Abstract
Purpose
To determine in vivo biomechanical properties of articular cartilage and cartilage repair tissue of the patella, using biochemical MRI by means of quantitative T2 mapping.
Methods
Twenty MR scans were achieved at 3T MRI, using a new 8-channel multi-function coil allowing controlled bending of the knee. Multi-echo spin-echo T2 mapping was prepared in healthy volunteers and in age- and sex-matched patients after matrix-associated autologous chondrocyte transplantation (MACT) of the patella. MRI was performed at 0° and 45° of flexion of the knee after 0 min and after 1 h. A semi-automatic region-of-interest analysis was performed for the whole patella cartilage. To allow stratification with regard to the anatomical (collagen) structure, further subregional analysis was carried out (deep–middle–superficial cartilage layer). Statistical analysis of variance was performed.
Results
During 0° flexion (decompression), full-thickness T2 values showed no significant difference between volunteers (43 ms) and patients (41 ms). Stratification was more pronounced for healthy cartilage compared to cartilage repair tissue. During 45° flexion (compression), full-thickness T2 values within volunteers were significantly increased (54 ms) compared to patients (44 ms) (p < 0.001). Again, stratification was more pronounced in volunteers compared to patients. The volunteer group showed no significant increase in T2 values measured in straight position and in bended position. There was no significant difference between the 0- and the 60-min MRI examination. T2 values in the patient group increased between the 0- and the 60-min examination. However, the increase was only significant in the superior cartilage layer of the straight position (p = 0.021).
Conclusion
During compression (at 45° flexion), healthy patellar cartilage showed a significant increase in T2-values, indicating adaptations of water content and collagen fibril orientation to mechanical load. This could not be observed within the patella cartilage after cartilage repair (MACT) of the patella, most obvious due to a lack of biomechanical adjustment.
Level of evidence
III.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human articular cartilage contributes to the physiological function of joints. Articular cartilage lesions are a common pathology, particularly in the knee (joint). The spontaneous repair capacity of hyaline cartilage is limited. In general, there is no complete regeneration of hyaline cartilage, but rather the development of repair tissue with potentially inferior properties compared to hyaline cartilage [16]. Consequently, there is a higher risk of developing osteoarthritis (OA) in the injured joint [10]. The ability to detect this usually progressive pathology of the joint at an early stage is important for therapy planning. In addition, a high-quality follow-up visualization is important after cartilage repair procedures. The advantage of MRI is the opportunity to assess and evaluate cartilage non-invasively. In contrast to arthroscopic biopsies, MRI does not have the associated surgical morbidity [32].
Thus, non-invasive conventional magnetic resonance imaging is the method of choice for the evaluation of articular cartilage in the knee. It has also been shown to be sensitive for the detection of morphologic changes in cartilage repair zones [1, 24, 30]. In addition to the morphological aspects, an assessment of the biochemical characteristics of cartilage is possible with MRI [7, 18, 20]. The biomechanical properties of cartilage have their origin in the composition of the extracellular matrix, which contains about 75 % water, about 20 % collagen, and about 5 % proteoglycan aggregates [23].
Cartilage can be divided into different layers according to the orientation of collagen within a three-dimensional network. The superficial zone is characterized by flattened chondrocytes, low quantities of proteoglycan, and high quantities of collagen fibrils. Those fibrils are arranged parallel to the articular surface. In the middle zone, the chondrocytes are round and embedded in a random arrangement of the collagen fibres. The proteoglycan quantity is high. The deep zone shows a characteristic low cell density, thick collagen fibrils perpendicular to the bone, and columns of chondrocytes. The layer under the deep zone is partly mineralized and connects cartilage and subchondral bone [11].
One of the most important treatment options in cartilage repair is matrix-associated autologous chondrocyte transplantation (MACT) [18, 19]. It has been shown in 12- and 24-month follow-up arthroscopic evaluations after MACT that the repair tissue can be described as hyaline or “hyaline-like” [8].
Nevertheless, in the clinical follow-up, MACT of the patella shows inferior results compared to the femoral condyles [4, 5]. One reason for the less favourable results of cartilage repair in the patellofemoral joint are differences in contact pressure due to movement, where flexion of the knee increases the pressure and the patellofemoral force [12].
