Abstract
Introduction
The treatment of larger osteochondral lesions in the knee is still a clinical challenge. One promising strategy to overcome this problem could be surgical repair by using a cell-free multilayered nano-composite scaffold.
Method
In this prospective cohort study eight consecutive patients which suffered from a single osteochondral lesion (≥1.5 cm2) on the femoral condyle were enrolled. The repair potential of the implant was assessed by using MRI based biochemical MR sequences (T2 mapping) as well as semi-quantitative morphological analyses (MOCART score) at 18 months after the surgery. The clinical outcome was determined at six, 12, 18, and 24 month follow ups by using IKDC, Tegner-Lysholm, and Cincinnati knee scores.
Results
Seven out of eight patients showed a complete integration of the scaffold into the border zone and five out of eight patients excellent or good subchondral ossification of the implant at 18 months following implantation. The surface of the repair tissue was found to be intact in all eight patients. T2 mapping data and the zonal T2 index significantly differed in the repair tissue compared to the healthy control cartilage (P < 0.001) which indicates a limited quality of the repair cartilage. The clinical outcome scores consistently improved during the follow up period without reaching statistical significance.
Conclusions
Osteochondral repair by implanting the MaioRegen® scaffold provides a successful osteoconduction and filling of the cartilage defect. However there is evidence for a limited repair cartilage tissue quality at 18 months after the surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Full thickness cartilage lesions within the knee are a frequent pathology and often require surgical intervention [1]. In most cases the subchondral bone is affected and plays a major role in the restoration of a congruent joint surface [2]. Untreated osteochondral defects increase in size and lead to osteoarthritis. In the last decades numerous surgical repair techniques have been developed [3, 4]: articular cartilage repair by (matrix-assisted) autologous chondrocyte implantation ((M)ACI) showed beneficial short and mid-term clinical results [5]. Unfortunately most techniques require two surgical procedures, do not address the subchondral bone, and are limited by increased costs. Osteochondral repair by using autografts/mosaicplasty [6] or donor allografts [7] also replace the affected subchondral bone. Important limitations for mosaicplasty are the donor site morbidity and difficulties in treating larger sized lesions. Allograft implantation is often limited by commercial availability and additionally still raises patient safety concerns.
Osteochondral lesion repair by using a cell-free three layered biomimetic scaffold seems to overcome these limitations [8]. The implant is easy to use intra-operatively and low priced compared to cell based techniques. It is cell-free which reduces the chance of an immune reaction or the transfer of an infectious disease. Short and mid-term data showed promising results regarding the clinical outcome and the scaffold integration [9, 10]. However a recent study of six knee and four ankle patients found limited osteoconduction, incomplete cartilage repair but significantly improved clinical scores one to three years after the surgery [11]. As a consequence the authors raised concerns about the biological repair potential of the scaffold.
Biochemical MRI sequences raise the potential of determining not only the morphology but also the tissue quality of the repair cartilage [12, 13]. Quantitative analyses including cartilage T2 relaxation time measurements (T2 mapping) are a well established technique to determine the water and collagen fiber content of the cartilage. As a consequence T2 mapping could be perfectly used to determine the biological repair process following implantation of a biomimetic scaffold into an osteochondral lesion.
Up to now knowledge about the tissue quality after osteochondral repair by using the cell-free biomimetic scaffold is scarce. Conflicting reports on the biological repair potential of the implant need to be reassessed.
The aim of this study was to determine repair tissue quality, scaffold integration as well as the short term clinical outcome following implantation of the nano-composite multilayered biomimetic scaffold in the knee. The osseous as well as cartilaginous repair potential of the scaffold is determined by using MRI based biochemical MR sequences (T2 mapping) as well as semi-quantitative morphological analyses (MOCART score) at 18 months after the surgery.
Materials and methods
Patients
The study protocol and all measurements were approved by the Federal Review Board (No. 40/2012) and the Clinical Trial Registry of the N.I.H. (No. NCT02345564).
