Abstract
Purpose
To seek differences of Achilles tendon hardness between insertional tendinopathy (IT) and asymptomatic controls by using computer-assisted quantification on axial-strain sonoelastography (ASE).
Methods
The study consisted of 37 non-athletic patients presenting with Achilles tendon pain in one or two tendons. Both tendons were examined clinically. Among the 74 tendons, 16 were diagnosed and categorized into an IT group and 29 into an asymptomatic group. The remaining 29 tendons were excluded due to non-insertional tendinopathy, ruptures, previous surgery or mixed disorders. The tendons in the IT and asymptomatic groups were examined with both ASE and conventional ultrasound. Computer-assisted quantification on ASE was conducted to extract parameters of tendon hardness, including the 20th percentile (H20), median (H50) and skewness (Hsk) of the hardness within tendon, as well as the ratio of the mean hardness within tendon to that outside tendon (Hratio).
Results
The H20 (p = 0.003), H50 (p = 0.004) and Hratio (p = 0.002) were larger and Hsk (p = 0.001) was smaller at distal thirds of IT tendons than those of asymptomatic tendons. For differentiation between two groups, the Hsk achieved the best value (0.815) of area under the receiver operating characteristic curve, with a sensitivity of 81.3 %, a specificity of 86.2 % and an accuracy of 84.4 %.
Conclusions
Computer-assisted quantification on ASE shows that IT tendons are harder than asymptomatic tendons. It might act as a potentially useful technique for identification and risk stratification of IT patients and thus be valuable in day-by-day clinical practice for monitoring IT progression and for evaluating therapeutic effects.
Level of evidence
III.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Achilles tendinopathy frequently occurs in athletes and soldiers, but also in the general public. As a real-time, efficient and low-cost imaging modality, ultrasound (US) is beneficial in the diagnosis of Achilles tendinopathy [18]. Axial-strain sonoelastography (ASE) is a newly developed technique of ultrasonography for measurement of tissue hardness, which involves manual axial compression of tissue using the hand-held US transducer to generate tissue strains (deformations) [24, 34]. The role of ASE on imaging of Achilles tendinopathy has increased in recent years [7, 12, 17, 24, 25, 30]. Compared with conventional US, ASE provides additional information regarding the biomechanical properties of tendon [7, 8, 14, 17, 24, 30, 32, 34, 36].
There are two types of Achilles tendinopathy, insertional tendinopathy (IT) and non-insertional tendinopathy (NIT). IT occurs at the insertion of an Achilles tendon onto calcaneus, while NIT affects the middle portion of an Achilles tendon. Previous studies on ASE of Achilles tendinopathy are often focused on NIT or carried out on mixed population [7, 17, 24, 25]. However, aetiology, histopathology and injury mechanism differ between IT and NIT [37, 38], which may result in their different patterns of hardness. The NIT tendon usually involves degeneration and disordered arrangement of collagen fibres, focal hypercellularity and neovascularization, which could soften the tendon [15–17]. But the IT is most often with formation of bone spurs and calcifications in the tendon proper at the insertion site [37, 38], which may increase the hardness of the tendon [6, 11]. To date, the role of ASE in evaluating the hardness of the IT conditions is still unclear.
Former research employed various elastography grading systems for qualitative identification of tendon disorders. No standard grading protocol has been established [16, 23]. Furthermore, the elasticity grades are generally scored by radiologists and are not yet automated, which limits the intra- or inter-observer reproducibility. Quantitative assessment of ASE images with computer assistance is potentially useful for more objective measurement of Achilles tendon hardness.
The purpose of this study was to propose a computer-assisted method for quantifying tendon hardness on ASE and apply it to comparing Achilles tendons with IT and asymptomatic tendons. To our knowledge, this study was the first to report different hardness patterns between IT and asymptomatic tendons. The computer-assisted ASE could be potentially used in daily clinical practice for monitoring IT progression and for evaluating treatment effects.
