Abstract
Purpose
To evaluate intercompartmental load intraoperatively with a sensor after conventional gap balancing with a tensiometer during total knee arthroplasty (TKA).
Methods
Fifty sensor-assisted TKA procedures were performed prospectively between August and September 2018 with a cruciate-retaining prosthesis. After applying a modified measured resection technique, conventional balancing between resected surfaces was achieved. The equal and rectangular flexion–extension gaps were confirmed using a tensiometer at 90° and 5°–7° (due to posterior tibial slope) of knee flexion. Then, the load distribution was evaluated intraoperatively with a sensor placed on trial implants in the positions of knee flexion (90° flexion) and extension (10° flexion).
Results
The proportion of coronal load imbalance (medial load − lateral load ≥ ± 15 lb) was 56% in extension and 32% in flexion (p = 0.023). The proportion of sagittal load imbalance (extension load − flexion load ≥ ± 15 lb) was 36% in the medial compartment and 4% in the lateral compartment (p < 0.001). An additional procedure for load balancing was performed in 74% of knees.
Conclusions
Coronal and sagittal load imbalances existed as determined by the sensor even after the achievement of appropriate conventional gap balance. The additional rebalancing procedure was performed for balanced loads in 74% of the knees after conventional balancing. The use of an intraoperative load sensor offers the advantage of direct evaluation of the load on TKA implants.
Level of evidence
IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conventional gap balancing is performed to establish equal flexion and extension gaps and achieve identical gap width in medial and lateral tibiofemoral compartments after bony resection in total knee arthroplasty (TKA) [23, 27]. This method has been generally accepted as a basic principle for soft tissue balancing in TKA [21, 23], despite some controversy over the permissible range of gap asymmetry [12, 27]. Various techniques for soft tissue procedures have been introduced based on this concept [13]. Instruments such as a spacer block, tensiometers, and navigation systems were developed and shown to provide objective and precise conventional gap balancing [15, 17]. However, soft tissue imbalance still remains a major cause of postoperative dissatisfaction [25]. As such, more surgeons have raised concerns over whether conventional gap balancing is the best way to achieve appropriate balance in TKA [16, 19].
A recently introduced load sensor is an implant-specific device and can be placed in either real or trial implants intraoperatively to allow for soft tissue balancing in conditions similar to those of post-TKA. Many previous studies have reported that TKA cases with appropriate load balancing show more favorable postoperative clinical outcomes [4, 7, 8]. The key goal in the TKA procedure will be the provision of an appropriate and balanced load to TKA implants (especially polyethylene inserts); establishing a rectangular and equal gap during surgery may be an insufficient method to achieve this goal.
Few studies have evaluated intercompartmental load distribution after a conventionally balanced knee in the TKA procedure. Manning et al. [19] reported that soft tissue gap balance using a tensiometer failed to achieve load balance according to sensor evaluation in three of seven TKA procedures. The authors demonstrated that isolated use of a typical tensiometer may fail to reliably equilibrate the load across medial and lateral compartments. However, that study was conducted on cadaveric specimens without degenerative osteoarthritis and deformity and could not represent accurately the clinical situation of TKA for osteoarthritic patients.
The purpose of the present study is to evaluate intercompartmental loads intraoperatively using a sensor placed on trial implants after completing conventional gap balancing, which was the establishment of an equal and rectangular flexion–extension gap, with a tensiometer. It was hypothesized that there would be significant differences between the medial and lateral, or flexion and extension intercompartmental loads even after making of an equal and rectangular flexion–extension gap using the tensiometer.
Materials and methods
Patients
The present study was conducted prospectively. Fifty sensor-assisted TKAs were performed between August and September 2018, with implantation of NexGen cruciate-retaining (CR) prostheses (Zimmer, Warsaw, IN, USA) by a senior surgeon (Fig. 1). The inclusion criteria were primary TKA due to Kellgren–Lawrence grade 4 degenerative osteoarthritis with varus deformities. The varus deformity was defined as the deformity with varus alignment of the femoral and tibial mechanical axes on the orthoroentgenogram [34]. The exclusion criteria were inflammatory arthritis; a history of knee infection, fracture, dislocation, or ligament injury; knee instability or a history of reconstructive ligament surgery or high-tibial osteotomy; severe coronal deformity (> 20°); and/or severe flexion contracture (> 20°). The preoperative demographics and deformities are presented in Table 1.
