Abstract
Purpose
The posterolateral corner (PLC) is more likely to be injured in combination with the posterior cruciate ligament (PCL) or the anterior cruciate ligament than in isolation. This leads to instability of the knee and loss of function. We hypothesised that combined PCL and PLC reconstruction would restore sufficient stability to allow improvement in patient symptoms and function.
Methods
19 patients who underwent arthroscopic-assisted single-bundle PCL and PLC reconstruction by a single surgeon were analysed retrospectively. The PLC reconstruction was a modified Larson reconstruction of the lateral collateral ligament and the popliteofibular ligament.
The IKDC and Tegner scores were used to assess outcome. Dial test and varus laxity were used to assess improvements in clinical laxity. Posterior laxity was tested using the KT-1000.
Results
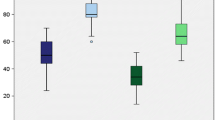
The mean follow-up was 38 months (±(2× standard deviations), ±12.3). There were no postoperative complications. All patients had less than 5 mm posterior step-off. 17 of 19 patients had negative dial and varus stress tests. Measured range of motion was reduced by a mean of 10°, but patients did not report any daily activities restrictions. Tegner scores improved from a median pre-operative value of 2 (range 1–4) to 6 (4–9) at final follow-up. The mean postoperative IKDC score was 86 (±11).
Conclusions
Subjectively, the knee stability achieved allowed daily activities. However, there were remaining abnormalities in range of motion, posterior drawer and rotational laxity, suggesting that normal knee laxity was not restored.
Level of evidence
IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isolated posterior lateral corner (PLC) injuries are rare, with reported rates of 2% [11] and 5% [30] in acute knee injury patients presenting to specialised clinics. It is more frequent for PLC injuries to be part of a multi-ligament injury pattern with the PLC injured with either the posterior cruciate ligament (PCL) or anterior cruciate ligament (ACL), with rates of approximately 10% in patients presenting with an acute post-traumatic knee haemarthrosis [30]. Isolated PCL injuries are usually well tolerated [48], probably due to the presence of secondary restraints to posterior translation [3, 18], and can be managed conservatively with satisfactory results [48, 52]. Combined injuries to the PLC and PCL lead to significant disability with increased laxity in valgus stress, external rotation and posterior drawer [1, 3, 16, 17]. Chronic injuries can lead to abnormal varus thrust gait [3, 11], significant changes in articular contact pressures at the medial and patellofemoral compartments [49], and progressive secondary osteoarthritis [46]. Surgical management for acute injuries has a better prognosis, but patients are often diagnosed at the chronic stage [11, 22, 30]. Anatomical and non-anatomical or isometric reconstruction techniques have been described for chronic combined PCL and PLC injuries, with variable results [13, 14, 26, 29, 33, 34, 38, 39, 47, 52, 53].
To help elucidate the optimum treatment for combined chronic PCL and PLC injuries, we report our results for combined reconstruction using an approximated isometric reconstruction of the popliteofibular ligament (PFL) and a single-bundle PCL reconstruction. This method of PLC reconstruction was used as it includes reconstruction of the single isometric part of the PLC [51]. The hypothesis was that this combined PCL and PLC reconstruction technique would reduce abnormal knee laxity and improve functional outcome.
Materials and methods
Between 2003 and 2007, we identified 23 consecutive patients with combined PCL and PLC injuries who underwent the index surgical reconstruction. Exclusion criteria included concomitant ACL rupture, varus thrust gait, malalignment compared to opposite normal knee on bilateral long-leg radiographs, common peroneal nerve lesion or significant arthritis. Osteoarthritis was assessed using the Kellgren–Lawrence grade, and patients with grade 3 or 4 were excluded [44]. This leads to the exclusion of 4 patients (3 with concomitant ACL rupture and 1 with significant osteoarthritis). Therefore, 19 patients underwent PCL and PLC reconstruction and were analysed retrospectively. All were available for long-term follow-up. There were 13 men and 6 women. The median age at surgery was 29 years (range 17–41 years), with a mean interval between the index injury and surgery of 11 months (±(2× standard deviations), ±9.3). The average follow-up was 38 months (±12.3). The mechanism of injury was road-traffic accidents (RTA) in 12 cases, soccer in 4 cases, skiing in 2 cases and 1 injury due to basketball. Approval was obtained from the local ethics committee, and all patients gave informed consent to be included in the study.