Materials and methods
This study was a retrospective analysis of a prospectively collected database for records of patients who underwent ASE of tendons. The study included 37 non-athletic patients (26 male and 11 female), who complained of Achilles tendon pain in one or both tendons and were examined clinically and with conventional US and ASE. All patients reported occasional sports activities but without participation in professional or amateur tournaments. For each patient, clinical evaluation was conducted on bilateral Achilles tendons (n = 74) by a sports medicine surgeon with 14 years of experience to classify them into 29 asymptomatic and 45 symptomatic tendons (Table 1).
The Achilles tendons were divided into three sections for clinical evaluation [7]: (1) proximal (musculotendinous junction), (2) middle (2–6 cm above insertion at the calcaneus) and (3) distal (insertion). The clinical evaluation included an assessment of tenderness, oedema, crepitus, and nodularity or thickening of each Achilles tendon section to differentiate symptomatic from asymptomatic tendon thirds [7, 24]. The symptomatic tendons all presented with pain, and the asymptomatic tendons had no history of tendon disorders or painful episodes on any section. For clinical diagnosis of tendinopathy, the patients had to have documented pain upon palpation over the tendon and pain with resisted activation of the tendon [22]. The tendons with tendinopathic findings confined on distal sections were finally identified as IT.
Among 45 symptomatic tendons, 16 were clinically diagnosed as IT (Table 1). Twenty-nine were diagnosed as NIT, IT plus NIT, ruptures or surgically repaired ruptures and were excluded from further research (Table 1), while their contralateral asymptomatic or IT tendons were reserved. Therefore, the tendons under further investigation consisted of two groups: 16 symptomatic with IT and 29 asymptomatic, presumably healthy.
Among the 16 IT tendons, there were seven with calcification detected at the distal sections on conventional US and nine without. Thus, the IT group was divided into two subgroups, calcified and non-calcified.
Ultrasound image acquisition
The subjects were examined in a prone position with the foot hanging over the edge of the examination bed using the HI VISION Preirus system (Hitachi Medical, Tokyo, Japan) equipped with a linear array transducer (L74 M, 5–13 MHz). Both conventional US and ASE were performed on Achilles tendons by a radiologist with 11 years of experience in musculoskeletal US, who was blinded to the clinical findings. Longitudinal and transversal imaging was performed for each third.
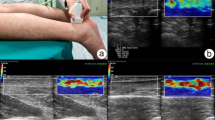
During ASE examination, light repetitive compression was applied over an Achilles tendon with the hand-held transducer. The transducer pressure was applied vertically, perpendicular to the Achilles tendon, and was adjusted so that the real-time feedback visual indicator of pressure on the screen depicted a sinus curve [16], as shown in lower left of Fig. 1a. The elastograms were represented in colour overlaying the B-mode greyscale image, using a colour map from red to blue indicating high to low strains, i.e. low to high hardness (Fig. 1a).
Example for illustrating the computer-assisted quantification of tendon hardness on axial-strain sonoelastography (ASE). The imaging system provides dual-modality visualization in a full screen a consisting of two parts: the right part depicts a greyscale B-mode image and the left is a composite colour image that is a pure colour elastogram overlaying the same B-mode image. The pure colour elastogram (b) is retrieved by subtracting the B-mode image from the composite image. The tendon borders are delineated by using interactive software (c). The pure colour elastogram is transformed to a greyscale image (d). The location of the tendon is depicted in a binary template (e)
The sinus curve should be stable at an almost constant amplitude and frequency for at least four cycles. For each scan plane, a representative image was selected from cine loops, captured and stored in DICOM format for image interpretation including computer-assisted quantification. A representative image should adequately depict tendon boundaries and regular structured peritendinous layers including paratenon, muscles and bursae, so that tissue movement would not affect intratendinous colouring [8].
Computer-assisted quantification of tendon hardness
A computer-assisted quantification program was developed for tendon hardness using MATLAB R2007a (MathWorks, Natick, MA, USA). The computer-assisted quantification was conducted by the same radiologist who had performed the scans. Then three months later, this radiologist assessed the same images for evaluating the intra-observer variability. Meanwhile, after receiving four-hour special training on musculoskeletal anatomy and imaging, a computer scientist specialized in computerized medical image analysis performed the same assessment for evaluating the inter-observer variability.