Methods
All primary CR TKA procedures were performed with a modified measured resection technique with patellar resurfacing. A medial parapatellar approach was used with a midline skin incision. All osteophytes were removed from the femur and tibia. An intramedullary guide was used for distal femoral resection, and the transepicondylar axis was used for femoral component rotation. The size of the femoral component was selected using the anterior-referencing method. An extramedullary guide was used for tibial resection. The tibial slope was usually set to 5°–7° of posterior slope in the sagittal plane. The reference line for tibial rotation was accurately aimed at a line passing through the medial third of the tibial tubercle and the second metatarsal or middle of the talus. The posterior cruciate ligament (PCL) was preserved and protected during tibial resection by vertical sawing in front of the PCL insertion site [35].
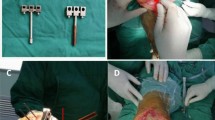
Following resection, soft tissue balancing was performed using a conventional method. The flexion and extension gaps were assessed at 90° and 5°–7° (the degree of extension was influenced by the posterior tibial slope) of knee flexion with a force-controlled tensiometer system (B. Braun Aesculap, Tuttlingenm, Germany) to assess an equal rectangular flexion–extension gap [38]. An absolute distraction force of 120–150 N was applied [14, 38]. The tensiometer consisted of an instrument showing gap size (mm) and a distractor (Fig. 2a). The medialized offset tensiometer enabled gap measurement in patella reduction. The device allowed the surgeon to choose a reproducible amount of tension across each medial and lateral compartment and to measure the gap sizes of each compartment independently. To minimize error from creep elongation of soft tissue, the joint distraction force was loaded more than three times until the joint gap was maintained at a constant level.
Evaluation of balanced intercompartmental gap and load. a Medial offset tensiometer consisting of an instrument showing gap size (mm) and a distractor. b Intraoperative load sensor after trial implantation and patella relocation. c Record of medial and lateral load data at 10°, 45°, and 90° of knee flexion. We choose the load data from a sensor at 10° and 90° of knee flexion to match the gap data collected with a tensiometer. At the bottom right, there is a table capturing the loads of medial and lateral intercompartments at 10°, 45°, and 90° of knee flexion during the collection of load data. The contact point rotation indicated femorotibial contact points and the degree of rotation of the component contact points in the medial and lateral compartments (blue points). Surgeons can assess how well the components are rotated relative to each other
Rectangular gaps were achieved by releasing the contractured soft tissue until the gap difference was less than 1 mm. Equal flexion and extension gaps were established with tibial slope modification and distal femoral additional resection until the difference was less than 1 mm (Table 2) [32].
Next, CR-type trial implants were placed, and an implant-specific load sensor (VERASENSE™; Orthosensor, Dania Beach, FL, USA) was inserted (Fig. 2b) [33]. The precision of the load sensor was validated in a previous study that reported high linear correlation (coefficient of determination = 1; correlation coefficient = between 0.96 and 1.03) between applied and measured forces [5]. A keeled tibial trial device was used to avoid mismatch of the tibiofemoral contact point between trial and real implants. Real-time intraoperative loads were evaluated with the sensor and recorded (Fig. 2c). Medial and lateral loads were measured at 10° and 90° of knee flexion with patella reduction and with one or two towel clips. Load data at 10° of knee flexion were used as the extension load because (1) intercompartmental loads artificially increase when the posterior capsule and screw home mechanism is fully engaged during full- or hyper-extension; these positions do not reflect conditions during normal gait [9]; and (2) 10° of knee flexion was similar with the degree of extension used in conventional gap balancing compared with full- or hyper-extension. Load data at 90° of knee flexion were used as flexion load based on the general concept [6, 9, 25]. The position of knee extension (10° of knee flexion) was achieved with placement of one hand of the surgeon on the heel of the operative leg and the other hand on the posterior aspect of the knee. The flexion position (90° of knee flexion) was achieved in thigh-up position to prevent axial pressure. The flexion angle was determined with a sterilized metal goniometer. For precise evaluation, the load was measured twice at each flexion angle to confirm reproducibility. The device was re-zeroed prior to the second measurement to adjust for plastic deformation, which can affect load measurements. The test–retest differences were less than 3 lb in each compartment, and the intraclass correlation coefficient (ICC) of the test–retest values was greater than 0.8. The values of ICC were 0.87 and 0.83 for medial and lateral intercompartmental loads in knee flexion and 0.89 and 0.82 for medial and lateral intercompartmental loads in knee extension, respectively. The average loading values of the two measurements with one decimal were used for statistical analysis.