All patients underwent pre-operative evaluation with clinical examination, weight-bearing Roentogenograms, MR scans and arthroscopy. The posterior sag and posterior drawer tests at 90° were used to assess PCL integrity [3, 21, 22]. The posterior drawer test was graded using Clancy’s classification [8]. In all subjects, the posterior drawer was correctable. The posterolateral corner was assessed using the dial test in the supine position and the varus stress test. Finally, range of motion was recorded. All tests were compared with the normal contralateral knee. For the dial test, cadaveric data suggest that isolated PLC injuries lead to an average increase in external rotation of 13° at 30° flexion, with an increase of 21° at 90° flexion with combined PLC and PCL injuries [1, 16, 17]. The patella–tubercle angle (PTA) was used to measure knee external rotation throughout [2, 25]. The posteriorly displaced tibia was reduced to an anatomical position, prior to the dial test being undertaken. This increases tibial external rotation, reducing the possibility of misdiagnosing PCL–PLC injuries [25, 50]. The varus stress test was carried out at 0° (extension) and 30° knee flexion, corresponding to the role of the lateral collateral ligament (LCL) as the primary restraint to tibial adduction at these flexion angles [3, 16, 17]. The clinical examination was repeated under anaesthesia, at the initial arthroscopy and at reconstruction.
Weight-bearing Roentogenograms were used to exclude osteoarthritis and malalignment. The Roentogenograms were also used to exclude the presence of Segond fractures and the arcuate sign.
All subjects underwent pre-operative MR scans using standard images, supplemented with thin-slice coronal oblique T1-weighted images through the entire fibular head [27].
At arthroscopy, the PLC injury was confirmed by increased lateral opening and the ‘drive-through sign’ [27]. A normal ACL was confirmed and significant osteoarthritis excluded. Meniscal tears, if present, were resected. No subjects were suitable for meniscal repair.
All PCL and PLC reconstructions were performed by a single orthopaedic surgeon with a special interest in knee ligament injuries [CZ]. Intra-operative radiographic guidance was not used. The PCL was reconstructed using a tibialis anterior tendon allograft. The femoral tunnel was produced with an outside-in technique. The tibial tunnel exit was at the PCL tibial insertion site, no more than 15 mm below the articular surface. The tendon graft was whip-stitched at both ends with Fiberwire® (Arthrex Inc, Naples, Florida, USA). The tendon was doubled up, diameter measured and then passed from the tibial tunnel to the femoral tunnel. The graft was fixed using a bio-absorbable screw (BioRCI Smith & Nephew, Andover, MA). The PCL graft was fixed in the femoral tunnel. After reconstructing the PLC, the PCL tibial tunnel was fixed with the knee in 70° of flexion and in anterior drawer.
