Abstract
Purpose
The introduction of patient-specific instruments (PSI) for guiding bone cuts could increase the incidence of malalignment in primary total knee arthroplasty. The purpose of this study was to assess the agreement between one type of patient-specific instrumentation (Zimmer PSI) and the pre-operative plan with respect to bone cuts and component alignment during TKR using imageless computer navigation.
Methods
A consecutive series of 30 femoral and tibial guides were assessed in-theatre by the same surgeon using computer navigation. Following surgical exposure, the PSI cutting guides were placed on the joint surface and alignment assessed using the navigation tracker. The difference between in-theatre data and the pre-operative plan was recorded and analysed.
Results
The error between in-theatre measurements and pre-operative plan for the femoral and tibial components exceeded 3° for 3 and 17 % of the sample, respectively, while the error for total coronal alignment exceeded 3° for 27 % of the sample.
Conclusion
The present results indicate that alignment with Zimmer PSI cutting blocks, assessed by imageless navigation, does not match the pre-operative plan in a proportion of cases. To prevent unnecessary increases in the incidence of malalignment in primary TKR, it is recommended that these devices should not be used without objective verification of alignment, either in real-time or with post-operative imaging. Further work is required to identify the source of discrepancies and validate these devices prior to routine use.
Level of evidence
II.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patient-specific cutting guides have been introduced as an alternative to traditional jigs and computer navigation for making bone cuts in total knee arthroplasty (TKA). Despite a range of potential benefits, with particular emphasis on reduced inventory and faster throughput, the cost-effectiveness of PSI may depend on significant reduction in revision rates in primary TKA [19].
A key risk factor for early revision is poor final alignment of the components in all three planes of knee movement [7]. Recent comparisons between patient-specific cutting guides and quantitative assessments of post-operative alignment have revealed the presence of outliers with respect to coronal alignment [15, 17, 18]. The proportion of outliers outside 3° of neutral ranged from 14 % [15] to 44 % [18] in the coronal plane. However, these studies used the patient-specific guides to perform the procedure and assessed the implanted component alignment post-operatively using CT or radiographs.
Given the risk of discrepancies between the achieved alignment and the target, the ethical considerations of performing cuts with untested guides and examining the achieved alignment post-operatively are not trivial. Therefore, the ability of these devices to achieve bone cuts during TKR as per the pre-operative plan should be assessed in real time, using accepted methods of assessing alignment and cut depth, with the option to override the cutting guide a key requirement [5]. Computer navigation is a well-established method for achieving accurate component alignment in the coronal and sagittal planes, and discrepancies between the pre-operative plan and the alignment given by the new technology assessed by computer navigation in-theatre have been identified [13]. Importantly, this method of validation provides real-time feedback in-theatre and an opportunity for the surgeon to override the blocks if accuracy is unsatisfactory.
The purpose of this study, therefore, was to assess the agreement between one type of patient-specific cutting guide (Zimmer, PSI) and the pre-operative plan with respect to bone cuts and component alignment during TKR using computer navigation. It was hypothesised that the intra-operative measurements would agree with the pre-operative plan within a 2° threshold for a majority (>90 %) of the sample.
Materials and methods
During a one-year period (2011–2012), the senior author performed a consecutive series of 30 primary TKAs in 25 patients between 50 and 90 years with disabling knee osteoarthritis. Inclusion criteria included acceptable medical risk and failed non-operative management of knee osteoarthritis, with candidates referred for evaluation to a research assistant for inclusion to the study. Patients that were able to undergo pre-operative MRI, wait 4–6 weeks for surgery, and accepted the new technology were included. Exclusion criteria included metallic hardware 15 cm proximal or distal to the knee joint, prior ipsilateral long bone fracture with extra-articular deformity or a known sensitivity to the materials in the cutting guides. The data collection protocol was approved by the local Human Research Ethics Committee. All patients provided written informed consent prior to enrolment in the study.