The inclusion criteria were:
-
written informed consent of all participants
-
surgery scheduled between January 1, 2012 and December 31, 2012
-
single focal osteochondral lesion on the femoral condyles which affects both cartilage and subchondral bone
-
lesion size ≥ 1.5 cm2 at the surface/maximal depth of 1.5 cm
-
age between 15 and 55 years
The exclusion criteria were:
-
history of rheumatoid arthritis
-
local infection
-
history of previously performed knee surgery
-
presence of contraindications to perform an MRI
Finally eight patients (two female and six male, mean age of 37 years, age ranging from 15 to 51 years) participated in the study. The osteochondral lesions were diagnosed by using standard MRIs three to six months prior to the surgery. The lesions were located on the medial condyle in five patients and on the lateral condyle in three patients. The mean lesion size was 2.07 cm2 (ranging from 1.5 to 3.75 cm2). Clinical outcome measurements were performed pre-operatively and at six, 12, 18, and 24 months after the surgery. Baseline measurements were performed using the Tegner-Lysholm knee score as well as the International Knee Documentation Committee (IKDC) score. Follow up examinations after the surgery were completed by additionally using the Cincinnati knee scale. In all cases MRI including the biochemical MR sequences (T2 mapping) was performed at 18 months after the surgery.
Scaffold
In this study a cell-free three layered biomimetic scaffold (MaioRegen® Finceramica, Faenza S.p.A., Italy) was assessed. The implant is designed for the treatment of osteochondral defects and is approved for clinical use in Austria. It consists of a porous nano-structured three-layered bio matrix designed to mimic the physiological osteochondral tissue. Being resorbable it is thought to facilitate the regenerative process by being gradually replaced by native tissue [8].
Surgical procedure
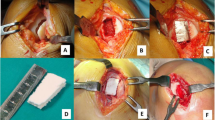
For surgery patients were placed in supine position and the knees were scrubbed and draped for knee arthroscopy. A femoral tourniquet was used. A peri-patellar mini-arthrotomy was performed to enable local debridement of the osteochondral lesion. Subchondral bone was partially removed to create a well-shaped cavity exhibiting 8-10 mm depth referenced to the intact cartilage surface. The MaioRegen® scaffold was shaped according to the defect size and implanted using the press fit technique. Additionally the implant was fixed by the use of fibrin glue.
After surgery all patients underwent a standardized treatment protocol. Early mobilization was carried out. Range of motion was restricted and stepwise increased during the first six weeks. Partial weight bearing was allowed after six weeks and full weight bearing after 12 weeks.
Magnetic resonance imaging
MRI was performed at 18 months after surgery. All images were acquired on a 3.0 Tesla whole body Magnetom TimTrio scanner with a gradient strength of 40 mT/m (Siemens Healthcare, Erlangen, Germany) using an 8-channel knee array coil (IN vivo, Gainesville, FL, USA). MRI parameters are separately shown below (see Table 1).
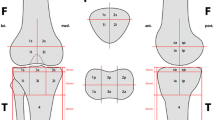
All image analyses were performed on a Leonardo workstation. Morphological evaluation was based on the MOCART scoring system [14]. Its reliability and reproducibility were demonstrated in previous studies [13, 15]. In this study the corrected MOCART scoring system [16] was used. To address the osseous and cartilaginous healing potential of the MaioRegen® implant a semi-quantitative assessment (MOCART score) was provided (see Tables 2 and 3). The evaluation was conducted by three independent raters.
T2 relaxation times were independently assessed based on a region-of-interest (ROI) analysis by the three readers. Three consecutive slices, covering the center implant area were selected. Within those the superficial, deep, and global layer ROIs covering the entire extent of the repair tissue were placed. Three slices depicting healthy control cartilage in a corresponding anatomical region (in terms of biomechanical strain and orientation to the magnetic field) of the same knee were independently selected by the readers to serve as a matched internal control.