The aim of this study was to determine the in vivo biomechanical/biochemical properties of articular cartilage and cartilage repair tissue of the patella using kinematic quantitative MR imaging. Our study design was based on the hypothesis that cartilage after MACT should ideally have the same biochemical and biomechanical properties as hyaline cartilage. Differences in cartilage contact areas reflected by changes in T2 in the patella cartilage in patients after MACT of the patella were compared to those parameters in healthy volunteers (as a possible clinical marker of the overall status of transplant maturation).
T2 mapping after MACT could become a clinically relevant parameter for the prognosis of clinical outcomes after cartilage transplantation and hence could give early information about transplant maturation or failure.
Materials and methods
Inclusion criteria for the volunteer group were no history of severe trauma of the knee or surgery of the lower extremity. For inclusion into the study, the patients also had to have a single, symptomatic, full-thickness cartilage defect treated with MACT. MACT is a two-step surgical approach for the treatment of middle to large full-thickness cartilage defects using a hyaluronan-based scaffold (Hyalograft®C, Fidia Advanced Biopolymers, Abano Terme, Italy). The defects were located on the patella. Exclusion criterion for the patient group was a defect size at the time of MACT surgery <4 cm2, as one aim of this study was to include patients with a large area of possibly altered cartilage in the patella.
Both the volunteer and the patient group consisted of five subjects each. The mean age of the volunteers was 24.8 ± 2.2, all are males; the patients’ mean age was 34.3 ± 4.2 years, with four males and one female. Exclusion criterion for both groups was a BMI >30 kg/m2.
Twenty magnetic resonance scans were performed on a 3T whole-body system (Trio, Siemens Healthcare, Erlangen, Germany). An eight-channel multi-function coil (NORAS, Würzburg, Germany) was used in order to allow controlled bending of the knee.
Two examinations were performed in each of the five patients and each of the five volunteers, as follows:
Multi-echo spin-echo T2 was obtained at 0° and 45° of flexion on two consecutive days in healthy volunteers and patients after matrix-associated autologous chondrocyte transplantation of the patella.
On one day, the first T2 scanning/measurement series was performed after 0 min with a flexion of 0°. The second measurement on day one, with the knee straight, began after 60 min.
The other day started similarly with a series of T2 measurements while the knee was flexed to 45° and measured after 0 and after 60 min.
The approximately 1-h measurement session was performed at the same time of the day and after a comparable amount of activity before the measurements.
The patients and volunteers had to stand and walk before the examination. Two of five patients and volunteers, respectively, began the scanning with the knee straight, and half of each group began with the flexed position and switched to the other position the following day in order to avoid a bias because of the order of measurements.
The extended knee (flexion of 0°) was seen as the model of full decompression of the patellofemoral cartilage. Flexion of the knee to 45° simulated the application of pressure to the patellar cartilage.
With each examination, the quantitative T2 measurements were performed at 0 and after 60 min to assess the unloading during MRI. Studies that have reported using this approach show that unloading is a sensitive measurement for cartilage alteration based on the biomechanical status [2, 17].
Magnetic resonance imaging
The protocol for both groups was identical. For T2 relaxation, an axial multi-echo gradient echo was obtained. The repetition time (TR) was set as 2.060 s. The echo times (TE) were 16.4, 32.8, 49.2, 65.6, 82.0, and 96.4 ms. Field of view (FOV) was 180 × 200 mm, with a 320 × 288 matrix size. A bandwidth (BW) of 130 Hz/pixel was used, and the slice thickness was 1 mm. The flip angle was 180°. The total acquisition time was 6:43 min.
To assess the morphological condition of the patella, the image protocol consisted of the following parameters for a morphological 3D-DESS sequence:
TR was 15.1 ms, and echo time (TE) was 5.11 ms. A flip angle (FA) of 25° was used. The field of view (FOV) was 150 × 150 mm, and the matrix size was 250 × 250. The voxel size was 0.6 × 0.6 × 0.6 mm, with a total scan time of 5:39 min.
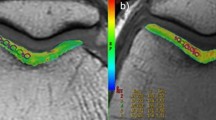
With respect to the main magnetic field, for the extended knee (0°), the orientation of the MR slices (slab) was about 90° (relative to the magnetic field), whereas for the image acquisition of the 45° bent knee, the slab was near a possible magic angle of about 55° (relative to the main magnetic field), with respect to the quantitative T2 mapping examination (see Fig. 1).