The Hitachi ASE system provides dual-modality visualization in a full screen (Fig. 1a). The right part is a greyscale B-mode image, while the left part is a composite colour image in the red–green–blue (RGB) format, displayed as a translucent colour ASE image superimposed on the greyscale B-mode image. Thus, a pure colour elastogram was obtained by subtracting the B-mode image from the composite image, but still in RGB format (Fig. 1b) [42, 43]. The strain distribution was then reconstructed by computing the hue value from the pure elastogram [43], ranging from 0 (red, softest) to 5/6 (blue, hardest), depicted as the greyscale image shown in Fig. 1d. Areas appearing as black holes or black shades on ASE colour images (Fig. 1a, b) had invalid hue values, and they were automatically detected and excluded from further calculation and analysis (Fig. 1d, depicted in magenta).
Software was developed for interactive delineation of a tendon border on either the ASE colour image (left of Fig. 1a) or the B-mode greyscale image (right of Fig. 1a), and it would automatically simultaneously map the outlined contour back to the other image. User interactions were as simple as clicks on either image while selecting several points on the tendon border (red dots in Fig. 1c) [40], and the B-spline interpolation of the discrete points was used to get a smooth closed curve (yellow curves in Fig. 1c, d).
The hardness of tendons was quantified by using the statistics of tendon hue values on ASE [41], including the mean (Hmean), skewness (Hsk), 20th percentile (H20) and 50th percentile (median; H50) of the hue values within tendon, as well as the ratio of the mean hardness within tendon to that outside tendon (Hratio). Here, Hsk measures the asymmetry of the probability distribution of tendon hardness. A negative Hsk value indicates that the mass of the distribution is concentrated on the right of the hardness histogram, and a more negative Hsk value indicates a harder tendon.
Morphological parameters were also automatically computed, including the thickness (THK), cross-sectional area (CSA) and eccentricity (ECC) of tendon. ECC ranges from 0 to 1, with a larger value denoting a more oblate shape of tendon. The CSA and ECC were only calculated at the transversal planes.
Every patient had given written informed consent for the ASE examination in the prospective database collection. The Institutional Review Board of Huashan Hospital, Fudan University, approved this study and waived the requirement for written informed consent for the retrospective analysis of the database.
Statistical analysis
All statistical analysis was performed with MATLAB. Tendon hardness and morphological parameters were expressed as mean ± SD because they were normally distributed via the Lilliefors test. Patient age was expressed as median and interquartile range (IQR) because of its non-normal distribution.
Parameters of tendon thirds were compared by using unpaired two-sample t tests between two groups, i.e. IT and asymptomatic controls. Quantitative parameters calculated on transversal and longitudinal planes were both compared between groups. The receiver operating characteristic (ROC) analysis was conducted for evaluating the differentiation performance of the parameters between two groups. The intra- and inter-observer agreement for each quantitative parameter was evaluated with Pearson’s correlation (r). The difference of age between groups was assessed with the Kruskal–Wallis test and the difference of sex with the Chi-square test. A p value less than 0.05 was considered to be statistically significant. The sample sizes of groups were statistically determined [4].
Results
Patient statistics and quantification repeatability
The median age of the patients was 33 years (IQR 14.5 and range 14–80). The median age of the IT group (12 male and four female) was 32 years (IQR 13 and range 15–53), and that of the asymptomatic (20 male and nine female) was 33 years (IQR 14.3 and range 14–80). There was no significant difference of patient age or sex between the two groups.
The correlation coefficients (r) measuring the intra-observer agreement were all larger than 0.86 for five hardness parameters (p < 0.001), while the r-values denoting the inter-observer agreement were all larger than 0.77 (p < 0.001).
Comparisons of hardness and morphology between groups
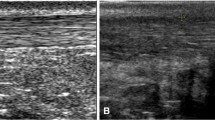
At transversal scans, Hmean, H20, H50, Hsk and Hratio of the distal portions in IT tendons were significantly larger than those in asymptomatic tendons (Table 2), indicating that IT tendons had harder distal portions than asymptomatic controls. The results at longitudinal scans were similar to these found at transversal scans. In Fig. 2, four typical IT tendons were harder than one asymptomatic tendon by visual observation and quantitative comparison.