Statistics
All statistical calculations were performed using IBM SPSS Statistics for Windows Version 22.0.0.2 (IBM, Armonk, NY, USA). Two way mixed intra class correlation coefficients (ICC) and their 95 % confidence intervals (CI) were used as an index of rater agreement. ICCs were interpreted according to the criteria of Landis and Koch [17]. Changes in clinical scores were assessed using repeated measures ANOVA. In order to compare repair tissue and healthy control cartilage T2 values of all three raters were averaged and a paired student’s t-test was calculated for each region separately. Due to the small sample size no multiplicity corrections were performed to avoid an increasing error of the second type. P-values less than 0.05 were considered statistically significant.
Results
Patient follow up
All eight patients underwent MRI assessment. One out of eight patients attended only one clinical follow up examination. From this patient only the imaging data could be used. Due to persistent pain and swelling two underwent a re-arthroscopy seven and 24 months after the first surgery, respectively. In the first case the re-arthroscopy showed a novel chondral lesion on the lateral condyle. In the second case an infrapatellar ossicle was removed. The scaffolds within the originally filled osteochondral defects were stable without any signs of secondary dislocation. Smooth and slight hypertrophic repair cartilage filled up the original defect in both cases (see Fig. 1a and b).
Morphological evaluation
The mean corrected MOCART score of our study population at 18 months follow up was 69 (ranging from 60 to 100). Seven out of eight patients showed a complete integration of the scaffold into the border zone and five out of eight patients excellent or good subchondral ossification of the implant at 18 months following implantation (see Tables 2 and 3). The surface of the repair tissue was found to be intact in all eight patients. An inhomogenous structure of the repair tissue was found in seven out of eight patients.
T2 mapping and evaluation of the zonal T2 index
In this study T2 mapping (see Fig. 2a and b) and evaluation of the zonal T2 index was performed. T2 relaxation times from ROI-analysis are shown below (see Fig. 3). T2 values within the deep ROI significantly differed in the repair tissue compared to the healthy control cartilage. To further quantify the zonal variation of T2 values within a ROI a “zonal T2 index” was calculated. T2 relaxation times in the deep zone were divided by the T2 relaxation times in the superficial zone. Mean zonal T2 index in the healthy control cartilage was 0.6874 which corresponds to the increase of T2 values from the deep to the superficial zone. This represents a typical hyaline cartilage collagen organization. Mean zonal T2 index in the repair tissue was 0.9874 and differed significantly compared to the healthy control cartilage (p < 0.001).
Clinical outcome
All three clinical outcome scores which were used in this study consistently improved over time without reaching statistical significance (Cincinnati p = 0.53, Tegner-Lysholm p = 0.176, IKDC = 0.30) (see Fig. 4a–c). Based on the given improvement and variance of our study cohort, a number of 71 cases for the Cincinatti score, 15 cases for the Tegner-Lysholm score, and 30 cases for the IKDC score would be necessary to reach a significant result between the first and the last evaluation at 24 months.
Discussion
An important finding of this study was the beneficial osteoconduction as well as the successful filling of the cartilaginous defect of the MaioRegen® scaffold 18 months after implantation. Patients in this study showed a mean corrected MOCART score of 69 which confirms data of a previously published case series [18]. The semi-quantitative assessment of the subchondral bone layer showed a complete integration into the border zone in seven out of eight patients. This finding was supported by the assessment of the intra-articular joint status during re-arthroscopy of two patients.
Subchondral oedema was frequently seen in most of the cases. However, data of a study of patients who underwent matrix-assisted autologous chondrocyte transplantation (MACT) found no correlation between the presence of a subchondral oedema and the short term clinical outcome [19]. It cannot be ruled out that the subchondral oedema after the implantation of osteochondral scaffolds is part of the normal healing process without having any deleterious prognostic value.