The patient-specific cutting blocks evaluated in this study were the Patient Specific Instrumentation (PSI) system from Zimmer™. A pre-operative MRI protocol was conducted (General Electric 3T Magnet, 8-channel transmit/receive extremity coil) comprising of a T1-weighted 3D sagittal knee scan (TE 3 ms, TR 12 ms, field of view 22 cm, slice thickness 2 mm, matrix 2,280 × 4,560 pixels), T1-weighted axial scans of the ankle (TE 9 ms, TR 700 ms, FOV 22 cm, slice thickness 5 mm, matrix 680 × 1,360) and hip (TE 6 ms, TR 920 ms, FOV 22 cm, slice thickness 5 mm, matrix 755 × 1,510 pixels), as well as a lower-resolution sagittal knee sequence (TE 4 ms, TR 14 ms, slice thickness 4 mm, FOV 22 cm, matrix 307 × 614 pixels). The images were sent to Materialise (Leuven, Belgium) for segmentation and digital construction of the femur and tibia models. The rendered bone models and digital templates of the prosthesis components were uploaded to a proprietary operative planner for review by the operating surgeon. Prior to surgery, the surgeon visualised the proposed bone resections in three dimensions and made adjustments to resection depth and implant positioning as appropriate. Once the pre-operative plan was finalised, the information was returned using the electronic interface for manufacturing of the PSI guides to fit the articular surfaces of the distal femur and proximal tibia.
Total knee arthroplasty was undertaken according to the surgeon’s standard technique. Intra-operative alignment data were collected using the ORTHOSoft Total Knee Navigation system (Zimmer Inc., Warsaw, USA). Average accuracy of an imageless navigation system using an infrared camera to detect marker positions has been reported to be within 1 mm for distance and 0.4° for angles [11]. With the patient supine on the operating table and the operated limb prepared, registration of landmarks and joint centres was conducted in a standard manner, as previously described [23]. Appropriate soft tissue was removed from the knee with osteophytes left in situ where present, prior to careful positioning of the PSI cutting guides (Zimmer Inc., Warsaw, USA) in accordance with manufacturer’s instructions. Pins were placed after pre-drilling and cutting guides applied to the articular surface (Fig. 1). The handheld navigation tracker was then placed in the cutting slot to replicate the saw blade, and the alignment in sagittal and coronal planes was recorded, as well as the cutting depths for the medial and lateral femoral condyles. The femoral four-in-one finishing guide was pinned, and the process repeated to assess rotation. The tibial guide was then assessed in the same way, without rotation (Fig. 2). Cuts were made with the PSI system only if the PSI-defined bone cuts and alignment were within 2° or 1 mm of the pre-operative plan in each plane. Otherwise, the surgeon overrode the PSI and performed the procedure utilising navigation information.
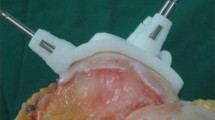
Illustration of the intra-operative measurement method with the knee exposed and the PSI positioned on the femoral surface and pinned in place. The fit between the PSI block and the femoral surface can be seen at the end of the white arrow. Navigation marker clusters are fixed to the tibia (left) and thigh (right) in preparation for measurement
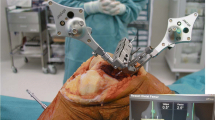
Positioning of the navigation tracker in the cutting slot following removal of the PSI block from the tibia and replacement with the cutting guide. The tracker can be seen well placed within the cutting slot at the tip of the white arrow and the marker clusters in place to the left and right of the joint
Statistical analysis
The intra-operative measurements and the pre-operative plan were compared in a manner previously described [13]. The femoral and tibial alignment measurements were summed to produce virtual total alignment errors in the coronal and sagittal planes. Following data checking and tests of normality, descriptive statistics (mean, standard deviation and range) were calculated, with one-sample t tests used to determine whether the mean difference between the plan and intra-operative measurements differed significantly from zero. The proportion of differences within ±3° and ±2° were calculated for alignment, as well as ±2 and ±1 mm for cut depth. Prediction intervals for a single future measurement were also calculated to identify the range of values the difference between pre-operative plan and intra-operative measurement would likely fall within [13]. An alpha error rate of 5 % and confidence interval of 95 % was set a priori for one-sample t tests, and a confidence interval of 99 % was set for the prediction interval. All statistical analyses were performed with Minitab (version 16, Minitab Inc., MA, USA).