The two images (a, b) show the relative angle to the main magnetic field of the slab (white stack of slices, perpendicular to the patella cartilage). a The angle of the slices towards the main magnetic field is 90° in the straight knee. b In the bent knee (45°), the slices have an angle of about 55° relative to the main magnetic field and thus near to the possible magic angle, important for the T2 relaxation time mapping
Image analysis
T2 maps were obtained in-line by a pixel-wise, monoexponential, non-negative least squares (NNLS) fit analysis using the built-in MapIt software (Siemens Medical Solutions, Erlangen, Germany). Regions of interest (ROIs) were drawn manually by an experienced senior musculoskeletal radiologist, in consensus with an orthopaedic surgeon with a special interest in musculoskeletal MRI. Since we selected only MACT patients with an initial cartilage defect >4 cm2, and thus the size of the implants covered almost the whole surface of the patella, it was not possible to segment a region of morphologically normal-appearing cartilage within the same anatomical region as the control cartilage. The ROIs divided the full thickness of the cartilage repair tissue and the healthy cartilage into equal-sized deep, mid, and superficial layers in the central part of the patella. The region of interest was defined on three consecutive slices covering the implant.
Clinical outcomes
Clinical outcomes for each patient were assessed using the Lysholm score [29], a knee scoring system that includes eight sub-criteria, three of which were functional and five of which were subjective. With a total of 100 possible points that can be achieved, the Lysholm knee scale is a condition-specific outcome measurement tool validated for chondral disorders of the knee [14]. Clinical testing was performed preoperatively and on the same day as the MRI examination.
Ethical approval for this study was granted by the local ethics commission [Medical University of Vienna (IRB approval 627/2007)], and written informed consent was obtained from all volunteers and patients before enrolment into the study.
Statistical analysis
Continuous variables are given as mean ± standard deviation. Statistical tests were used to perform the data analyses. Quantitative evaluation was accomplished using a one-way ANOVA with random factors. SPSS version 16.0 (SPSS Institute, Chicago, IL, USA) was used. Differences with a p value <0.05 were considered statistically significant. For reproducibility measurements, the intraclass correlation coefficient (ICC) was analysed.
Results
Mean T2 values for all zones and the average values for all three zones were evaluated. Differences between layers and dependence on the degree of flexion were compared.
Stratification
Three zones were determined in each region of interest. Comparing the zones within one ROI, the notable stratification in T2 values in the cartilage layer was more pronounced in the healthy cartilage (deep = 35, middle = 42, superficial = 52) compared to cartilage repair tissue (deep = 36, middle = 39, superficial = 44).
Stratification during flexion was more pronounced in volunteers (48; 55; 59 ms) compared to patients (42; 43; 47 ms), as well. This distribution was comparable at 0 and after 60 min of unloading the knee during the MR examination. Examples of T2 mapping images of a volunteer and a patient are shown in Figs. 2 and 3. Additionally, T2 maps (0 min) of all patients and volunteers are added in Figs. 4 and 5.
A set of images of a volunteer measurement is presented. T2 mapping of the patellar cartilage of the straight (upper line) and the bent knee (bottom line). a PD-weighted TSE sequence (morphological imaging) b T2 map, first measurement right at the beginning of the MR measurement and c T2 map, second measurement after 60 min of rest
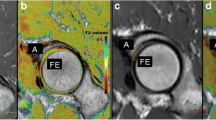
A set of images of a patient measurement is presented. T2 mapping of the patellar cartilage of the straight (upper line) and the bent knee (bottom line). The area of MACT is marked with arrows. a PD-weighted TSE sequence (morphological imaging), b T2 map, first measurement right at the beginning of the MR measurement and c T2 map, second measurement after 60 min of rest
Volunteers
All results from the volunteer group are provided in Table 1. In the volunteer group, for both the measurement with the knee in a straight position (flexion 0°) and the measurement in the bended position (flexion 45°), there was no significant increase between the 0- and the 60-min MRI examination (n.s.).
Patients
All results from the patient group are presented in Table 2. In the patient group, there was an increase in T2 values between the 0- and the 60-min MRI examinations in all assessed areas. However, this increase was only significant in the superior cartilage layer of the straight (flexion 0°) position (p = 0.021).
When comparing the straight (flexion 0°) position to the bended position (flexion 45°) of the knee joint, there was no significant increase in the T2 values for the middle and the superior cartilage layer, or for the full-thickness cartilage measurement (n.s.). There was no significant increase in T2 values during flexion in the deep cartilage layer (n.s.).
The post-operative MOCART score was 79.0 ± 14.3 points. The preoperative Lysholm score for the patient group was 51.0 ± 6.8. The clinical results post-operatively showed significant better results with a Lysholm score of 78.8 ± 10.4 points (p < 0.001).
