Abstract
Purpose
The aim of this retrospective study was to evaluate the clinical and radiological results of a nano-composite multi-layered three-dimensional biomaterial scaffold for treatment of osteochondral lesions (OCL) of the knee. It was a particular radiological interest to analyse the osseointegration, filling of the defects and the bone tracer uptake (BTU), and it was hypothesised that this scaffold, which was created to mimic the entire osteo-cartilaginous unit, is integrated within the bone 12 months postoperatively and comes along with improved patients symptoms and function.
Methods
Fourteen patients (male:female = 11:3, mean age ± SD 33.1 ± 10.7 years) treated for OCL (size 1.0–3.5 cm2) were clinically and radiologically evaluated at 1 year postoperatively. The data were prospectively collected including SPECT/CT, Tegner and Lysholm scores. BTU was anatomically localised and volumetrically quantified in SPECT/CT. Defect filling was analysed in CT. Spearman’s rho and Wilcoxon test were used for correlation of BTU in SPECT/CT and clinical scores (p < 0.05).
Results
A significant improvement in Lysholm knee score (p < 0.001) and slight deterioration in Tegner score were found (p < 0.01). A complete filling of the defect was shown in 14%, a partial filling in 14% and only minor filling was seen in 72%. A significant correlation (p < 0.001) was found between location of osteochondral lesions and increased BTU. At the lesion sites pre- and postoperative BTU was markedly increased and did not show any decrease at 12-month follow-up. Median Tegner and mean Lysholm scores did not correlate with BTU at any time.
Conclusions
Treatment of OCL in the knee joint with a nano-composite multi-layered three-dimensional biomaterial scaffold resulted in a significant clinical improvement at 1-year follow-up. However, osseointegration was still ongoing at 12-month follow-up.
Level of evidence
Case series, Level IV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various surgical treatment options such as Pridie drilling, open or arthroscopic fixation using screws or pins, excision of the osteochondral fragment, microfracturing, osteochondral grafting (with autograft or allograft), and autologous chondrocyte implantation (ACI) as well as tissue-engineered construct (TEC) derived from synovial mesenchymal stem cells (MSCs) and hydroxyapatite artificial bone have been proposed as treatment for osteochondral lesions (OCL). However, to date there is a common gold standard yet to be established [1, 2, 4, 10, 11, 17, 35, 36, 52,53,54, 57, 60].
Pure fragment excision leaves a clinically relevant defect. Pridie drilling or microfracturing also leaves the defect mostly unfilled, and clinical results are not promising for OCL [5, 44, 59]. The problem of ACI is the fact that it needs to be done in a sandwich technique and also requires a two-stage setting. To date, there is insufficient evidence for the superiority of ACI to other treatment strategies for treating OCL in the knee [5, 7]. Allografts have a low availability in many European countries, whereas in the USA fresh allograft tissue is commonly available [22]. Autografts come along with a considerable harvesting morbidity [48].
For these reasons, biomimetic scaffolds were introduced for treatment of OCL. The theoretical advantage is that these scaffolds are commonly available off the shelf and can be implanted in a one-stage surgery. The scaffold used in the present study is a cell-free three-layered collagen–hydroxyapatite biomimetic scaffold. Excellent short-to-midterm results were reported [20, 39, 51, 58].
Few authors have also evaluated the osteochondral repair potential of this biomimetic scaffold using MRI [8, 13, 16, 21, 39, 40]. Some authors found no improvement at any time point [13], others found improved MOCART values over time [8, 21, 39, 40].
In the last decades, single photon emission computerised tomography/computerised tomography (SPECT/CT), which as a three-dimensional hybrid imaging modality consists of a 3D bone scan and a conventional CT, has been increasingly used in patients with painful knees [25,26,27,28, 30,31,32,33,34, 49, 55, 56]. In particular, its ability to visualise the in vivo bone loading and to correlate such findings to biomechanical considerations has been highlighted [25, 32, 33, 47, 49, 50]. In contrast to MRI, SPECT/CT is able to provide information on alignment, structural pathologies, osseous physiology and metabolism together [12, 30]. In recent studies of Konala et al. [42] and Hirschmann et al. [27], the diagnostic value of SPECT/CT in patients who underwent treatment for OCL was highlighted. The scaffold used consists of hydroxyl-apatite and collagen, and osseointegration of this scaffold can be monitored by SPECT/CT as the tracer is targeted towards hydroxyl-apatite produced by active osteoblasts.