Afterload evaluation, additional procedures for final load balancing, including soft tissue release (medial collateral ligament or posterior capsule), modification of tibial cut surface, or distal femoral bone resection, were performed with the sensor. These procedures were performed until loads of the medial and lateral compartments were < 5 lb and 40 lb, respectively, and the differences in load between medial and lateral compartments and between flexion and extension knee positions were < 15 lb [8, 20, 28].
IRB (institutional review board) approval
This study was approved by the IRB of our hospital (Kyung Hee University hospital; KHUH 2018-06-039). Informed consent was obtained from all patients before the study.
Statistical analysis
The loads in the medial and lateral compartments at both knee flexion and extension were normally distributed (Kolmogorov–Smirnov test; n.s., respectively).
Coronal load imbalance was defined as a difference between medial and lateral loads greater than or equal to 15.0 lb (medial load − lateral load ≥ ± 15.0 lb) before additional procedures for final load balancing [8, 20, 28]. Medial-tight coronal load imbalance was defined as a medial load 15.0 lb or above greater than the lateral load (medial load − lateral load ≥ 15.0 lb). Sagittal load imbalance was defined as a difference between extension and flexion loads greater than or equal to 15.0 lb (extension load − flexion load ≥ ± 15.0 lb) before additional procedures for final load balancing [8, 20, 28]. Extension-tight coronal load imbalance was defined as an extension load 15.0 lb or above greater than the flexion load (extension load − flexion load ≥ 15.0 lb).
The medial and lateral compartmental loads were compared at extension and flexion of the knee (paired t test). Separately, the proportions of coronal load imbalance and medial-tight coronal load imbalance were also compared between extension and flexion positions (McNemar test). Additionally, the loads between extension and flexion positions of the knees were compared in each medial and lateral compartment (paired t test), and the amount of load increase during knee extension (extension load − flexion load) was compared between medial and lateral compartments (paired t test). The proportions of sagittal load imbalance and extension-tight sagittal load imbalance were also compared between the medial and lateral compartments (McNemar test).
It was investigated whether demographic factors (age, sex, and body mass index) and preoperative deformity (preoperative mechanical axis and flexion contracture) affected the coronal and sagittal load imbalances (logistic regression analysis).
A priori power analysis of our cohort was performed to determine the sample size affording sufficient power, with the proportions of coronal and sagittal load imbalances as the primary outcome. The analysis was performed to achieve power for detecting significant differences in the proportion of the coronal imbalances between the knee flexion and extension, and the proportion of the sagittal imbalances between the medial and lateral compartments. The alpha and power values were set at 0.05 and 80%, respectively. The results of sample size calculation showed the need for 48 pairs for coronal load imbalance and 23 pairs for sagittal load imbalance. Consequently, our sample size was determined to have appropriate power.
Results
Load distribution evaluations
The average load of the medial compartment was greater than that of the lateral compartment in both extension and flexion of the knee (Fig. 3; both p < 0.001). The proportions of coronal load imbalance and medial-tight coronal load imbalance were higher in extension than in flexion (p = 0.023 and 0.035, respectively) (Table 3) (Fig. 4).
Scatterplot showing coronal load imbalance in flexion and extension of the knee after tensiometer-assisted conventional gap balancing. The formula of the red line: Load in the medial compartment − load in the lateral compartment = 15.0 lb; The formula of the blue line: load in the medial compartment − load in the lateral compartment = − 15.0 lb. The plot below the red line indicates the cases with medial-tight coronal imbalance, while the plot above the blue line indicates the cases with latera-tight coronal imbalance
Load in each the medial and lateral compartment was greater in the extension of the knee than in flexion (Fig. 3; both p < 0.001). The increase in load amount was greater in the medial compartment than in the lateral compartment when the knee was extended compared to flexed (9.7 lb vs. 4.0 lb; p < 0.001). The proportions of sagittal load imbalance and extension-tight sagittal load imbalance were higher in the medial compartment compared with the lateral compartment (p < 0.001, respectively) (Table 4) (Fig. 5).