To validate the reliability of our measurements, we analysed the plausibility of the results measured by the ICC. The analysis of reliability of our T2 mapping results showed an intraclass correlation coefficient between 0.923 and 0.955 for the volunteers and between 0.905 and 0.951 for the patients.
Discussion
The most important finding of the present study was that MACT cartilage of the patella seems to have lower adaption abilities towards pressure than healthy cartilage.
In this study, differences in the patellar cartilage during flexion could be detected in healthy joints compared to patients after MACT of the patella.
Flexion of the knee is seen to increase contact pressure and the patellofemoral force [12]. In most studies, contact areas have been measured in vitro. Salsich et al. [26], however, assessed the contact areas of the patellofemoral joint in vivo using MRI. Rubenstein et al. [25] examined the effects of compression on (bovine) articular cartilage and the associated appearance on MR images. According to their findings, the varying appearance and the signal intensity characteristics of cartilage under pressure may result from a combination of net water loss and alteration in collagen fibre arrangement.
Magnetic resonance T2 relaxation times can detect spatial variation in cartilage depth, probably due to the fact that T2 reflects the content and distribution of interstitial water and the solid matrix [21]. In healthy cartilage, T2 values increase from the deep to the superficial layers. This observation is based on the anisotropy of collagen fibres running perpendicular to cortical bone in the deep zone of cartilage [28]. The zonal increase in T2 values has been proven to indicate the presence of hyaline or hyaline-like cartilage after cartilage repair procedures in the knee [31, 34]. The combination of morphological and biochemical MRI after cartilage repair promises to detect the constitution of the repair tissue [33].
In the present study, we could demonstrate that in the volunteer group as well as in the patient group after MACT, T2 values increase from the deep to the superficial layers whether the knee was straight or bent. Thus, the findings for the T2 values of cartilage without the application of pressure were seen to be congruent with previous study results.
The effect of pressure on cartilage has been a subject of controversy in the literature. Mosher et al. [22] assessed T2 in the femorotibial cartilage of healthy volunteers before and after 30 min of running. They found a significant decrease in the T2 of the superficial zone of the femoral cartilage after running. One group evaluated the changes in the patellar cartilage after knee bends and during the subsequent recovery time [15]. During 45 min of rest after the knee bends, T2 values increased, as did cartilage thickness. Our findings of increasing T2 values under the application of pressure onto the patella of healthy subjects were seen—to a certain extent—consistent with these results. The increased T2 values of the second measurement after 45 min might conform to a state of relaxation in the cartilage.
Different types of loading and unloading result in different types of deformation of the patellar surface [9]. In general, if pressure is applied perpendicularly on the cartilage surface for a short time, collagen fibres in the radial region will be deformed under different angles. These changes lead to increased T2 values.
This might be one explanation for our results of increasing T2 values after loading in contrast to the decreased T2 values of other studies after longer or repeated loading. While high loading forces squeeze water out of the cartilage (T2 decrease) and regeneration leads to reabsorption of water (T2 increase), a light loading pressure might cause (in addition to the changes in collagen fibre orientation) an increase in the water content, as well.
The thinner the cartilage layer, particularly in the superficial zone, the less change in fibre orientation occurs due to loading, and T2 values do not change substantially. This might be one of the reasons for the lack of T2 changes, especially in the superficial zones of repair cartilage. In cartilage implants, the collagen orientation is, for the most part, not as regular and perpendicular to the bone zone as native cartilage. This may explain why the effect of loading on the T2 changes in our patients was lower than in the healthy subjects.
Hence, the implanted cartilage (MACT) seems to have less ability to adapt to changes in loading than the healthy cartilage, which is possibly the most important finding of this study.
Quantitative T2 mapping has been assessed with regard to the potential to serve as a non-invasive tool for the differentiation of cartilage repair tissue after surgical treatments such as MACT and microfracture therapy (MFX). After MACT, the mean T2 values showed no significant difference compared to normal controls without loading [32]. This implies that the structure of cartilage after MACT is very close to that of normal hyaline cartilage. Still, our findings could imply that the complete biomechanical properties of hyaline cartilage are not present in the patellar cartilage after MACT, based on the reported inability of the repair tissue to adapt to pressure.
The MACT cartilage in this study does not have the same degree of adaptation abilities as healthy cartilage. This conclusion has been underlined by the findings of Jones et al. [13]. They showed, in an animal model, that the hyaline-like cartilage after MACT showed a significantly decreased stiffness compared to healthy cartilage in the compression experiment.