A combined evaluation of osseointegration and clinical results of cell-free multi-layered nano-composite scaffold for treatment of osteochondral lesions of the knee using SPECT/CT is yet lacking in the current literature.
The aim of this retrospective study was to evaluate the clinical and radiological results of a nano-composite multi-layered three-dimensional biomaterial scaffold for treatment of osteochondral lesions (OCL) of the knee. It was a particular radiological interest to analyse the osseointegration, filling of the defects and the bone tracer uptake (BTU), and it was hypothesised that this scaffold, which was created to mimic the entire osteo-cartilaginous unit, is integrated within the bone at 12 months postoperatively and comes along with improved patients symptoms and function.
Materials and methods
Fourteen consecutive patients (mean age ± standard deviation 33 ± 10 years, range 18–47 years, right:left = 6:8, 11:3 = male:female) with knee pain due to OCL and with International Cartilage Repair Society (ICRS) grade 3 to 4 chondral and osteochondral lesions of the knee, as evaluated by MRI and confirmed intraoperatively, were included. All patients presented to a specialised knee clinic from 2010 to 2012. Osteoarthritis was graded preoperatively according to Kellgren–Lawrence, and patients with Kellgren–Lawrence >2 were excluded.
The defect size of the treated lesions determined during surgery was 1.0–3.5 cm2. OCLs involved the medial femoral condyle in eight patients and in each two patients the trochlea, the lateral femoral condyle and patellar facet.
SPECT/CT was used to evaluate fourteen patients after and ten patients before scaffold implantation. Restoration of the subchondral bone plate was described as no (<25%), partial (25–75%) and complete (75–100%). At 1-year SPECT/CT evaluation, a complete filling of the defect was shown in 14%, a partial filling in 14% and only minor filling was seen in 72%.
Four patients received an isolated filling of the osteochondral defect using a cell-free three-layered biomimetic scaffold, while ten patients had undergone the following associated treatments: Six patients underwent high tibial valgus osteotomy (one patient additional medial meniscal repair), one patient isolated medial meniscal repair, one patient tuberosity osteotomy, one patient autologous chondrocyte implantation and one patient ACL reconstruction and lateral meniscal repair.
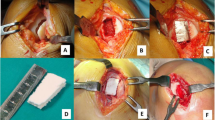
In all patients, a cell-free three-layered biomimetic scaffold (MaioRegen Finceramica, Faenza S.p.A., Italy) was used for reconstruction of the osteochondral defect. The scaffold consists of a porous nano-structured three-layered biomimetic matrix designed to mimic the physiological osteochondral tissue. Being resorbable, it is thought to facilitate the regenerative process by being gradually replaced by native tissue [39].
After an arthroscopic evaluation of the knee, a medial or lateral parapatellar arthrotomy was performed to expose the OCL. The sclerotic subchondral bone was partially removed using an osteotome, and the host bone was prepared to a depth of 8 mm. The scaffold was cut and sized and then implanted press fit into the defect.
After surgery, all patients underwent a standardised treatment protocol following the implant producing company’s guidelines: Early mobilisation using continuous passive motion and weight bearing was limited to maximum 15 km for 6 weeks. At this time, active functional training was started allowing full weight bearing.
Clinical outcome measurements were performed preoperatively and at 12 months after the surgery using the Tegner score and Lysholm knee score. In all cases, SPECT/CT was performed 12 months pre- and postoperatively. SPECT/CTs were prospectively collected and retrospectively analysed using a standardised protocol. All patients received a 500–700-MBq Technetium 99 m hydroxymethylene diphosphonate (HDP) injection (Malinckrodt, Wallerau, Switzerland). SPECT/CT was performed using a Symbia T16 (Siemens, Erlangen, Germany), which consists of a pair of low-energy, high-resolution collimators, a dual-head gamma camera and an integrated 16-slice CT scanner (collimation of 16 × 0.75 mm). A standardised imaging protocol was used which was based on the Imperial Knee Protocol [23]. CT slice thickness was 0.75 mm. SPECT was performed in the delayed phase three to four hours after bone tracer injection (matrix size, 128 × 128; angle step, 32; and time per frame, 25 s). BTU on SPECT/CT was analysed using a specialised software allowing 3D volumetric quantitative analysis of SPECT data [34]. A recently validated localisation scheme for anatomical localisation of the SPECT/CT tracer activity was used (Fig. 1) [50]. Maximum intensity values of BTU in each area of interest were volumetrically recorded. In a previous study, the localisation of BTU in an anatomical scheme and grading of BTU showed excellent intra- and inter-observer reliability [50].