Results
In the sample of 30 primary TKAs, 20 patients underwent unilateral procedures and 5 received simultaneous bilateral replacements. The 25 patients enrolled were a mixed-gender (females—10, males—15), elderly (69.4 ± 8.4 years) and overweight (1.7 ± 0.12 m; 85.2 ± 14.9 kg; 29.6 ± 5.1 BMI) sample. Pre-operative knee range of motion was 3.3 ± 6.8° degrees of fixed flexion to 118.4 ± 7.6° of flexion, with 1.9 ± 5.5° average varus alignment in full extension.
The mean difference between planned femoral component alignment and intra-operative measurements in the coronal and sagittal planes was not significantly different (p > 0.05) from zero (Table 1), in contrast to femoral rotation (p < 0.01) The range of individual differences for the femoral component alignments also differed between planes, with the coronal plane demonstrating the lowest range, followed by sagittal and rotation planes (Table 1). The mean difference for tibial slope was significantly different (p < 0.05) from zero, while the mean difference for the coronal plane was near zero. However, the ranges of individual differences were similar between planes (Table 1). When the alignment differences were summed for the femoral and tibial components, respectively, the averages for coronal and sagittal planes were not significantly different to zero, however, the ranges of individual differences were larger than for each component in isolation.
The femoral component alignment differences in the coronal plane demonstrated the highest proportion of cases within ±3° and ±2° (97 and 93 %, respectively), as well as the lowest prediction interval for a single future measurement. Femoral sagittal alignment as well as the coronal and slope alignment for the tibia showed higher proportions of outliers for the 3° and 2° thresholds as well as markedly wider prediction intervals (Table 1). Femoral rotation demonstrated the lowest proportions and widest prediction interval. Importantly, the total error reduced the proportion of the sample within the alignment thresholds and displayed an additive effect on the prediction interval (Table 1) with an interval for total sagittal error of 18.8°.
The mean difference between the planned femoral resection and the intra-operative measurements differed significantly from zero (p < 0.01), with a range between −1.5 and 4 mm (Table 2). The proportions of values were 90 and 64 % within 2 and 1 mm, respectively, with the smallest prediction interval for a single future measurement. The mean difference between the plan and intra-operative measurements was 0.1 ± 1.4 and 0.1 ± 2.2 mm, respectively. The medial plateau had a lower range of values (Table 2), with 89 % within ±2 mm compared to 70 % for the lateral tibial plateau. The proportions within ±1 mm were 52 and 45 % for the medial and lateral plateaus, respectively, while the prediction intervals were 7.8 and 12.2 mm, respectively.
Discussion
The primary finding of the present study was that the Zimmer PSI does not recreate the pre-operative plan in terms of alignment and bone cut depth for a proportion of cases. There remains limited information regarding the ability of PSI to achieve the pre-operative plan in-theatre. To date, OtisMed, Signature and Visionaire cutting blocks have been evaluated with post-operative imaging, while just two studies have evaluated Visionaire intra-operatively with computer navigation [4, 13]. Previous studies have reported incidences of coronal alignment >3° from neutral for TKRs performed with patient-specific cutting guides ranging from 9.4 % [1] to 44 % [18] (Table 3). The PSI system did not demonstrate a marked improvement in the incidence of outliers in final coronal alignment (27 %), comparing poorly with computer-assisted navigation (9 %), while demonstrating similar rates to conventional techniques (31.8 %) [14].
To date, just one study has examined the agreement between Visionaire and computer navigation in the sagittal plane [13]. The present results featured a reduced outlier incidence compared to [13] for both femoral flexion (10 vs 41 %) and tibial slope (13 vs 36 %) as well as total sagittal error at 3° and 2° thresholds. Nevertheless, the agreement between PSI and navigation was less for femoral rotation compared to Visionaire [13]. These findings are in contrast to a previous study that reported improved rotational accuracy over conventional instrumentation for TKA [9]. The reasons for the discrepancies between studies are not immediately clear, and further work is required to resolve this. Importantly, the system examined in this study demonstrated comparable agreement between the cutting guides and computer navigation for distal femoral resection for both ±2 and ±1 mm thresholds. However, these results should be interpreted cautiously given the differences between studies with respect to the patient-specific cutting system, the navigation system, the surgeon and the patient cohort.