A second important finding concerns the quality of the repair tissue within the surface area of the implant. According to the manufacturers’ information repair tissue quality comparable to hyaline like cartilage can be expected. This assumption is supported by histological data in animal studies [20, 21]. In this study we used the T2 mapping technique which permits the assessment of key matrix components like water concentration and collagen architecture/orientation [22–24]. There was no zonal variation of T2 values in the repair tissue. According to a previous work this “zonal variation” is a key marker for hyaline cartilage like collagen fibre organization [25]. Data of this previous study showed a significant difference between the “zonal variation” of the repair tissue following MACT and microfracturing. Interestingly there was no difference in the “zonal variation” of MACT repair tissue and healthy control cartilage. As a consequence the authors conclude that microfracturing leads to fibrous cartilage which was shown in numerous studies by taking biopsies. According to those data the repair tissue quality of our patients can be considered similar to fibrous cartilage. To further quantify this “zonal variation” a zonal T2 index was calculated. Mean zonal T2 index of the repair cartilage significantly differed to the healthy control cartilage. This indicates limited quality of the repair tissue. This finding was supported by the assessment of the implant surface during the two re-arthroscopies. The surface of the scaffolds showed slight hypertrophy, was significantly smoother that the adjacent cartilage, and looked like fibrous cartilage (see Fig. 1a and b).
Another finding of this study was a substantial increase in all clinical scoring systems compared to the pre-operative baseline (see Fig. 4a–c). Mean IKDC score in this study was 79.3 at 24 months after the surgery. This finding is supported by a previous study in which IKDC was found to be 75.42 at a 2 year follow up [9]. Nevertheless statistical significance could not be achieved due to the small sample size as demonstrated by statistical power calculations. There was no case of infection, wound problems or early post-operative complications. This might support the safe use of the implant.
Unfortunately this study bears some limitations. In this prospective case series only eight patients were enrolled. This sample size appeared to be insufficient to assess a significant clinical improvement after the surgery. Nevertheless the focus of this study laid on the imaging evaluation, in particular on the ossification of the subchodral part of the defect. Beyond that the biochemical evaluation of the repair tissue which reflects water content and collagen fiber organization was performed to assess the repair potential of the MaioRegen® implant. Another limitation is the absence of histological analyses. Data of this study strongly suggest a limited quality of the repair tissue in the superficial layer of the implant. However definitive evidence would only have been achieved by performing re-arthroscopies and the collection of histological samples in every (even symptom free) patient. This study protocol is hardly applicable due to ethical reasons.
In conclusion data of this study strongly support a successful osteoconduction and filling of the cartilage defect after osteochondral lesion repair by using a cell-free multilayered nano-composite scaffold (MaioRegen®). The surgical technique seems to be safe and effective with beneficial clinical outcome. However there is evidence that the quality of the superficial cartilage repair tissue is limited.
References
McCormick F, Harris JD, Abrams GD, Frank R, Gupta A, Hussey K, Wilson H, Bach B Jr, Cole B (2014) Trends in the surgical treatment of articular cartilage lesions in the United States: an analysis of a large private-payer database over a period of 8 years. Arthroscopy 30(2):222–226
Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, Kon E (2010) The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc 18(4):434–447
Marcacci M, Filardo G, Kon E (2013) Treatment of cartilage lesions: what works and why? Injury 44(Suppl 1):S11–S15
Bentley G, Bhamra JS, Gikas PD, Skinner JA, Carrington R, Briggs TW (2013) Repair of osteochondral defects in joints—how to achieve success. Injury 44(Suppl 1):S3–S10
Oussedik S, Tsitskaris K, Parker D (2015) Treatment of articular cartilage lesions of the knee by microfracture or autologous chondrocyte implantation: a systematic review. Arthroscopy 31(4):732–744