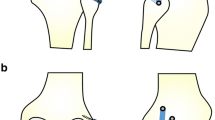
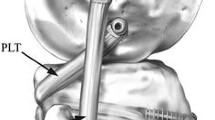
The PLC was reconstructed using a semitendinosus tendon allograft. An approximated isometric technique, based on the Larson PLC reconstruction [30], with a single femoral tunnel was performed in all patients. The tendon graft was whip-stitched at both ends with Fiberwire® (Arthrex Inc, Naples, Florida, USA) and sized. With the knee flexed at 90°, a straight-line incision was performed beginning from the posterior aspect of the lateral femoral epicondyle to the distal part of the fibular head. A 4 cm horizontal fascial incision was performed posterior to the fibular head, just anterior to and in line with the biceps tendon. The common peroneal nerve was identified and protected throughout. A guide wire was then passed from just anterior and distal to the insertion of the lateral collateral ligament (LCL) and directed proximally and medially to exit the posterior aspect of the fibula adjacent to the proximal tibio-fibular joint at the point of widest fibular head diameter [31]. A tunnel, corresponding to the diameter of the tendon graft, was drilled, and the graft passed through the tunnel. At this point, the lateral femoral epicondyle was identified, and a 4-cm iliotibial band incision was performed over this point. The LCL inserts to the posterior aspect of the lateral femoral condyle close to the epicondyle, proximal to the groove for the tendon of the popliteus [2, 16, 17]. Therefore, palpation of the epicondyle was used as an anatomical marker for the tunnel entry point. A guide wire was then introduced just anteriorly to the central origin of the LCL. The guide wire was slightly inclined from posterior to anterior and was directed proximo-medially to the medial epicondyle and adductor tubercle. A tunnel corresponding to the graft diameter was reamed to a depth of 35 mm. At this point, both ends of the graft were passed under the iliotibial band and then through the femoral tunnel. The graft was tensioned with the knee flexed at 30°, minimal internal rotation and in slight valgus. A greater amount of internal rotation was avoided to prevent problems with postoperative external–internal rotation. Graft fixation in the femoral tunnel was performed with a bio-absorbable screw (BioRCI-HA; Smith & Nephew, Andover, MA) one mm greater than the tunnel diameter. The posterior arm of the PLC reconstruction represented the popliteofibular ligament, and the anterior arm represented the lateral collateral ligament (Fig. 1).
Patients followed an identical rehabilitation programme with full weight bearing in a knee brace in extension for 2 weeks. Physical therapy was initially aimed at reducing swelling and reducing quadriceps atrophy by allowing straight leg raises and quadriceps exercises in the brace. Graduated range of motion exercises out of the brace was allowed aiming for minimum of 90° flexion prior to removal of the brace. Between weeks 2 and 6, range of motion was increased to try to achieve the range of the opposite normal knee. As range of motion and limb control improved, closed-chain kinetic exercises were introduced, but exercises that could compromise the graft, including varus and external rotation, were avoided. Once patients achieved full range of motion, and not before 8 weeks, graduated low-resistance exercises using a stationary bike were introduced. Open-chain kinetic exercises of the hamstrings were not allowed for the first 3 months. After 3 months, patients increased their endurance and strength training. Proprioception and core-strength exercises were also introduced. We aimed to allow patients to return to full sports activity, including pivoting sports, at the 9-month postoperative stage after assessment by an orthopaedic surgeon.
All patients were reviewed at regular intervals by an orthopaedic surgeon who had not been involved in the original assessment of the patients or the index operation. Clinical examination using the tests described above were repeated after patients had completed their rehabilitation and at each subsequent review. At the latest follow-up, patients were evaluated with the IKDC Subjective Evaluation Form [23] and weight-bearing radiographs to assess Kellgren–Lawrence scores. Additionally, KT-1000 measurements were taken using the posterior drawer at 90° flexion.
Results
There were no reported postoperative complications.
On examination, all 19 patients had posterior drawer step-off grade III and a positive dial and varus stress test pre-operatively. Postoperative examination revealed that 14 patients (14 of 19, 74%) had grade 0 to step-off, and the remaining 5 patients (5 of 19, 26%) had grade 1. In 17 patients (17 of 19, 89%), the dial test and varus stress test were negative, only 2 patients (2 of 19, 11%) had residual posterolateral laxity. Postoperative range of motion showed a mean reduction of 10° compared to the contralateral knee in flexion without causing any functional restriction, while the extension was symmetrical.