Osseointegration of the scaffold into host bone and defect filling was evaluated at 1 year postoperatively in SPECT/CT by a musculoskeletal radiologist.
All patients gave their informed consent, and the study was approved by the local ethical committee (EK 2015-396).
Statistical analysis
Data were analysed using IBM SPSS Statistics for Windows 22.0 (Armonk, NY: IBM Corp.). Wilcoxon signed-rank test was used as a non-parametric paired difference test for analysis of SPECT/CT bone tracer uptake and clinical outcome measures. To investigate relationships between two variables, Spearman’s rank correlation analysis was performed. The threshold of significance was set at a p value less than 0.05.
A post hoc power analysis showed the number of 14 patients to be sufficient to detect a moderate one-sided significant Spearman’s correlation of rho ≥0.45 between BTU and clinical findings.
Results
A statistically significant improvement in Lysholm knee score was found between preoperative and 1-year follow-up. The mean Lysholm score was 65.6 ± 12.6 before surgery and 90.1 ± 10.0 after 1 year (p < 0.001) (Fig. 2). The median Tegner score was 6.0 (range 3–9) before surgery and 4.5 (range 2–9) after 1 year (p < 0.01) (Fig. 3).
A high correlation (p < 0.001) was found between the location of OCL and increased BTU. Compared to the non-affected anatomical regions of the knee joint, the OCL showed significantly increased BTU pre- and postoperatively (Fig. 4). Comparing BTU values of the OCL pre- and postoperatively, there was no difference (mean 6.3 ± 3.9, respectively, 6.0 ± 4.4) (Figs. 5, 6).
Higher BTU values did not correlate with lower Tegner or Lysholm scores, neither pre- nor postoperatively.
Discussion
The most important findings of the present study were the following: The use of the biomimetic scaffold for treatment of OCL led to good clinical functional outcomes with regard to the Lysholm score, which is in agreement with previous studies [8, 13, 37, 39, 41]. In contrast, the sport and daily activity represented by the Tegner score slightly decreased, which might be due to two reasons. One is the fact that the follow-up time was rather short and it could be expected that there is still an increase in activity level over time. The second reason is that subchondral bone restoration is not entirely completed at this time [16, 21, 40]. This hypothesis is confirmed by the BTU findings and incomplete defect filling in SPECT/CT.
Increased BTU in and around the OCL site entails ongoing healing of the OCL through subchondral bone formation and remodelling. The bone tracer used is directed towards hydroxyl-apatite produced by active osteoblasts, representing increased loading and remodelling of the subchondral bone [14, 19, 27]. Histology of the subchondral bone in areas with increased BTU shows increased vascularity and new bone formation [6]. This is in line with an experimental study, which showed how the scaffold used is able to integrate within the bone and surrounding tissues. The authors further reported the regeneration of the entire damaged osteochondral unit including formation of bone and hyaline-like cartilaginous tissue [38, 58].
In most of the previous studies, MRI was used for morphological evaluation based on the MOCART scoring system [45]. To our knowledge, only few authors have compared MRI and SPECT/CT in patients after knee surgery [9, 24, 42, 43, 46]. It is a pertinent question what the pathophysiology behind BTU in SPECT/CT and bone oedema in MRI is and whether these represent similar or different windows into the underlying pathology. In a study comparing BTU in bone scans and bone oedema in MRI, Buck et al. [9] found that there is a correlation of both, but there are also patients with increased BTU and normal bone marrow signal. The study was done in patients complaining about chronic medial knee pain, which is a comparable patient sample as in the present study. BTU might be increased due to increased loading or due to a cartilage lesion. In traumatic cartilage lesions, MRI shows huge areas of bone oedema in the underlying subchondral bone. However, in chronic conditions the findings are not that consistent, which has been shown by Buck et al. SPECT/CT has a more important role in chronic knee joint conditions.