Scatterplot showing sagittal load imbalance in the medial and lateral compartments of the knee after tensiometer-assisted conventional gap balancing. The formula of the red line: Load in extension − load in flexion = 15.0 lb. The formula of the blue: Load in extension − load in flexion = − 15.0 lb. The plot below the red line indicates the cases with extension-tight sagittal imbalance, while the plot above the blue line indicates the cases with flexion-tight sagittal imbalance
A logistic regression model was not established to identify the factors affecting coronal load imbalance in either extension or flexion of the knee in terms of preoperative demographics or severity of deformities (significance of regression model = n.s., respectively); no model was established to identify the preoperative factors affecting sagittal load imbalance in both the medial and lateral compartments (significance of regression model = n.s., respectively).
Proportion of rebalanced knees with the sensor
The additional rebalancing procedure was performed with the sensor for balanced load distribution in 37 of 50 knees (74%) after conventional gap balancing with the tensiometer.
Discussion
The most important finding of the present study was that there were coronal and sagittal load imbalances in the evaluation using the sensor, even after the achievement of an appropriate gap balance using the tensiometer. The coronal load imbalance, especially regarding the medial-tight load, was more pronounced at knee extension. Separately, sagittal load imbalance, especially the extension-tight load, was more pronounced in the medial compartment. Rebalancing was performed for balanced load distribution in 74% of the knees.
The present study is first to report the load distribution according to a sensor after conventional gap balancing with a tensiometer when TKA is performed for osteoarthritic patients with degenerative arthritis and varus deformity.
Despite technologic and technical advances in TKA, overall patient satisfaction has not improved significantly in the last decade [30]. Many studies have reported a satisfaction rate less than 85% [2, 6]. Other studies have demonstrated soft tissue imbalance as a major cause of dissatisfaction, and subjective assessment of surgeons regarding balancing causes the soft tissue imbalance [6, 25]. Importantly, instruments available for objective conventional balancing, such as a tensiometer or navigation system, have been introduced, and previous studies reported that such instruments provide equal and rectangular extension and flexion gaps [15, 17]. Nevertheless, use of these instruments still did not significantly improve postoperative patient satisfaction [18, 30].
Several previous studies [16, 19] have discussed the limitations of conventional gap balancing techniques. Kinsey and Mahoney [16] reported that posterior tibial translation can cause artifactual widening of the flexion gap, which can be of sufficient magnitude to alter femoral component size selection. Manning et al. [19] suggested that equivalent mediolateral gap size does not necessarily indicate adequate mediolateral load balancing. In addition, establishment of elaborate equal and rectangular gaps with the tensiometer could be easily influenced by rotational changes of the device, due to different elastic constraints of the medial collateral ligament and the posterolateral corner [19].
Additionally, the conventional balancing method cannot reproduce the implanted knee condition [10, 11, 23]. Previous studies have reported that gap size significantly decreased and alignment was changed to the valgus direction during knee extension after femoral component insertion; conversely, gap size slightly increased and alignment changed to the varus direction during knee flexion [10, 11, 23]. The mechanism for the decrease in extension gap has been explained as the pushing of posterior soft tissue by the posterior condyle of the femoral component during extension; separately, the valgus change in alignment during knee extension is suggested to be due to a 3° externally rotated femoral component pushing the posterolateral structure more than the posteromedial one [10, 23]. The reason for the alteration in the flexion gap after femoral component placement has been not explained precisely in previous studies. It has just been reported that the anterior structure was more elastic than the posterior structure and seemed to not be significantly affected by the push of the femoral component [10, 23].