Tegner scores improved from a median pre-operative value of 2 (range 1–4) to 6 (4–9) at final follow-up. All patients with postoperative Clancy grade I exhibited KT-1000 measurements greater than 3 mm and IKDC scores lower than 80, except patient 3 who had an IKDC score of 86.
Assessment of the side-to-side differences in posterior drawer using the KT-1000 showed differences of 0–2 mm in 14 patients (14 of 19, 74%) and 3–5 mm in 5 patients (5 of 19, 26%). No patient showed differences greater than 5 mm. No patient showed a difference in anterior drawer greater than 3 mm using the KT-1000.
All patients had Kellgren–Lawrence grades 0–1 pre-operatively. At the last follow-up, radiographs revealed that 2 patients had grade 2 changes. These 2 patients did not show any significantly increased Tegner, IKDC or KT-1000 scores. Clinical examination also did not show increased laxity. However, both patients had undergone partial medial menisectomy at the time of reconstruction.
Discussion
The most important finding of the present study was that single-bundle PCL reconstruction with an isometric PLC reconstruction reduced abnormal knee laxity and improved functional outcome. Conservative management can be considered for isolated PCL injuries [1, 3, 12, 18, 34, 47]. However, the combination of a PLC and PCL injury produces a complex instability and loss of function of the knee [1, 3, 4, 13, 16, 17]. The absence of the PLC structures leads to abnormally high forces in PCL reconstructions [10, 19, 35, 37] and does not restore normal kinematics, particularly external rotation and varus [10, 37, 45]. The increased forces can lead to early failure of the PCL reconstruction [15, 20, 37]. Additionally, PLC and PCL sectioning leads to abnormally raised contact pressures in the medial compartment and the patellofemoral joint [49].
In this study, the PCL was reconstructed using a single-bundle approach and with the tunnels placed as close to the tibial and femoral attachments as possible. Cadaveric studies with isolated PCL tears have suggested better control of posterior laxity with double-bundle compared to single-bundle PCL reconstruction [20, 43], although there is not always a significant difference in posterior laxity using the single- and double-bundle techniques [6]. However, the femoral tunnel positions and tensioning protocols varied between all the studies. Biomechanical studies have suggested tibial tunnel PCL reconstructions can undergo elongation and thinning compared to tibial inlay techniques, due to the acute graft angle at the tibial insertion [7, 36]. This has led to the development of arthroscopic tibial inlay techniques, which have comparable results to open inlay techniques in terms of posterior translation and tibial external rotation in cadavers [54]. Using a tibial tunnel technique in this study, the KT-1000 measurements revealed side-to-side differences of 0–2 mm in 14 of 19 patients (74%) and 2–5 mm in 5 of 19 patients (26%). This compares to side-to-side differences of 1.8–2.1 mm by Fanelli and Edson [14] using a single-bundle PCL reconstruction. Significant improvements of posterior translation using a PCL reconstruction technique involving remnant PCL tensioning and single PCL anterolateral bundle reconstruction were reported as part of a combined PCL–PLC reconstruction [32], although the KT-1000 measurements were taken at 70° knee flexion. Posterior translation was assessed using the Clancy classification for posterior step-off, which allows clinical quantification and comparison with other reported series. In this series, 12 of 19 (63%) patients and 7 of 19 (37%) patients had grade 0 and 1 posterior step-off, respectively. This compares favourably to the results reported by Wajsfisz et al. [52] (16 of 21 patients (75%) grade 0 and 1), Fanelli and Edson [14] (29 of 41 (70%) grade 0, 11 of 41 (27%) grade 1) and Khanduja et al. [26] (7 of 19 (37%) grade 0, 11 of 19 (58%) grade 1). However, comparisons with the Clancy classification should be interpreted with caution, as it is not a validated assessment and may be prone to inter- and intra-observer errors. Overall, the posterior translation results suggest graft elongation had not occurred at latest follow-up. Taken together with the studies on isolated PCL injuries, it is difficult to extrapolate firm conclusions regarding the tibial tunnel and inlay reconstruction techniques in combined PLC and PLC injuries in the clinical setting.