A study of Dordevic et al. [18] showed a significant correlation of BTU in SPECT/CT with the degree and size of chondral lesions on MR images. Based on those findings, the correlation of increased BTU with OCL in this study may in connection with the incomplete reconstruction of the subchondral bone indicate the ongoing osseointegration 1 year postoperatively.
Whereas others found a complete filling of the osteochondral defect and complete osseointegration of the scaffold [8, 21, 39, 40], in the present study only a minority of patients (14%) showed a complete filling of the OCL defects or restoration of the subchondral bone plate at 12-month follow-up. Similar findings have been reported by Christensen et al., who have investigated the defect filling in OCL patients treated with the same scaffold using CT at 1 and 2.5 years postoperatively [13]. It appears that with the use of the scaffold used only minor defect filling can be achieved at 12-month follow-up. Clearly, the osseointegration process is not completed, and more time is needed for full osseointegration.
No direct correlation between BTU in SPECT/CT and outcome scores was found, which might be due to the small sample size. In addition, previous studies were not able to establish a clear correlation between clinical and radiological outcomes [15, 16, 21, 39, 40]. However, controversial results have been published in recent studies analysing the clinical value of SPECT/CT in patients with knee pain [3, 28, 29, 32]. It is currently still unclear whether BTU shows a significant correlation with clinical outcome parameters in patients with OCL. In our opinion, the persistent increased BTU represents an ongoing remodelling process.
The study bears a considerable number of limitations to be acknowledged. Firstly, the sample size was rather small. However, it represents the largest sample ever analysed in vivo osseointegration of the scaffold used. Secondly, the follow-up period is rather short, but this was done to minimise the radiation burden of SPECT/CT in these young patients. Thirdly, there was no control group, patients were variable in terms of OCL locations (femoral condyle, trochlea and patella) and need for additional surgery, which may be a confounding factor.
On the basis of this study, the scaffold used is a valuable single-stage treatment option for patients with OCL, although no entire defect filling and osseointegration were observed. In future, augmentation of cells and growth factors might improve osseointegration.
Conclusion
This study is the first combined evaluation of osseointegration and clinical results of cell-free multi-layered nano-composite scaffold for treatment of osteochondral lesions of the knee. Treatment of OCL in the knee joint with the scaffold used resulted in a significant clinical improvement at 1-year follow-up. However, an ongoing defect filling was found at 12-month follow-up.
References
Aglietti P, Buzzi R, Bassi PB, Fioriti M (1994) Arthroscopic drilling in juvenile osteochondritis dissecans of the medial femoral condyle. Arthroscopy 10:286–291
Aichroth P (1971) Osteochondritis dissecans of the knee. A clinical survey. J Bone Joint Surg Br 53:440–447
Al-Nabhani K, Michopoulou S, Allie R, Alkalbani J, Saad Z, Sajjan R, Syed R, Bomanji J (2014) Painful knee prosthesis: can we help with bone SPECT/CT? Nucl Med Commun 35:182–188
Anderson AF, Pagnani MJ (1997) Osteochondritis dissecans of the femoral condyles. Long-term results of excision of the fragment. Am J Sports Med 25:830–834
Bentley G, Bhamra JS, Gikas PD, Skinner JA, Carrington R, Briggs TW (2013) Repair of osteochondral defects in joints—how to achieve success. Injury 44(Suppl 1):S3–S10
Boegard T, Rudling O, Dahlstrom J, Dirksen H, Petersson IF, Jonsson K (1999) Bone scintigraphy in chronic knee pain: comparison with magnetic resonance imaging. Ann Rheum Dis 58(1):20–26