It is noteworthy that load distribution has not been properly explained by the previously described mechanisms for gap change after femoral component placement [10, 22, 23]. According to previous studies: (1) the average lateral load should have been greater than the medial load during knee extension; (2) the proportion of medial-tight coronal load imbalance should have been higher during knee flexion versus extension; (3) when the knee is extended from flexion, the increased load amount should have been greater in the lateral compartment; and (4) the proportion of sagittal load imbalance, especially extension-tight, should have been higher in the lateral compartment. However, as described previously, the load distribution in our study was different than those outlined in the previous studies.
Nagai et al. [24] reported that the medial structure was always stiffer than the lateral structure at all flexion angles from 0° to 135°. Considering Young’s modulus, a stiffer medial structure will result in more loading to the medial compartment when the polyethylene insert is placed in the conventionally balanced rectangular gap. In addition, even though the posteromedial structure is less affected by the femoral component in the extension position, a stiff medial structure can become even tenser than the lateral structure. This hypothesis could explain the greater average medial load and the higher proportion of medial-tight coronal load imbalance during knee extension; furthermore, it could also explain the greater amount of load increase and higher proportion of sagittal load imbalance, especially extension-tight, of the medial compartment after femoral component placement.
It would be better to consider the possible mismatch between the appropriate gap achieved with the tensiometer and the balanced load obtained with the sensor, especially, in male patients with advanced varus deformity whose stiffness of the medial structure is thought to be apparent [1, 29]. However, in the present study, we could not establish a regression model explaining the preoperative factors causing coronal or sagittal load imbalance. Although this could indicate that preoperative demographics or deformity might not affect the load distribution after conventional gap balancing, we thought that the relatively small sample size could be one of the important reasons for non-establishment of a model. Previous studies have reported that the extension gap was significantly affected by the femoral condyle when an advanced varus deformity was present [22, 36].
Even though there is a concern in terms of cost–benefit [33], it seems certain that the use of an intraoperative load sensor is beneficial for elaborate soft tissue balancing. Creation of a rectangular gap in the resected bony surfaces could not guarantee appropriate load balancing at the time of implantation in the present study. Use of an intraoperative sensor can overcome the limitations of conventional gap balancing and provide adequate load to the polyethylene insert.
Additionally, we think that patient-specific implants can decrease the risk of load imbalance after the achievement of appropriate conventional gap balance. Because the patient-specific implant allowed various sizes, shapes, and geometries of the prosthesis to match each individual patient’s anatomy, it may help to achieve optimal soft tissue balancing for each individual patient [31].
The present study has several limitations that should be noted. First, we did not investigate the “component gap” by evaluating gap size with the femoral component placed [23]. This may limit the demonstration of the limitation of gap balancing concept. Future studies are required to evaluate the load change in patients with a balanced “component gap” rather than with a balanced “surface gap”. Second, our study evaluated intercompartmental loads in placement of trial implants. However, the load distribution between knees with trial and final cemented implants might depend on cement thickness. Nodzo et al. [25] reported linear correlation of the load with trial and final cemented implants in the medial compartment, but no correlation in the lateral compartment. It would be interesting to investigate the load distribution with trial and final cemented implants after conventional gap balancing. Such a study may provide a method to prevent load changes due to cement thickness. Third, the present study cannot suggest whether gap or load balancing is clinically better. It will be necessary to compare clinical results between patients undergoing TKA procedures balanced with the two instruments. Fourth, the study sample was small. This could be a reason why we could not establish a regression model to find the factors affecting load imbalance. A larger cohort study will be required for further sophisticated evaluation to determine the affecting factors. Fifth, the present study was conducted involving CR TKA. The PCL contributes medial compartmental stabilization of the knee as a lateral ligament of the medial compartment [3, 26], and the anterior cruciate ligament (ACL) has an opposing function [37]. Removing the ACL with preservation of the PCL may cause load imbalance with higher loads in the medial compartment [37]. For this reason, there could be different load distribution in posterior stabilized TKA. Last, most procedures were performed in Asian female patients with varus deformity. This should be considered when extrapolating our findings to cases with valgus deformities and other populations.
Conclusion
Coronal and sagittal load imbalances existed as determined by the sensor even after the achievement of appropriate conventional gap balance. The additional rebalancing procedure was performed for balanced loads in 74% of the knees after conventional balancing. Use of an intraoperative load sensor offers the advantage of direct evaluation of the load on TKA implants.