PLC reconstructions can be broadly categorised into ‘anatomical’ or ‘isometric’ (or non-anatomical) reconstructions [5, 28, 29, 31]. The Larson reconstruction [31] is based on the observation that the fibular head is isometric to the femoral lateral condyle through 0–90° flexion. It is non-anatomical, as the reconstruction attaches to the lateral femoral condyle and does not reproduce the anatomical femoral insertion sites of the LCL and the PFL. A comparison of Larson’s reconstruction with biceps tenodesis and the popliteal bypass procedure [10] concluded that Larson’s procedure better restored posterior translation and external rotational laxity. LaPrade [29] described an ‘anatomical’ reconstruction of the main static stabilisers of the PLC, although the exact position of the PFL reconstruction has been questioned [5]. This reconstruction technique has been modified further to try to replicate the LCL, popliteus tendon and the popliteofibular ligament [28, 40]. After reviewing 57 failures of PLC reconstruction [39], it was suggested that the commonest cause was non-anatomical reconstructions. However, cadaveric studies have confirmed that the Larson reconstruction, in combination with a single-bundle PCL reconstruction, restores knee posterior translation, external rotation and varus laxity to normal [4, 5]. We used the semitendinosus tendon for reconstruction of the PLC to approximate the static isometric popliteofibular ligament portion of the PLC [51] and the LCL. The absence of postoperative complications would support the contention is sage and results in acceptable surgical morbidity. This contrasts to up to 7 of 19 patients (37%) requiring a further operation in a study using a modified Larson technique [26].
In cases of combined PCL–PLC injuries with an intact lateral collateral ligament (LCL), popliteofibular ligament reconstruction using tibialis anterior allograft and an approximated isometric technique combined with single-bundle tibial tunnel PCL reconstruction is reported to improve posterior translation, external rotation and the IKDC score [55]. The main reported advantage was that it was fixator-free, and hence less expensive. However, two diverging tunnels in the fibular head are used, increasing the risk of fractures in this cancellous bone and making the technique difficult in smaller knees, and an allograft is used [55]. Fractures of the patellar have been reported following medial patellofemoral ligament reconstruction using two diverging tunnel techniques [42]. PLC reconstruction using a modified Larson technique with a single-bundle hamstring ACL reconstruction for combined ACL and PLC injuries has been shown to improve subjective outcome, outcome scores and instrumented laxity tests [33]. Isolated PLC injuries are less common than combined injuries with the PCL or the anterior cruciate ligament [11]. Isolated anatomic-type reconstruction with separate LCL and PFL arms has been reported to show improved posterolateral rotatory laxity and IKDC scores [24]. An approximated isometric PLC repair in 10 patients with average follow-up of 27 months also showed improved outcome scores [9]. The use of either isolated technique in combined PCL–PLC injuries remains to be reported.
The combined PLC and PCL reconstructions did affect range of motion, with a 10° reduction in flexion and normal extension, which did not adversely affect the patient’s subjective assessment of their knee function. The external rotational laxity and varus stress laxity were corrected in 17 of 19 (89%) of patients. This compares favourably to reported residual posterolateral laxity in 8 of 21 (40%) patients using a Larson reconstruction, and a double-bundle PCL graft [52] and residual posterolateral laxity in 5 of 19 (26%) patients in another study using Larson reconstruction and a single-bundle PCL reconstruction [26]. This higher posterolateral laxity may be related to the increased cyclic loading in that study [26] from the longer follow-up time of 67 months compared to 38 months in the present study. In a larger series, residual posterolateral laxity was reported in only 1 of 41 patients (2%) [14]. However, this group included only 11 of 41 patients (27%) with normal residual laxity, and 29 of 41 (71%) patients who had tighter than normal knees on the dial test. The increased tightening may have resulted from the biceps tenodesis and posterolateral capsular shift procedures used for PLC reconstruction [8]. This, while improving stability, has the risk of over-tightening the posterolateral corner and may have contributed to the observed degenerative changes in 29 of 41 (71%) patients [14, 26].