Differences between the pre-operative plan and in-theatre measurements have important implications for TKA. The link between malalignment in component positioning and TKR longevity is well established [14], although the link between implant alignment and function remains less elucidated. The need to achieve accurate implant positioning in a reliable manner has driven the development and implementation of real-time navigation for TKR with reduced incidence of alignment outliers [8]. Therefore, the introduction of patient-specific cutting guides must be supported with evidence that the incidence of malalignment is not increased, thereby exposing patients to unnecessary risk of poor outcomes [2]. The present results highlight discrepancies between the planned alignment and the predicted alignment when assessed by computer navigation. Importantly, the results suggest that up to 1 in 4 patients may be exposed to malalignment in the coronal plane, compared to 1 in 10 using computer navigation alone [8, 14]. This uncertainty in alignment potentially requires greater surgeon intervention, thereby negating a number of the potential benefits of patient-specific cutting guides, such as reduced operative times [21].
The source of discrepancies between the pre-operative plan and in-theatre measurements remains unclear. The process of pre-operative imaging, model generation, planning and block manufacture is complex and involves numerous steps and personnel. Small errors anywhere along the production chain may culminate in considerable differences between the measured alignment and the plan. Specifically, limitations with the imaging protocol may be a potential source of error [13]. The low-resolution sequences used in this study to identify joint centres combined with a high-resolution scan with a limited field of view may have also contributed to inaccuracies, particularly in the sagittal and coronal planes. However, additional study is required to verify these hypotheses. An alternative explanation may be the stability of the blocks in-theatre, particularly when the block was pinned to the bone [21]. Even slight movements could rotate the block in any plane, leading to angular discrepancies to the planned alignment, although this remains difficult to verify without additional data. The differences identified in the present results could also be explained by co-variation in the errors associated with PSI and errors related to the navigation procedure. Nevertheless, this covariance may occur with any measurement system used to verify the alignment achieved with PSI and future studies should consider this when interpreting these data. Further work is required to identify the source of discrepancies between measurement systems and validate patient-specific devices prior to routine use in TKA.
The results of the present study should be interpreted in the context of its limitations. The verification of the PSI technology remains limited by the constraints inherent in current methods for assessing component alignment and resection depths in vivo. Computer navigation provides the only real-time method for assessing alignment of PSI, and the imageless approach used in this study has been validated [11]. However, the specific system used has not been validated, and the navigation process includes inherent error, particularly with respect to landmark identification [12]. Nevertheless, a method to perfectly determine component alignment for TKR remains elusive, with even CT-based visualisation and measurement criticised for inaccuracies in some planes [22]. The error associated with different validation methods also makes comparisons between the present study and previous assessments of PSI alignment difficult. A number of studies have used two-dimensional CT or radiographs to verify coronal alignment post-operatively [1, 18, 20], which differ to the navigation approach used here. Additional factors such as the surgeon, the patient anatomy and the specific navigation system also complicate comparison and should be considered before comparing between studies.
Conclusions
The present results indicate that alignment with PSI patient-specific cutting guides, assessed by computer navigation, does not match the pre-operative plan in a proportion of cases. To prevent unnecessary increases in the incidence of malalignment in primary TKR, it is recommended that these devices should not be used without objective verification of alignment, either in real-time or with post-operative imaging.