References
Boguszewski DV, Cheung EC, Joshi NB, Markolf KL, McAllister DR (2015) Male-female differences in knee laxity and stiffness: a cadaveric study. Am J Sports Med 43(12):2982–2987
Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD (2010) Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res 468(1):57–63
Brown TE (2009) Arthritis & arthroplasty. The knee. Saunders/Elsevier, Philadelphia, p xvii
Chow JC, Breslauer L (2017) The use of intraoperative sensors significantly increases the patient-reported rate of improvement in primary total knee arthroplasty. Orthopedics 40(4):e648–e651
Crottet D, Kowal J, Sarfert SA, Maeder T, Bleuler H, Nolte LP et al (2007) Ligament balancing in TKA: evaluation of a force-sensing device and the influence of patellar eversion and ligament release. J Biomech 40(8):1709–1715
Elmallah RK, Mistry JB, Cherian JJ, Chughtai M, Bhave A, Roche MW et al (2016) Can we really “feel” a balanced total knee arthroplasty? J Arthroplasty 31(9 Suppl):102–105
Geller JA, Lakra A, Murtaugh T (2017) The use of electronic sensor device to augment ligament balancing leads to a lower rate of arthrofibrosis after total knee arthroplasty. J Arthroplasty 32(5):1502–1504
Gustke KA, Golladay GJ, Roche MW, Elson LC, Anderson CR (2014) A new method for defining balance: promising short-term clinical outcomes of sensor-guided TKA. J Arthroplasty 29(5):955–960
Gustke KA, Golladay GJ, Roche MW, Elson LC, Anderson CR (2017) A targeted approach to ligament balancing using kinetic sensors. J Arthroplasty 32(7):2127–2132
Hananouchi T, Yamamoto K, Ando W, Fudo K, Ohzono K (2012) The intraoperative gap difference (flexion gap minus extension gap) is altered by insertion of the trial femoral component. Knee 19(5):601–605
Hayashi S, Murakami Y, Inoue H, Nobutou H, Nishida K, Mochizuki Y (2014) Gap measurement in posterior-stabilized total knee arthroplasty with or without a trial femoral component. Arch Orthop Trauma Surg 134(6):861–865
Higuchi H, Hatayama K, Shimizu M, Kobayashi A, Kobayashi T, Takagishi K (2009) Relationship between joint gap difference and range of motion in total knee arthroplasty: a prospective randomised study between different platforms. Int Orthop 33(4):997–1000
Hunt NC, Ghosh KM, Athwal KK, Longstaff LM, Amis AA, Deehan DJ (2014) Lack of evidence to support present medial release methods in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 22(12):3100–3112
Jim S (2014) New tensor/sensor technologies. Knee 21(6):985–986
Joseph J, Simpson PM, Whitehouse SL, English HW, Donnelly WJ (2013) The use of navigation to achieve soft tissue balance in total knee arthroplasty—a randomised clinical study. Knee 20(6):401–406
Kinsey TL, Mahoney OM (2018) Balanced flexion and extension gaps are not always of equal size. J Arthroplasty 33(4):1062–1068 (e1065)
Kwak DS, Kong CG, Han SH, Kim DH, In Y (2012) Development of a pneumatic tensioning device for gap measurement during total knee arthroplasty. Clin Orthop Surg 4(3):188–192
Lee DH, Park JH, Song DI, Padhy D, Jeong WK, Han SB (2010) Accuracy of soft tissue balancing in TKA: comparison between navigation-assisted gap balancing and conventional measured resection. Knee Surg Sports Traumatol Arthrosc 18(3):381–387
Manning WA, Blain A, Longstaff L, Deehan DJ (2018) A load-measuring device can achieve fine-tuning of mediolateral load at knee arthroplasty but may lead to a more lax knee state. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-018-5164-3
Meneghini RM, Ziemba-Davis MM, Lovro LR, Ireland PH, Damer BM (2016) Can intraoperative sensors determine the "target" ligament balance? Early outcomes in total knee arthroplasty. J Arthroplasty 31(10):2181–2187