Stress radiographs, with or without instrumentation, can be used to objectively quantify PCL deficiency [12, 51]. Kneeling view radiographs have been described as a method of measuring posterior knee laxity [41], but not rotational laxity. External rotational laxity can be measured clinically in healthy volunteers using a novel instrumented device [1]. The difficulty in utilising stress radiographs using instrumented devices for posterior translation is that these have not yet been validated in combined PCL and PLC deficiencies. This is particularly important as the combined injury produces complex laxities, while stress radiographs only measure anterior–posterior laxity. We would, therefore, be cautious in interpreting data from these methods. The instrumented device for measuring external rotational laxity was not available [1].
A limitation of this study was the lack of pre-operative IKDC Subjective Knee Form Scores to allow a comparison for postoperative outcome. Although the final follow-up IKDC score in this study averaged 86, indicating satisfactory knee function, the effect of surgery could not be quantified. In future, we aim to utilise both pre- and postoperative IKDC scores to allow a validated system to reflect patient’s subjective perception.
Another limitation was the number of patients which, although comparable to other similar studies [24, 26, 55], limited the power of our study. Studies of combined PCL and PLC reconstruction [14, 32] with larger sample sizes have been reported. Although we used the Kellgren–Lawrence scoring system to grade radiographically osteoarthritis, we do acknowledge that, although widely used in clinical practice and in research articles, this scoring system does have problems with both its definition and reliability [44]. The effect of combined PCL and PLC reconstruction on the development of subsequent osteoarthritis, malalignment and gait abnormalities would require studies with longer term follow-up, preferably with a control group and a larger sample size.
Conclusion
This study confirms the hypothesis with patients showing improvements in subjective and objective criteria after single-bundle PCL reconstruction with an isometric PLC reconstruction. From a clinical viewpoint, this technique and rehabilitation programme may reduce surgical morbidity and allow improvements in function.
References
Alam M (2009) Development of a clinical device for measuring rotational laxity of the knee. Master of Science Thesis, Imperial College London, University of London, pp 219–239
Alam M, Bull AM, Thomas RD, Amis AA (2011) Measurement of rotational laxity of the knee: in vitro comparison of accuracy between the tibia, overlying skin and foot. Am J Sports Med. doi:10.1177/0363546511424872
Amis AA, Bull AM, Gupte CM, Hijazi I, Race A, Robinson JR (2003) Biomechanics of the PCL and related structures: posterolateral, posteromedial and meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc 11:271–281
Apsingi S, Nguyen T, Bull AM, Unwin A, Deehan DJ, Amis AA (2008) Control of laxity in knees with combined posterior cruciate ligament and posterolateral corner deficiency: comparison of single-bundle versus double-bundle posterior cruciate ligament reconstruction combined with modified Larson posterolateral corner reconstruction. Am J Sports Med 36:487–494
Apsingi S, Nguyen T, Bull AM, Unwin A, Deehan DJ, Amis AA (2009) A comparison of modified Larson and ‘anatomic’ posterolateral corner reconstructions in knees with combined PCL and posterolateral corner deficiency. Knee Surg Sports Traumatol Arthrosc 17:305–312