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Brix M, Kaipel M, Kellner R, Schreiner M, Apprich S, Boszotta H, Windhager R, Domayer S, Trattnig S (2016) Successful osteoconduction but limited cartilage tissue quality following osteochondral repair by a cell-free multilayered nano-composite scaffold at the knee. Int Orthop 40:625–632
Buck FM, Hoffmann A, Hofer B, Pfirrmann CW, Allgayer B (2009) Chronic medial knee pain without history of prior trauma: correlation of pain at rest and during exercise using bone scintigraphy and MR imaging. Skelet Radiol 38:339–347
Cahill B (1985) Treatment of juvenile osteochondritis dissecans and osteochondritis dissecans of the knee. Clin Sports Med 4(2):367–384
Cain EL, Clancy WG (2001) Treatment algorithm for osteochondral injuries of the knee. Clin Sports Med 20:321–342
Chopra A (2007) 99mTc-methyl diphosphonate. In: Molecular imaging and contrast agent database (MICAD). National Center for Biotechnology Information (US) 2004–2013, Bethesda, MD
Christensen BB, Foldager CB, Jensen J, Jensen NC, Lind M (2016) Poor osteochondral repair by a biomimetic collagen scaffold: 1- to 3-year clinical and radiological follow-up. Knee Surg Sports Traumatol Arthrosc 24:2380–2387
Cook GJ, Ryan PJ, Clarke SE, Fogelman I (1996) SPECT bone scintigraphy of anterior cruciate ligament injury. J Nucl Med 37:1353–1356
de Windt TS, Welsch GH, Brittberg M, Vonk LA, Marlovits S, Trattnig S, Saris DB (2013) Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am J Sports Med 41:1695–1702
Delcogliano M, de Caro F, Scaravella E, Ziveri G, De Biase CF, Marotta D, Marenghi P, Delcogliano A (2014) Use of innovative biomimetic scaffold in the treatment for large osteochondral lesions of the knee. Knee Surg Sports Traumatol Arthrosc 22:1260–1269
Donaldson LD, Wojtys EM (2008) Extraarticular drilling for stable osteochondritis dissecans in the skeletally immature knee. J Pediatr Orthop 28:831–835
Dordevic M, Hirschmann MT, Rechsteiner J, Falkowski A, Testa E, Hirschmann A (2016) Do chondral lesions of the knee correlate with bone tracer uptake by using SPECT/CT? Radiology 278:223–231
Dye SF, Chew MH (1994) The use of scintigraphy to detect increased osseous metabolic activity about the knee. Instr Course Lect 43:453–469
Emmerson BC, Gortz S, Jamali AA, Chung C, Amiel D, Bugbee WD (2007) Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med 35:907–914
Filardo G, Kon E, Di Martino A, Busacca M, Altadonna G, Marcacci M (2013) Treatment of knee osteochondritis dissecans with a cell-free biomimetic osteochondral scaffold: clinical and imaging evaluation at 2-year follow-up. Am J Sports Med 41:1786–1793
Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, Kon E (2010) The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc 18:434–447
Henckel JRR, Lozhkin K, Harris S, Baena FM, Barrett AR, Cobb JP (2006) Very low-dose computed tomography for planning and outcome measurement in knee replacement. The imperial knee protocol. J Bone Joint Surg Br 88:1513–1518
Hirschmann A, Hirschmann MT (2016) Chronic knee pain: clinical value of MRI versus SPECT/CT. Semin Musculoskelet Radiol 20:3–11
Hirschmann MT, Adler T, Rasch H, Hugli RW, Friederich NF, Arnold MP (2010) Painful knee joint after ACL reconstruction using biodegradable interference screws—SPECT/CT a valuable diagnostic tool? A case report. Sports Med Arthrosc Rehabil Ther Technol 2:24
Hirschmann MT, Davda K, Iranpour F, Rasch H, Friederich NF (2011) Combined single photon emission computerised tomography and conventional computerised tomography (SPECT/CT) in patellofemoral disorders: a clinical review. Int Orthop 35:675–680
Hirschmann MT, Davda K, Rasch H, Arnold MP, Friederich NF (2011) Clinical value of combined single photon emission computerized tomography and conventional computer tomography (SPECT/CT) in sports medicine. Sports Med Arthrosc 19:174–181
Hirschmann MT, Henckel J, Rasch H (2013) SPECT/CT in patients with painful knee arthroplasty—what is the evidence? Skelet Radiol 42:1201–1207
