton fixation on the femoral side. Arthroscopy 14:810–815
Onodera J, Yasuda K, Masuda T, Tanabe Y, Kitamura N, Yagi T et al (2017) Is the grafted tendon shifted anteriorly in the femoral tunnel at the postremodeling phase after anterior cruciate ligament reconstruction? A clinical MRI study. Orthop J Sports Med 5:2325967117711120
Rahnemai-Azar AA, Sabzevari S, Irarrazaval S, Chao T, Fu FH (2016) Anatomical individualized ACL reconstruction. Arch Bone Jt Surg 4:291–297
Rangger C, Daniel DM, Stone ML, Kaufman K (1993) Diagnosis of an ACL disruption with KT-1000 arthrometer measurements. Knee Surg Sports Traumatol Arthrosc 1:60–66
Sasaki Y, Chang SS, Fujii M, Araki D, Zhu J, Marshall B et al (2016) Effect of fixation angle and graft tension in double-bundle anterior cruciate ligament reconstruction on knee biomechanics. Knee Surg Sports Traumatol Arthrosc 24:2892–2898
Schiavone Panni A, Denti M, Franzese S, Monteleone M (1993) The bone-ligament junction: a comparison between biological and artificial ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 1:9–12
Silva A, Sampaio R, Pinto E (2010) Femoral tunnel enlargement after anatomic ACL reconstruction: a biological problem? Knee Surg Sports Traumatol Arthrosc 18:1189–1194
Surer L, Michail K, Koken M, Yapici C, Zhu J, Marshall BD et al (2017) The effect of anterior cruciate ligament graft rotation on knee biomechanics. Knee Surg Sports Traumatol Arthrosc 25:1093–1100
Suzuki T, Shino K, Otsubo H, Suzuki D, Mae T, Fujimiya M et al (2014) Biomechanical comparison between the rectangular-tunnel and the round-tunnel anterior crucciate ligament reconstruction procedures with a bone-patellar tendon-bone graft. Arthroscopy 30:1294–1302
Tachibana Y, Mae T, Shino K, Ohori T, Amano H, Yoshikawa H et al (2018) Femoral tunnel enlargement after anatomic anterior cruciate ligament reconstruction: bone-patellar tendon-bone/single rectangular tunnel versus hamstring tendon/double tunnels. J Orthop Sci 23:1011–1018
Tang X, Marshall B, Wang JH, Zhu J, Li J, Smolinski P et al (2019) Lateral meniscal posterior root repair with anterior cruciate ligament reconstruction better restores knee stability. Am J Sports Med 47:59–65
Tsuda E, Fukuda Y, Loh JC, Debski R, Fu FH, Woo SL (2002) The effect of soft-tissue graft fixation in anterior cruciate ligament recontruction on graft-tunnel motion under anterior tibial loading. Arthroscopy 18:960–967
Vignos MF, Smith CR, Roth JD, Kaiser JM, Baer GS, Kijowski R et al (2020) Anterior cruciate ligament graft tunnel placement and graft angle are primary determinants of internal knee mechanics after reconstructive surgery. Am J Sports Med 48:3503–3514
Wilson TC, Kantaras A, Atay A, Johnson DL (2004) Tunnel enlargement after anterior cruciate ligament surgery. Am J Sports Med 32:543–549
Woo SL, Debski RE, Withrow JD, Janaushek MA (1999) Biomechanics of knee ligaments. Am J Sports Med 27:533–543
Woo SL, Fisher MB (2009) Evaluation of knee stability with use of a robotic system. J Bone Joint Surg Am 91(Suppl 1):78–84
Funding
No external funding was received for this study.
Author information
Authors and Affiliations
Contributions
JZ and BM carried out the study and participated in data collection and interpretation. XT provided technical support and design. ML participated in study conception and design, provided critical revision of the manuscript. FF and PS helped in the analysis and interpretation of data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Institutional approval (Committee for Oversight of Research and Clinical Training Involving Decedents #453) was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, J., Marshall, B., Tang, X. et al. ACL graft with extra-cortical fixation rotates around the femoral tunnel aperture during knee flexion. Knee Surg Sports Traumatol Arthrosc 30, 116–123 (2022). https://doi.org/10.1007/s00167-021-06703-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-021-06703-8