Mihalko WM, Saleh KJ, Krackow KA, Whiteside LA (2009) Soft-tissue balancing during total knee arthroplasty in the varus knee. J Am Acad Orthop Surg 17(12):766–774
Minoda Y, Sakawa A, Aihara M, Tada K, Kadoya Y, Kobayashi A (2007) Flexion gap preparation opens the extension gap in posterior cruciate ligament-retaining TKA. Knee Surg Sports Traumatol Arthrosc 15(11):1321–1325
Muratsu H, Matsumoto T, Kubo S, Maruo A, Miya H, Kurosaka M et al (2010) Femoral component placement changes soft tissue balance in posterior-stabilized total knee arthroplasty. Clin Biomech (Bristol, Avon) 25(9):926–930
Nagai K, Muratsu H, Matsumoto T, Miya H, Kuroda R, Kurosaka M (2014) Soft tissue balance changes depending on joint distraction force in total knee arthroplasty. J Arthroplasty 29(3):520–524
Nodzo SR, Franceschini V, Gonzalez Della Valle A (2017) Intraoperative load-sensing variability during cemented, posterior-stabilized total knee arthroplasty. J Arthroplasty 32(1):66–70
Park SJ, Seon JK, Park JK, Song EK (2009) Effect of PCL on flexion-extension gaps and femoral component decision in TKA. Orthopedics 32(10 Suppl):22–25
Risitano S, Indelli PF (2017) Is "symmetric" gap balancing still the gold standard in primary total knee arthroplasty? Ann Transl Med 5(16):325
Risitano S, Karamian B, Indelli PF (2017) Intraoperative load-sensing drives the level of constraint in primary total knee arthroplasty: surgical technique and review of the literature. J Clin Orthop Trauma 8(3):265–269
Rossi R, Cottino U, Bruzzone M, Dettoni F, Bonasia DE, Rosso F (2019) Total knee arthroplasty in the varus knee: tips and tricks. Int Orthop 43(1):151–158
Scott WN, Diduch DR, Long WJ (2018) Insall & Scott surgery of the knee, vol 2, 6 edn. Elsevier, Philadelphia, pp 1766–1769
Scott WN, Diduch DR, Long WJ (2018) Insall & Scott surgery of the knee, vol 2, 6 edn. Elsevier, Philadelphia, pp 1520–1525
Seo SS, Kim CW, Seo JH, Kim DH, Kim OG, Lee CR (2017) Effects of resection of posterior condyles of femur on extension gap of knee joint in total knee arthroplasty. J Arthroplasty 32(6):1819–1823
Song SJ, Kang SG, Lee YJ, Kim KI, Park CH (2018) An intraoperative load sensor did not improve the early postoperative results of posterior-stabilized TKA for osteoarthritis with varus deformities. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-018-5314-7
Song SJ, Kang SG, Park CH, Bae DK (2018) Comparison of clinical results and risk of patellar injury between attune and PFC sigma knee systems. Knee Surg Relat Res 30(4):334–340
Song SJ, Park CH, Bae DK (2019) What to know for selecting cruciate-retaining or posterior-stabilized total knee arthroplasty. Clin Orthop Surg 11(2):142–150
Sugama R, Kadoya Y, Kobayashi A, Takaoka K (2005) Preparation of the flexion gap affects the extension gap in total knee arthroplasty. J Arthroplasty 20(5):602–607
Tanaka K, Muratsu H, Mizuno K, Kuroda R, Yoshiya S, Kurosaka M (2007) Soft tissue balance measurement in anterior cruciate ligament-resected knee joint: cadaveric study as a model for cruciate-retaining total knee arthroplasty. J Orthop Sci 12(2):149–153
Yoon JR, Oh KJ, Wang JH, Yang JH (2015) Does patella position influence ligament balancing in total knee arthroplasty? Knee Surg Sports Traumatol Arthrosc 23(7):2012–2018
Acknowledgements
Zimmer Biomet Company provided Verasense sensors free of charge.
Funding
No extenral funding was used.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board of our hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, S.J., Lee, H.W., Kim, K.I. et al. Load imbalances existed as determined by a sensor after conventional gap balancing with a tensiometer in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 28, 2953–2961 (2020). https://doi.org/10.1007/s00167-019-05699-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-019-05699-6