Hirschmann MT, Iranpour F, Davda K, Rasch H, Hugli R, Friederich NF (2010) Combined single-photon emission computerized tomography and conventional computerized tomography (SPECT/CT): clinical value for the knee surgeons? Knee Surg Sports Traumatol Arthrosc 18:341–345
Hirschmann MT, Konala P, Iranpour F, Kerner A, Rasch H, Friederich NF (2011) Clinical value of SPECT/CT for evaluation of patients with painful knees after total knee arthroplasty—a new dimension of diagnostics? BMC Musculoskelet Disord 12:36
Hirschmann MT, Mathis D, Afifi FK, Rasch H, Henckel J, Amsler F, Wagner CR, Friederich NF, Arnold MP (2013) Single photon emission computerized tomography and conventional computerized tomography (SPECT/CT) for evaluation of patients after anterior cruciate ligament reconstruction: a novel standardized algorithm combining mechanical and metabolic information. Knee Surg Sports Traumatol Arthrosc 21:965–974
Hirschmann MT, Mathis D, Rasch H, Amsler F, Friederich NF, Arnold MP (2013) SPECT/CT tracer uptake is influenced by tunnel orientation and position of the femoral and tibial ACL graft insertion site. Int Orthop 37:301–309
Hirschmann MT, Schon S, Afifi FK, Amsler F, Rasch H, Friederich NF, Arnold MP (2013) Assessment of loading history of compartments in the knee using bone SPECT/CT: a study combining alignment and 99mTc-HDP tracer uptake/distribution patterns. J Orthop Res 31:268–274
Hirschmann MT, Wagner CR, Rasch H, Henckel J (2012) Standardized volumetric 3D-analysis of SPECT/CT imaging in orthopaedics: overcoming the limitations of qualitative 2D analysis. BMC Med Imaging 12:5
Hughston JC, Hergenroeder PT, Courtenay BG (1984) Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg Am 66:1340–1348
Kivisto R, Pasanen L, Leppilahti J, Jalovaara P (2002) Arthroscopic repair of osteochondritis dissecans of the femoral condyles with metal staple fixation: a report of 28 cases. Knee Surg Sports Traumatol Arthrosc 10:305–309
Kon E, Delcogliano M, Filardo G, Altadonna G, Marcacci M (2009) Novel nano-composite multi-layered biomaterial for the treatment of multifocal degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc 17:1312–1315
Kon E, Delcogliano M, Filardo G, Fini M, Giavaresi G, Francioli S, Martin I, Pressato D, Arcangeli E, Quarto R, Sandri M, Marcacci M (2010) Orderly osteochondral regeneration in a sheep model using a novel nano-composite multilayered biomaterial. J Orthop Res 28:116–124
Kon E, Delcogliano M, Filardo G, Pressato D, Busacca M, Grigolo B, Desando G, Marcacci M (2010) A novel nano-composite multi-layered biomaterial for treatment of osteochondral lesions: technique note and an early stability pilot clinical trial. Injury 41:693–701
Kon E, Filardo G, Di Martino A, Busacca M, Moio A, Perdisa F, Marcacci M (2014) Clinical results and MRI evolution of a nano-composite multilayered biomaterial for osteochondral regeneration at 5 years. Am J Sports Med 42:158–165
Kon E, Vannini F, Buda R, Filardo G, Cavallo M, Ruffilli A, Nanni M, Di Martino A, Marcacci M, Giannini S (2012) How to treat osteochondritis dissecans of the knee: surgical techniques and new trends: AAOS exhibit selection. J Bone Joint Surg Am 94:e1–e8
Konala P, Iranpour F, Kerner A, Rasch H, Friederich NF, Hirschmann MT (2010) Clinical benefit of SPECT/CT for follow-up of surgical treatment of osteochondritis dissecans. Ann Nucl Med 24:621–624
Maas O, Joseph GB, Sommer G, Wild D, Kretzschmar M (2015) Association between cartilage degeneration and subchondral bone remodeling in patients with knee osteoarthritis comparing MRI and (99 m)Tc-DPD-SPECT/CT. Osteoarthr Cartil 23(10):1713–1720
Magnussen RA, Dunn WR, Carey JL, Spindler KP (2008) Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res 466:952–962
Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, Trattnig S (2004) Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol 52:310–319