While global T2 times and histological findings have confirmed the presence of hyaline-like properties in cartilage after MACT, the mechanical properties seem to differ, at least during the first years after transplantation. Further long-term studies on this topic are needed to investigate outcomes, as well as the possible improvement of the mechanical properties of the graft after longer follow-ups. Battaglia et al. reported in a 5-year follow-up after autologous chondrocyte implantation in the talus that the clinical and morphological results improved over time. Further follow-up studies will be needed to evaluate whether the biomechanical properties of transplanted cartilage improve [3].
The used model of pressure required the flexion of the knee in order to develop pressure on the patellar cartilage. A possible side effect of the change in position of the articular surface towards the scanner might be the development of a so-called magic angle, and thus, a possible increase in our T2 values. Shiomi et al. [27] described a significant increase in T2 values after bending of the knee in an animal model due to the effect of the magic angle. The orientation of fibres is crucial in determining the magic angle effect, which manifests as an increase in signal intensity of tendons, ligaments, and cartilage at 55°, compared to the static magnetic field (B0) [6]. Although the MR measurements of 45° knee flexion in this study might provoke a magic angle effect, we still see clear differences between the repair tissue (patients) and the native cartilage (controls).
The limitations of the present study are a relatively low number of patients and the varying points of time during follow-up. However, we performed 20 MR measurements in these patients and volunteers, and this initial study should be viewed as a proof of principle. In addition, the lack of histological correlation with the MR findings should be mentioned. Nevertheless, all patients had a good clinical outcome at the time of enrolment into this study.
Due to the fact that the repair sites almost covered the whole cartilage surface of the patella, it was not possible to define a region of morphologically normal-appearing cartilage within the same anatomical region. Therefore, the comparison between healthy cartilage and cartilage after MACT was only possible between subjects (patients vs. controls), and not within the same subject. This can be seen as another limitation of our approach. The lower T2 changes in the cartilage under pressure after MACT provided indirect information about the possible lack of adaptive capability towards load stress. However, we did not biomechanically prove this directly with additional tests. This could be a topic for further investigations
Conclusion
During compression (at 45°-flexion), healthy patellar cartilage showed a significant increase in T2 values, indicating adaptations of water content and collagen fibril orientation near a possible magic angle of 55°. This could not be observed within cartilage repair tissue, due to an obvious lack of biomechanical adjustment. This initial study is, to our knowledge, the first in vivo approach designed to understand the biomechanical properties of cartilage repair tissue and possibly predict the limited long-term outcome of this surgical treatment, especially in the patellofemoral joint.
References
Alparslan L, Winalski CS, Boutin RD et al (2001) Postoperative magnetic resonance imaging of articular cartilage repair. Semin Musculoskelet Radiol 5(4):345–363
Apprich S, Mamisch TC, Welsch GH et al (2011) Quantitative T2 mapping of the patella at 3.0T is sensitive to early cartilage degeneration, but also to loading of the knee. Eur J Radiol 81(4):e438–e443
Battaglia M, Vannini F, Buda R et al (2011) Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: mid-term T2-mapping MRI evaluation. Knee Surg Sports Traumatol Arthrosc 19(8):1376–1384
Brittberg M, Faxen E, Peterson L (1994) Carbon fiber scaffolds in the treatment of early knee osteoarthritis. A prospective 4-year followup of 37 patients. Clin Orthop Relat Res 307:155–164
Brittberg M, Lindahl A, Nilsson A et al (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331(14):889–895
Bydder M, Rahal A, Fullerton GD et al (2007) The magic angle effect: a source of artifact, determinant of image contrast, and technique for imaging. J Magn Reson Imaging 25(2):290–300
Chung CB, Frank LR, Resnick D (2001) Cartilage imaging techniques: current clinical applications and state of the art imaging. Clin Orthop Relat Res 391(Suppl):S370–S378
D’Anchise R, Manta N, Prospero E et al (2005) Autologous implantation of chondrocytes on a solid collagen scaffold: clinical and histological outcomes after two years of follow-up. J Orthopaed Traumatol 6:36–43