Distal thirds of Achilles tendons from four subjects with insertional tendinopathy (a–d) and one subject with an asymptomatic tendon (e). The tendinopathic tendons shaded blue and green (a–d) are harder than the asymptomatic tendon which is predominantly green and mixed with small yellow stripes and blue patches (e). Arrows in (a) and (b) indicate calcification. a The quantitative parameters H50 = 0.62, Hratio = 1.30, Hsk = −2.23 and THK = 0.50 cm. b H50 = 0.55, Hratio = 1.14, Hsk = −0.83 and THK = 0.70 cm. c H50 = 0.56, Hratio = 1.42, Hsk = −1.26 and THK = 0.86 cm. d H50 = 0.60, Hratio = 1.27, Hsk = −1.54 and THK = 0.38 cm. e H50 = 0.33, Hratio = 0.67, Hsk = 0.20 and THK = 0.43 cm. H50 and Hsk denote the 50th percentile (median) and skewness of the hardness within tendon, Hratio denotes the mean hardness within tendon to that outside tendon, and THK represents the thickness. The THK in the asymptomatic tendon (e, 0.43 cm) was only a little smaller than that in an IT tendon (a, 0.50 cm) and even larger than that in another IT tendon (d, 0.38 cm); the differences of H50, Hratio and Hsk values between two groups of tendons were much more salient
Compared with asymptomatic tendons, IT tendons tended to be significantly thinker and less oblate (more round) shaped (Table 2). However, the p-values of the morphological parameters were all larger than those of the hardness parameters.
There was a tendency that from the asymptomatic group to the non-calcified subgroup and then to the calcified subgroup, the distal portions became harder (H20 = 0.25, 0.34 and 0.35, respectively; Hsk = −0.28, −0.73, and −0.84) and thicker (THK = 0.45, 0.48, and 0.62 cm). H20 and Hsk were significantly different between the calcified subgroup and the asymptomatic group (p = 0.021 and 0.007), as well as between the non-calcified subgroup and the asymptomatic group (p = 0.022 and 0.026), while THK only had a significant difference between the calcified subgroup and the asymptomatic group (p = 0.004), but not between the non-calcified subgroup and the asymptomatic group.
In terms of H50, IT tendons were significantly harder than asymptomatic tendons at middle portions (0.50 ± 0.09 vs. 0.43 ± 0.10, p = 0.014), but not significantly at proximal portions. In IT tendons, there was no difference between three sections (n.s.), neither was there in asymptomatic tendons (n.s.).
Sensitivity, specificity and correlation
For discrimination between two groups, all five hardness parameters had larger AUCs than three morphological parameters (Table 3; Fig. 3). Hsk achieved the best AUC value of 0.815 with a sensitivity of 81.3 %, a specificity of 86.2 % and an accuracy of 84.4 %.
H50 (r = 0.42; p = 0.004), Hmean (r = 0.38; p = 0.009) and Hsk (r = −0.35; p = 0.017) were marginally but significantly correlated with CSA at distal sections in the entire population. There were no significant correlations between hardness parameters and morphological parameters at middle or proximal sections.
Discussion
The most important finding of the present study is to report that symptomatic IT tendons are significantly harder than asymptomatic controls, presumably healthy tendons, by using a set of quantitative parameters derived from ASE with computer-assisted methods. ASE combined with computer-assisted quantification is recognized as a feasible technique in the assessment of insertional Achilles tendinopathy, providing quantitative information about mechanical properties of tendons. This quantitative hardness information would be potentially valuable for identification and risk stratification of insertional Achilles tendinopathic patients. Thus, in daily clinical practice, it might be useful for monitoring the progression of IT and for evaluating the effects of surgical and non-surgical treatment, such as open and arthroscopic surgeries, injections, eccentric training and extracorporeal shockwave therapy.