References
Bali K, Walker P, Bruce W (2012) Custom-fit total knee arthroplasty: our initial experience in 32 knees. J Arthroplasty 27(6):1149–1154
Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM (2012) Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br 94 (11 suppl A):95–99
Boonen B, Schotanus MG, Kort NP (2012) Preliminary experience with the patient-specific templating total knee arthroplasty. Acta Orthop 83(4):387–393
Conteduca F, Iorio R, Mazza D, Caperna L, Bolle G, Argento G, Ferretti A (2012) Are MRI-based, patient matched cutting jigs as accurate as the tibial guides? Int Orthop 36(8):1589–1593
Conteduca F, Iorio R, Mazza D, Caperna L, Bolle G, Argento G, Ferretti A (2012) Evaluation of the accuracy of a patient-specific instrumentation by navigation. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-012-2098-z
Daniilidis K, Tibesku CO (2013) Frontal plane alignment after total knee arthroplasty using patient-specific instruments. Int Orthop 37(1):45–50
Fang D, Ritter MA (2009) Malalignment: forewarned is forearmed. Orthopedics 32(9):681–682
Hetaimish BM, Khan MM, Simunovic N, Al-Harbi HH, Bhandari M, Zalzal PK (2012) Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty 27(6):1177–1182
Heyse TJ, Tibesku CO (2012) Improved femoral component rotation in TKA using patient-specific instrumentation. Knee. doi:10.1016/j.knee.2012.10.009
Klatt BA, Goyal N, Austin MS, Hozack WJ (2008) Custom-fit total knee arthroplasty (OtisKnee) results in malalignment. J Arthroplasty 23(1):26–29
Lustig S, Fleury C, Goy D, Neyret P, Donell ST (2011) The accuracy of acquisition of an imageless computer-assisted system and its implication for knee arthroplasty. Knee 18(1):15–20
Lustig S, Fleury C, Servien E, Demey G, Neyret P, Donell ST (2011) The effect of pelvic movement on the accuracy of hip centre location acquired using an imageless navigation system. Int Orthop 35(11):1605–1610
Lustig S, Scholes CJ, Oussedik SI, Kinzel V, Coolican MR, Parker DA (2013) Unsatisfactory accuracy as determined by computer navigation of VISIONAIRE patient-specific instrumentation for total knee arthroplasty. J Arthroplasty 28(3):469–473
Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K (2007) Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty 22(8):1097–1106
Ng VY, DeClaire JH, Berend KR, Gulick BC, Lombardi AV Jr (2012) Improved accuracy of alignment with patient-specific positioning guides compared with manual instrumentation in TKA. Clin Orthop Relat Res 470:99–107
Noble JW Jr, Moore CA, Liu N (2012) The value of patient-matched instrumentation in total knee arthroplasty. J Arthroplasty 27(1):153–155
Nunley RM, Ellison BS, Ruh EL, Williams BM, Foreman K, Ford AD, Barrack RL (2012) Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res 470:889–894
Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL (2012) Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res 470:895–902
Slover JD, Rubash HE, Malchau H, Bosco JA (2012) Cost-effectiveness analysis of custom total knee cutting blocks. J Arthroplasty 27(2):180–185
Spencer BA, Mont MA, McGrath MS, Boyd B, Mitrick MF (2009) Initial experience with custom-fit total knee replacement: intra-operative events and long-leg coronal alignment. Int Orthop 33(6):1571–1575
Stronach BM, Pelt CE, Erickson J, Peters CL (2013) Patient-specific total knee arthroplasty required frequent surgeon-directed changes. Clin Orthop Relat Res 471:169–174
Victor J, Van Doninck D, Labey L, Innocenti B, Parizel PM, Bellemans J (2009) How precise can bony landmarks be determined on a CT scan of the knee? Knee 16(5):358–365
Widmer BJ, Scholes CJ, Lustig S, Conrad L, Oussedik SI, Parker DA (2013) Intraoperative computer navigation parameters are poor predictors of function 1 year after total knee arthroplasty. J Arthroplasty 28(1):56–61
Acknowledgments
This study was supported financially by Zimmer Inc. and the Sydney Orthopaedic Research Institute. The authors would like to acknowledge Dr Joe Costa and Dr Jean Christian Balestro for their help with data collection. They would also like to acknowledge Mrs Amy Brierley and Mr Joe Lynch for their help with patient recruitment and data management.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scholes, C., Sahni, V., Lustig, S. et al. Patient-specific instrumentation for total knee arthroplasty does not match the pre-operative plan as assessed by intra-operative computer-assisted navigation. Knee Surg Sports Traumatol Arthrosc 22, 660–665 (2014). https://doi.org/10.1007/s00167-013-2670-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-013-2670-1