Bergfeld JA, Graham SM, Parker RD, Valdevit AD, Kambic HE (2005) A biomechanical comparison of posterior cruciate ligament reconstructions using single- and double-bundle tibial inlay techniques. Am J Sports Med 33:976–981
Bergfeld JA, McAllister DR, Parker RD, Valdevit AD, Kambic HE (2001) A biomechanical comparison of posterior cruciate ligament reconstruction techniques. Am J Sports Med 29:129–136
Bisson LJ, Clancy WG (2001) Isolated posterior cruciate ligament injury and posterolateral laxity. In: Chapman M (ed) Chapman’s orthopaedic surgery, 3rd edn. Lippincott Williams & Wilkins, Philadelphia, pp 2393–2416
Camarda L, Condello V, Madonna V, Cortese F, D’Arienzo M, Zorzi C (2010) Results of isolated posterolateral corner reconstruction. J Orthop Traumatol 11:73–79
Cooley VJ, Harrington RM, Larson RV (1996) Effect of lateral ligament reconstruction on intra-articular posterior cruciate ligament graft force and knee motion. University of Washington research report, pp 37–41
DeLee JC, Riley MB, Rockwood CA Jr (1983) Acute posterolateral rotatory instability of the knee. Am J Sports Med 11:199–207
Dowd GS (2004) Reconstruction of the posterior cruciate ligament. Indications and results. J Bone Joint Surg [Br] 86:480–491
Fanelli GC (2006) Surgical treatment of lateral posterolateral instability of the knee using biceps tendon procedures. Sports Med Arthrosc 14:37–43
Fanelli GC, Edson CJ (2004) Combined posterior cruciate ligament-posterolateral reconstructions with Achilles tendon allograft and biceps femoris tendon tenodesis: 2- to 10-year follow-up. Arthroscopy 20:339–345
Freeman RT, Duri ZA, Dowd GS (2002) Combined chronic posterior cruciate and posterolateral corner ligamentous injuries: a comparison of posterior cruciate ligament reconstruction with and without reconstruction of the posterolateral corner. Knee 9:309–312
Gollehon DL, Torzilli PA, Warren RF (1987) The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J Bone Joint Surg [Am] 69:233–242
Grood ES, Stowers SF, Noyes FR (1988) Limits of movement in the human knee. Effect of sectioning the posterior cruciate ligament and posterolateral structures. J Bone Joint Surg [Am] 70:88–97
Gupte CM, Bull AM, Thomas RD, Amis AA (2003) The meniscofemoral ligaments: secondary restraints to the posterior drawer. Analysis of anteroposterior and rotary laxity in the intact and posterior-cruciate-deficient knee. J Bone Joint Surg [Br] 85:765–773
Harner CD, Höher J, Vogrin TM, Carlin GJ, Woo SL (1998) The effects of a popliteus muscle load on in situ forces in the posterior cruciate ligament and on knee kinematics. A human cadaveric study. Am J Sports Med 26:669–673
Harner CD, Janaushek MA, Kanamori A, Yagi M, Vogrin TM, Woo SL (2000) Biomechanical analysis of a double-bundle posterior cruciate ligament reconstruction. Am J Sports Med 28:144–151
Hughston JC, Andrews JR, Cross MJ, Moschi A (1976) Classification of knee ligament instabilities. Part I. The medial compartment and cruciate ligaments. J Bone Joint Surg [Am] 58:159–172
Hughston JC, Jacobson KE (1985) Chronic posterolateral rotatory instability of the knee. J Bone Joint Surg [Am] 67:351–359
Irrgang JJ, Anderson AF, Boland AL (2001) Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med 29:600–613
Jakobsen BW, Lund B, Christiansen SE, Lind MC (2010) Anatomic reconstruction of the posterolateral corner of the knee: a case series with isolated reconstructions in 27 patients. Arthroscopy 26:918–925
Jung YB, Lee YS, Jung HJ, Nam CH (2009) Evaluation of posterolateral rotatory knee instability using the dial test according to tibial positioning. Arthroscopy 25:257–261