Hardness parameters exhibited a more significant difference between IT and asymptomatic tendons than did morphological parameters (Table 2) and also achieved better discrimination performance with ROC analysis (Table 3). Furthermore, alterations from the asymptomatic group to the non-calcified subgroup and then to the calcified subgroup were more evident using hardness parameters than morphological ones. These results suggest that tendon hardening may happen before changes in tendon morphology and that ASE can reliably and reproducibly detect tendon hardening before the conventional US finds calcification or other morphological alterations. The finding that ASE can observe increased hardness in non-calcified subgroup also suggests that ASE may be capable of detecting early calcification and spur formation.
Compared with the asymptomatic tendons, the median hardness (H50) of the IT tendons increased by 20 % at distal sections (0.51 vs. 0.42, p = 0.004; Table 2) and 18 % at middle sections (0.50 vs. 0.43, p = 0.014), but there was no significant difference between two groups at proximal sections. The increase in hardness from normal to IT tendons became less significant from distal to middle and then to proximal sections. It could be hypothesized that the hardening process of IT begins from distal sections, evolves to middle sections and finally approaches proximal sections so as to stiffen the entire tendon. However, the hypothesis needs to be tested by using longitudinal studies.
Previous studies on the hardness of Achilles tendinopathic tendons usually used mixed population including both IT and NIT tendons. Sconfienza et al. [30] found that tendons with chronic overuse-associated pain were harder than asymptomatic tendons. However, others reported that symptomatic patients had softer tendons compared with healthy controls [1, 7, 24]. These contradictory findings might be explained as due to heterogeneous subjects across studies; different proportions of IT and NIT patients in the mixed population might lead to completely different results. To our knowledge, the present study is the first to investigate the regional-specific changes in Achilles tendon hardness by including only IT tendons in the tendinopathic group, but no NIT tendons. This study has shown increased hardness on IT tendons, most likely attributable to calcification and spur formation. However, NIT tendons may tend to be soft due to mucoid degeneration disordered collagen fibres, and cellular, vascular and neural proliferation [15–17]. Therefore, future work will be focused on investigating the different hardness patterns between IT and NIT tendons.
Ageing alters tendon mechanical properties [27, 33, 45]. Recent studies found that the hardness of Achilles tendon increased with increasing age in healthy subjects [28, 35]. In the present study, the ages of subjects had no significant difference between two groups, indicating that our findings were not biased by the subject age. Furthermore, considering the association between the incidence of tendinopathy and ageing [33, 45], tendon hardening with ageing observed in those recent studies seems to coincide with our argument of increased hardness in IT tendons but be in contrary to the findings of decreased hardness in tendinopathic tendons in other literature [1, 7, 24]. Anyhow, the effect of ageing on tendon hardness should be elucidated with longitudinal studies.
Tendon hardening is recently observed in patellar tendinopathy with sonoelastography [44], which is in accordance with our finding in Achilles tendinopathy. In addition to Achilles tendinopathy, Achilles tendons have been evaluated with sonoelastography in various populations, such as patients with ankylosing spondylitis [36], foot ulcers [13], clubfoot [20, 21], ruptured Achilles tendons [5, 9, 14] or repaired ruptures [19, 32]. On these new application areas, computer-assisted quantification of sonoelastography will be expected to yield more objective measurement of tendon hardness.
Four terms, hardness, stiffness, elasticity and elastic modulus, have been interchangeably used across studies [1, 9, 17, 23, 30, 34]. Hardness is a general term describing the ability of a material to resist plastic deformation, while strictly speaking the latter three terms have more specific meanings. Stiffness is defined as the ratio of the force applied on a material to its consequent displacement [39]. Elasticity is the ability of a material to resist a stress and return to its original shape and size after the stress is removed. Elasticity is quantified by elastic modulus, which is the ratio of the stress to its resulting strain [29, 31]. A larger elastic modulus stands for smaller elasticity. ASE does not measure elastic modulus or stiffness but strains, which are a quantity representing hardness, and its measurement has been extensively validated with phantoms and excised tissues [2, 26]. Therefore, the term “hardness” is used throughout the article for appropriate description [23].