Eckstein F, Lemberger B, Gratzke C et al (2005) In vivo cartilage deformation after different types of activity and its dependence on physical training status. Ann Rheum Dis 64(2):291–295
Felson DT (2004) Risk factors for osteoarthritis: understanding joint vulnerability. Clin Orthop Relat Res 427(Suppl):S16–S21
Goodwin DW, Zhu H, Dunn JF (2000) In vitro MR imaging of hyaline cartilage: correlation with scanning electron microscopy. Am J Roentgenol (AJR) 174(2):405–409
Huberti HH, Hayes WC (1984) Patellofemoral contact pressures. The influence of q-angle and tendofemoral contact. J Bone Joint Surg Am 66(5):715–724
Jones CW, Willers C, Keogh A et al (2008) Matrix-induced autologous chondrocyte implantation in sheep: objective assessments including confocal arthroscopy. J Orthop Res 26(3):292–303
Kocher MS, Steadman JR, Briggs KK et al (2004) Reliability, validity, and responsiveness of the Lysholm knee scale for various chondral disorders of the knee. J Bone Joint Surg Am 86-A(6):1139–1145
Liess C, Lusse S, Karger N et al (2002) Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthr Cartil 10(12):907–913
Madry H, van Dijk CN, Mueller-Gerbl M (2010) The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc 18(4):419–433
Mamisch TC, Trattnig S, Quirbach S et al (2010) Quantitative T2 mapping of knee cartilage: differentiation of healthy control cartilage and cartilage repair tissue in the knee with unloading—initial results. Radiology 254(3):818–826
Marcacci M, Berruto M, Brocchetta D et al (2005) Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res 435:96–105
Marlovits S, Kutscha-Lissberg F, Aldrian S et al (2004) Autologous chondrocyte transplantation for the treatment of articular cartilage defects in the knee joint. Techniques and results. Radiologe 44(8):763–772
Marlovits S, Trattnig S (2006) Cartilage repair. Eur J Radiol 57(1):1–2
Mosher TJ, Dardzinski BJ, Smith MB (2000) Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2-preliminary findings at 3 T. Radiology 214(1):259–266
Mosher TJ, Smith HE, Collins C et al (2005) Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology 234(1):245–249
Poole AR, Kojima T, Yasuda T et al (2001) Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res 391(Suppl):S26–S33
Recht M, White LM, Winalski CS et al (2003) MR imaging of cartilage repair procedures. Skeletal Radiol 32(4):185–200
Rubenstein JD, Kim JK, Henkelman RM (1996) Effects of compression and recovery on bovine articular cartilage: appearance on MR images. Radiology 201(3):843–850
Salsich GB, Ward SR, Terk MR et al (2003) In vivo assessment of patellofemoral joint contact area in individuals who are pain free. Clin Orthop Relat Res 417:277–284
Shiomi T, Nishii T, Myoui A et al (2010) Influence of knee positions on T2, T*2, and dGEMRIC mapping in porcine knee cartilage. Magn Reson Med 64(3):707–714
Smith HE, Mosher TJ, Dardzinski BJ et al (2001) Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging 14(1):50–55
Tegner Y, Lysholm J (1985) Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 198:43–49
Trattnig S, Ba-Ssalamah A, Pinker K et al (2005) Matrix-based autologous chondrocyte implantation for cartilage repair: noninvasive monitoring by high-resolution magnetic resonance imaging. Magn Reson Imaging 23(7):779–787
Watrin-Pinzano A, Ruaud JP, Cheli Y et al (2004) Evaluation of cartilage repair tissue after biomaterial implantation in rat patella by using T2 mapping. MAGMA 17(3–6):219–228
Welsch GH, Mamisch TC, Domayer SE et al (2008) Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures—initial experience. Radiology 247(1):154–161
Welsch GH, Mamisch TC, Zak L et al (2010) Evaluation of cartilage repair tissue after matrix-associated autologous chondrocyte transplantation using a hyaluronic-based or a collagen-based scaffold with morphological MOCART scoring and biochemical T2 mapping: preliminary results. Am J Sports Med 38(5):934–942
White LM, Sussman MS, Hurtig M et al (2006) Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology 241(2):407–414
Conflict of interest
All authors disclose that there are no financial and/or personal relationships with other individuals or organizations that could inappropriately influence (bias) this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pachowsky, M.L., Trattnig, S., Wondrasch, B. et al. In vivo evaluation of biomechanical properties in the patellofemoral joint after matrix-associated autologous chondrocyte transplantation by means of quantitative T2 MRI. Knee Surg Sports Traumatol Arthrosc 22, 1360–1369 (2014). https://doi.org/10.1007/s00167-013-2527-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-013-2527-7