McCoy B, Miniaci A (2012) Osteochondral autograft transplantation/mosaicplasty. J Knee Surg 25(2):99–108
Chahal J, Gross AE, Gross C, Mall N, Dwyer T, Chahal A, Whelan DB, Cole BJ (2013) Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy 29(3):575–588
Kon E, Delcogliano M, Filardo G, Pressato D, Busacca M, Grigolo B, Desando G, Marcacci M (2010) A novel nano-composite multi-layered biomaterial for treatment of osteochondral lesions: technique note and an early stability pilot clinical trial. Injury 41(7):693–701
Berruto M, Delcogliano M, de Caro F, Carimati G, Uboldi F, Ferrua P, Ziveri G, De Biase CF (2014) Treatment of large knee osteochondral lesions with a biomimetic scaffold: results of a multicenter study of 49 patients at 2-year follow-up. Am J Sports Med 42(7):1607–1617
Kon E, Filardo G, Di Martino A, Busacca M, Moio A, Perdisa F, Marcacci M (2014) Clinical results and MRI evolution of a nano-composite multilayered biomaterial for osteochondral regeneration at 5 years. Am J Sports Med 42(1):158–165
Christensen BB, Foldager CB, Jensen J, Jensen NC, Lind M (2015) Poor osteochondral repair by a biomimetic collagen scaffold: 1- to 3-year clinical and radiological follow-up. Knee Surg Sports Traumatol Arthrosc 18
Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS (2013) Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthr Cartil 21(10):1474–1484
Trattnig S, Millington SA, Szomolanyi P, Marlovits S (2007) MR imaging of osteochondral grafts and autologous chondrocyte implantation. Eur Radiol 17(1):103–118
Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, Trattnig S (2004) Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur Radiol 52(3):310–319
Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S (2006) Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol 57(1):16–23
Trattnig S, Ohel K, Mlynarik V, Juras V, Zbyn S, Korner A (2015) Morphological and compositional monitoring of a new cell-free cartilage repair hydrogel technology—GelrinC by MR using semi-quantitative MOCART scoring and quantitative T2 index and new zonal T2 index calculation. Osteoarthr Cartil 23(12):2224–2232
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Filardo G, Kon E, Di Martino A, Busacca M, Altadonna G, Marcacci M (2013) Treatment of knee osteochondritis dissecans with a cell-free biomimetic osteochondral scaffold: clinical and imaging evaluation at 2-year follow-up. Am J Sports Med 41(8):1786–1793
Filardo G, Kon E, Di Martino A, Perdisa F, Busacca M, Tentoni F, Balboni F, Marcacci M (2014) Is the clinical outcome after cartilage treatment affected by subchondral bone edema? Knee Surg Sports Traumatol Arthrosc 22(6):1337–1344
Kon E, Delcogliano M, Filardo G, Fini M, Giavaresi G, Francioli S, Martin I, Pressato D, Arcangeli E, Quarto R, Sandri M, Marcacci M (2010) Orderly osteochondral regeneration in a sheep model using a novel nano-composite multilayered biomaterial. J Orthop Res 28(1):116–124
Kon E, Mutini A, Arcangeli E, Delcogliano M, Filardo G, Nicoli Aldini N, Pressato D, Quarto R, Zaffagnini S, Marcacci M (2010) Novel nanostructured scaffold for osteochondral regeneration: pilot study in horses. J Tissue Eng Regen Med 4(4):300–308
Glaser C (2005) New techniques for cartilage imaging: T2 relaxation time and diffusion-weighted MR imaging. Radiol Clin N Am 43(4):641–653
Recht MP, Goodwin DW, Winalski CS, White LM (2005) MRI of articular cartilage: revisiting current status and future directions. AJR Am J Roentgenol 185(4):899–914
Lusse S, Claassen H, Gehrke T, Hassenpflug J, Schunke M, Heller M, Gluer CC (2000) Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging 18(4):423–430
Welsch GH, Mamisch TC, Domayer SE, Dorotka R, Kutscha-Lissberg F, Marlovits S, White LM, Trattnig S (2008) Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures--initial experience. Radiology 247(1):154–161
Acknowledgments
The authors deeply appreciate Michael Weber (MD) for his help with statistics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
The authors declare that there are no existing conflicts of interest.
Rights and permissions
About this article
Cite this article
Brix, M., Kaipel, M., Kellner, R. et al. Successful osteoconduction but limited cartilage tissue quality following osteochondral repair by a cell-free multilayered nano-composite scaffold at the knee. International Orthopaedics (SICOT) 40, 625–632 (2016). https://doi.org/10.1007/s00264-016-3118-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-016-3118-2