There are several limitations. First, asymptomatic tendons contralateral to symptomatic ones composed the control group in this retrospective study. Healthy population should be recruited as controls in a future prospective study to eliminate the factor that the contralateral asymptomatic tendons may also have early subclinical changes in hardness [7]. Second, the sample size is small especially for calcified IT tendons, which renders our results preliminary. However, for such a pilot study, the subject number is statistically calculated to be acceptable to yield reliable results [4]. Third, ASE uses freehand probe-axial compression/decompression for imaging, assessing only axial hardness of tendons. Combining both axial and lateral sonoelastography techniques may be more sensitive to changes in the material properties of tendon’s highly anisotropic structures [3]. Fourth, ASE is a modality of strain elastography measuring relative elasticity of tissues. A technique for measurement of elastic modulus, such as shear-wave elastography, would provide absolute elasticity measurement [1, 5, 9, 10, 19, 28, 44]. Fifth, the preliminary findings on ASE need to be confirmed with histopathology in the future.
Conclusions
It is shown that in patients with insertional Achilles tendinopathy, the computer-assisted ASE can reliably and reproducibly detect increased hardness prior to conventional ultrasound. As a quantitative objective method, the computer-assisted ASE might act as a potentially valuable tool in day-by-day clinical practice to provide tendon hardness information, which could be important for monitoring the progression of IT and for evaluating the effects of surgical and non-surgical treatment.
References
Aubry S, Nueffer J-P, Tanter M, Becce F, Vidal C, Michel F (2015) Viscoelasticity in achilles tendonopathy: quantitative assessment by using real-time shear-wave elastography. Radiology 274(3):821–829
Bamber J, Cosgrove D, Dietrich C, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas J, D’Onofrio M, Drakonaki E (2013) Efsumb guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: basic principles and technology. Ultraschall Med 34(2):169–184
Brown PG, Alsousou J, Cooper A, Thompson MS, Noble JA (2013) The AutoQual ultrasound elastography method for quantitative assessment of lateral strain in post-rupture achilles tendons. J Biomech 46(15):2695–2700
Campbell MJ, Julious SA, Altman DG (1995) Estimating sample sizes for binary, ordered categorical, and continuous outcomes in two group comparisons. BMJ 311(7013):1145–1148
Chen X-M, Cui L-G, He P, Shen W-W, Qian Y-J, Wang J-R (2013) Shear wave elastographic characterization of normal and torn achilles tendons a pilot study. J Ultrasound Med 32(3):449–455
Currey JD (1999) The design of mineralised hard tissues for their mechanical functions. J Exp Biol 202(23):3285–3294
De Zordo T, Chhem R, Smekal V, Feuchtner G, Reindl M, Fink C, Faschingbauer R, Jaschke W, Klauser AS (2010) Real-time sonoelastography: findings in patients with symptomatic achilles tendons and comparison to healthy volunteers. Ultraschall Med 31(4):394–400
De Zordo T, Fink C, Feuchtner GM, Smekal V, Reindl M, Klauser AS (2009) Real-time sonoelastography findings in healthy achilles tendons. Am J Roentgenol 193(2):W134–W138
DeWall RJ, Jiang J, Wilson JJ, Lee KS (2014) Visualizing tendon elasticity in an ex vivo partial tear model. Ultrasound Med Biol 40(1):158–167
DeWall RJ, Slane LC, Lee KS, Thelen DG (2014) Spatial variations in achilles tendon shear wave speed. J Biomech 47(11):2685–2692
Doschak MR, Zernicke RF (2005) Structure, function and adaptation of bone-tendon and bone-ligament complexes. J Musculoskelet Neuronal Interact 5(1):35–40