Mathis DT, Hirschmann A, Falkowski AL, Kiekara T, Amsler F, Rasch H, Hirschmann MT (2017) Increased bone tracer uptake in symptomatic patients with ACL graft insufficiency: a correlation of MRI and SPECT/CT findings. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-017-4588-5
Mathis DT, Rasch H, Hirschmann MT (2015) In vivo bone tunnel remodeling in symptomatic patients after ACL reconstruction: a retrospective comparison of articular and extra-articular fixation. Muscles Ligaments Tendons J 5:316–324
McCoy B, Miniaci A (2012) Osteochondral autograft transplantation/mosaicplasty. J Knee Surg 25:99–108
Mucha A, Dordevic M, Hirschmann A, Rasch H, Amsler F, Arnold MP, Hirschmann MT (2015) Effect of high tibial osteotomy on joint loading in symptomatic patients with varus aligned knees: a study using SPECT/CT. Knee Surg Sports Traumatol Arthrosc 23:2315–2323
Mucha A, Dordevic M, Testa EA, Rasch H, Hirschmann MT (2013) Assessment of the loading history of patients after high tibial osteotomy using SPECT/CT—a new diagnostic tool and algorithm. J Orthop Surg Res 8:46
Pallante AL, Bae WC, Chen AC, Gortz S, Bugbee WD, Sah RL (2009) Chondrocyte viability is higher after prolonged storage at 37 °C than at 4 °C for osteochondral grafts. Am J Sports Med 37(Suppl 1):24S–32S
Pascual-Garrido C, Friel NA, Kirk SS, McNickle AG, Bach BR Jr, Bush-Joseph CA, Verma NN, Cole BJ (2009) Midterm results of surgical treatment for adult osteochondritis dissecans of the knee. Am J Sports Med 37(Suppl 1):125S–130S
Peterson L, Minas T, Brittberg M, Lindahl A (2003) Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am 85-A(Suppl 2):17–24
Ramirez A, Abril JC, Chaparro M (2010) Juvenile osteochondritis dissecans of the knee: perifocal sclerotic rim as a prognostic factor of healing. J Pediatr Orthop 30:180–185
Rasch H, Falkowski AL, Forrer F, Henckel J, Hirschmann MT (2013) 4D-SPECT/CT in orthopaedics: a new method of combined quantitative volumetric 3D analysis of SPECT/CT tracer uptake and component position measurements in patients after total knee arthroplasty. Skelet Radiol 42:1215–1223
Schon SN, Afifi FK, Rasch H, Amsler F, Friederich NF, Arnold MP, Hirschmann MT (2014) Assessment of in vivo loading history of the patellofemoral joint: a study combining patellar position, tilt, alignment and bone SPECT/CT. Knee Surg Sports Traumatol Arthrosc 22:3039–3046
Shimomura K, Moriguchi Y, Ando W, Nansai R, Fujie H, Hart DA, Gobbi A, Kita K, Horibe S, Shino K, Yoshikawa H, Nakamura N (2014) Osteochondral repair using a scaffold-free tissue-engineered construct derived from synovial mesenchymal stem cells and a hydroxyapatite-based artificial bone. Tissue Eng Part A 20:2291–2304
Tampieri A, Sandri M, Landi E, Pressato D, Francioli S, Quarto R, Martin I (2008) Design of graded biomimetic osteochondral composite scaffolds. Biomaterials 29:3539–3546
Wasiak J, Clar C, Villanueva E (2006) Autologous cartilage implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst Rev 3:CD003323
Wright RW, McLean M, Matava MJ, Shively RA (2004) Osteochondritis dissecans of the knee: long-term results of excision of the fragment. Clin Orthop Relat Res 424:239–243
Acknowledgements
The authors would like to thank Mr. Patrick Jaeger for the graphical formatting.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Funding
A financial grant from Fin-ceramica faenza spa, Faenza RA, Italia was recieved for statistical analysis.
Ethical approval
Ethical approval was obtained from the Ethikkommission Nordwest- und Zentralschweiz (EKNZ, Basel EK 2015-396). All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Mathis, D.T., Kaelin, R., Rasch, H. et al. Good clinical results but moderate osseointegration and defect filling of a cell-free multi-layered nano-composite scaffold for treatment of osteochondral lesions of the knee. Knee Surg Sports Traumatol Arthrosc 26, 1273–1280 (2018). https://doi.org/10.1007/s00167-017-4638-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-017-4638-z