Drakonaki EE, Allen GM, Wilson DJ (2012) Ultrasound elastography for musculoskeletal applications. Br J Radiol 85(1019):1435–1445
Evranos B, Idilman I, Ipek A, Polat SB, Cakir B, Ersoy R (2015) Real-time sonoelastography and ultrasound evaluation of the achilles tendon in patients with diabetes with or without foot ulcers: a cross sectional study. J Diabetes Complicat 29(8):1124–1129
Gehmert S, Jung E, Kügler T, Klein S, Gehmert S, Zeitler K, Loibl M, Prantl L (2012) Sonoelastography can be used to monitor the restoration of achilles tendon elasticity after injury. Ultraschall Med 33(6):581
Klauser AS, Faschingbauer R, Jaschke WR (2010) Is sonoelastography of value in assessing tendons? Semin Musculoskelet Radiol 14(3):323–333
Klauser AS, Miyamoto H, Bellmann-Weiler R, Feuchtner GM, Wick MC, Jaschke WR (2014) Sonoelastography: musculoskeletal applications. Radiology 272(3):622–633
Klauser AS, Miyamoto H, Tamegger M, Faschingbauer R, Moriggl B, Klima G, Feuchtner GM, Kastlunger M, Jaschke WR (2013) Achilles tendon assessed with sonoelastography: histologic agreement. Radiology 267(3):837–842
Lee KS (2012) Musculoskeletal sonography of the tendon. J Ultrasound Med 31(12):1879–1884
Martin JA, Biedrzycki AH, Lee KS, DeWall RJ, Brounts SH, Murphy WL, Markel MD, Thelen DG (2015) In vivo measures of shear wave speed as a predictor of tendon elasticity and strength. Ultrasound Med Biol 41(10):2722–2730
Masala S, Manenti G, Antonicoli M, Morosetti D, Claroni G, Guglielmi G, Simonetti G (2014) Real time evaluation of monolateral clubfoot with sonoelastography. Radiol Med 119(8):601–606
Masala S, Manenti G, Antonicoli M, Morosetti D, Claroni G, Simonetti G (2012) Real time evaluation of monolateral clubfoot with sonoelastography. Preliminary results. Muscles Ligaments Tendons J 2(1):49–52
Mautner K, Colberg RE, Malanga G, Borg-Stein JP, Harmon KG, Dharamsi AS, Chu S, Homer P (2013) Outcomes after ultrasound-guided platelet-rich plasma injections for chronic tendinopathy: a multicenter, retrospective review. PM&R 5(3):169–175
Ooi CC, Malliaras P, Schneider ME, Connell DA (2014) “Soft, hard, or just right?” Applications and limitations of axial-strain sonoelastography and shear-wave elastography in the assessment of tendon injuries. Skelet Radiol 43(1):1–12
Ooi CC, Schneider ME, Malliaras P, Chadwick M, Connell DA (2015) Diagnostic performance of axial-strain sonoelastography in confirming clinically diagnosed achilles tendinopathy: comparison with b-mode ultrasound and color doppler imaging. Ultrasound Med Biol 41(1):15–25
Ooi CC, Schneider ME, Malliaras P, Jones D, Saunders S, McMahon A, Connell D (2016) Sonoelastography of the achilles tendon: prevalence and prognostic value among asymptomatic elite australian rules football players. Clin J Sport Med. doi:10.1097/JSM.0000000000000265
Ophir J, Alam SK, Garra BS, Kallel F, Konofagou EE, Krouskop T, Merritt CR, Righetti R, Souchon R, Srinivasan S (2002) Elastography: imaging the elastic properties of soft tissues with ultrasound. J Med Ultrason 29(4):155–171
Reeves ND (2006) Adaptation of the tendon to mechanical usage. J Musculoskelet Neuronal Interact 6(2):174–180
Ruan Z, Zhao B, Qi H, Zhang Y, Zhang F, Wu M, Shao G (2015) Elasticity of healthy achilles tendon decreases with the increase of age as determined by acoustic radiation force impulse imaging. Int J Clin Exp Med 8(1):1043–1050
Schepull T, Aspenberg P (2015) Healing of human achilles tendon ruptures: radiodensity reflects mechanical properties. Knee Surg Sports Traumatol Arthrosc 23(3):884–889
Sconfienza LM, Silvestri E, Cimmino MA (2010) Sonoelastography in the evaluation of painful achilles tendon in amateur athletes. Clin Exp Rheumatol 28(3):373–378
Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, Castera L, Choi BI, Chou Y-H, Cosgrove D, Dietrich CF, Ding H, Amy D, Farrokh A, Ferraioli G, Filice C, Friedrich-Rust M, Nakashima K, Schafer F, Sporea I, Suzuki S, Wilson S, Kudo M (2015) Wfumb guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol 41(5):1126–1147
Tan S, Kudas S, Ozcan AS, Ipek A, Karaoglanoglu M, Arslan H, Bozkurt M (2012) Real-time sonoelastography of the achilles tendon: pattern description in healthy subjects and patients with surgically repaired complete ruptures. Skelet Radiol 41(9):1067–1072
Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HR (2013) Capacity for sliding between tendon fascicles decreases with ageing in injury prone equine tendons: a possible mechanism for age-related tendinopathy. Eur Cells Mater 25:48–60
Tudisco C, Bisicchia S, Stefanini M, Antonicoli M, Masala S, Simonetti G (2015) Tendon quality in small unilateral supraspinatus tendon tears. Real-time sonoelastography correlates with clinical findings. Knee Surg Sports Traumatol Arthrosc 23(2):393–398
Turan A, Teber MA, Yakut ZI, Unlu HA, Hekimoglu B (2015) Sonoelastographic assessment of the age-related changes of the achilles tendon. Med Ultrason 17(1):58–61
Turan A, Tufan A, Mercan R, Teber MA, Tezcan ME, Bitik B, Goker B, Haznedaroglu S (2013) Real-time sonoelastography of achilles tendon in patients with ankylosing spondylitis. Skelet Radiol 42(8):1113–1118
van Dijk CN, van Sterkenburg MN, Wiegerinck JI, Karlsson J, Maffulli N (2011) Terminology for achilles tendon related disorders. Knee Surg Sports Traumatol Arthrosc 19(5):835–841
Wiegerinck JI, Kerkhoffs GM, van Sterkenburg MN, Sierevelt IN, van Dijk CN (2013) Treatment for insertional achilles tendinopathy: a systematic review. Knee Surg Sports Traumatol Arthrosc 21(6):1345–1355
Wu Z, Hua Y, Li H, Chen S, Li Y (2015) Biomechanical comparison of three methods for distal achilles tendon reconstruction. Knee Surg Sports Traumatol Arthrosc 23(12):3756–3760
Zhang Q, Li CL, Han H, Yang LJ, Wang YY, Wang WP (2014) Computer-aided quantification of contrast agent spatial distribution within atherosclerotic plaque in contrast-enhanced ultrasound image sequences. Biomed Signal Process Control 13:50–61
Zhang Q, Li CL, Zhou ML, Liao Y, Huang CC, Shi J, Wang YY, Wang WP (2015) Quantification of carotid plaque elasticity and intraplaque neovascularization using contrast-enhanced ultrasound and image registration-based elastography. Ultrasonics 62:253–262
Zhang Q, Xiao Y, Chen S, Wang C, Zheng H (2015) Quantification of elastic heterogeneity using contourlet-based texture analysis in shear-wave elastography for breast tumor classification. Ultrasound Med Biol 41(2):588–600
Zhang X, Xiao Y, Zeng J, Qiu W, Qian M, Wang C, Zheng R, Zheng H (2014) Computer-assisted assessment of ultrasound real-time elastography: initial experience in 145 breast lesions. Eur J Radiol 83(1):e1–e7
Zhang ZJ, Ng GY, Lee WC, Fu SN (2014) Changes in morphological and elastic properties of patellar tendon in athletes with unilateral patellar tendinopathy and their relationships with pain and functional disability. Plos One 9(10):e108337
Zhou B, Zhou Y, Tang K (2014) An overview of structure, mechanical properties, and treatment for age-related tendinopathy. J Nutr Health Aging 18(4):441–448
Acknowledgments
This work was supported by the National Natural Science Foundation of China (61401267 and 61471231). The authors wish to thank Dr. Liangjun Zhang for his editorial contributions and the anonymous referees for their careful review.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Q., Cai, Y., Hua, Y. et al. Sonoelastography shows that Achilles tendons with insertional tendinopathy are harder than asymptomatic tendons. Knee Surg Sports Traumatol Arthrosc 25, 1839–1848 (2017). https://doi.org/10.1007/s00167-016-4197-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-016-4197-8