Khanduja V, Somayaji HS, Harnett P, Utukuri M, Dowd GS (2006) Combined reconstruction of chronic posterior cruciate ligament and posterolateral corner deficiency. A two- to nine-year follow-up study. J Bone Joint Surg [Br] 88:1169–1172
LaPrade RF, Gilbert TJ, Bollom TS, Wentorf F, Chaljub G (2000) The magnetic resonance imaging appearance of individual structures of the posterolateral knee. A prospective study of normal knees and knees with surgically verified grade III injuries. Am J Sports Med 28:191–199
LaPrade RF, Johansen S, Agel J, Risberg MA, Moksnes H, Engebretsen L (2010) Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg [Am] 92:16–22
LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A (2004) An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med 32:1405–1414
LaPrade RF, Wentorf FA, Fritts H, Gundry C, Hightower CD (2007) A prospective magnetic resonance imaging study of the incidence of posterolateral and multiple ligament injuries in acute knee injuries presenting with a hemarthrosis. Arthroscopy 23:1341–1347
Larson RV, Sidles JA, Beals CT (1996) Isometry of lateral collateral and popliteofibular ligaments and a technique for reconstruction. University of Washington research report, pp 42–44
Lee KH, Jung YB, Jung HJ, Jang EC, Song KS, Kim JY, Lee SH (2011) Combined posterolateral corner reconstruction with remnant tensioning and augmentation in chronic posterior cruciate ligament injuries: minimum 2-year follow-up. Arthroscopy 27:507–515
Lee SH, Jung YB, Jung HJ, Song KS, Ko YB (2010) Combined reconstruction for posterolateral rotatory instability with anterior cruciate ligament injuries of the knee. Knee Surg Sports Traumatol Arthrosc 18:1219–1225
Mariani PP, Becker R, Rihn J, Margheritini F (2003) Surgical treatment of posterior cruciate ligament and posterolateral corner injuries. An anatomical, biomechanical and clinical review. Knee 10:311–324
Markolf KL, Graves BR, Sigward SM, Jackson SR, McAllister DR (2007) Effects of posterolateral reconstructions on external tibial rotation and forces in a posterior cruciate ligament graft. J Bone Joint Surg [Am] 89:2351–2358
Markolf KL, Zemanovic JR, McAllister DR (2002) Cyclic loading of posterior cruciate ligament replacements fixed with tibial tunnel and tibial inlay methods. J Bone Joint Surg [Am] 84:518–524
Mauro CS, Sekiya JK, Stabile KJ, Haemmerle MJ, Harner CD (2008) Double-bundle PCL and posterolateral corner reconstruction components are codominant. Clin Orthop Relat Res 466:2247–2254
McCarthy M, Camarda L, Wijdicks CA, Johansen S, Engebretsen L, LaPrade RF (2010) Anatomic posterolateral knee reconstructions require a popliteofibular ligament reconstruction through a tibial tunnel. Am J Sports Med 38:1674–1681
McGuire DA, Wolchok JC (2003) Posterolateral corner reconstruction. Arthroscopy 19:790–793
Noyes FR, Barber-Westin SD (2007) Posterolateral knee reconstruction with an anatomical bone-patellar tendon-bone reconstruction of the fibular collateral ligament. Am J Sports Med 35:259–273
Osti L, Papalia R, Rinaldi P, Denaro V, Bartlett J, Maffulli N (2009) The kneeling view: evaluation of the forces involved and side-to-side difference. Knee 16:463–465
Panni AS, Alam M, Cerciello S, Vasso M, Maffulli N (2011) Medial patellofemoral ligament reconstruction with a divergent patellar transverse 2-tunnel technique. Am J Sports Med. doi:10.1177/0363546511420079
Race A, Amis AA (1998) PCL reconstruction. In vitro biomechanical comparison of ‘isometric’ versus single and double-bundled ‘anatomic’ grafts. J Bone Joint Surg [Br] 80:173–179
Schiphof D, Boers M, Bierma-Zeinstral SMA (2008) Differences in descriptions of Kellgren and Lawrence grades of knee osteoarthritis. Ann Rheum Dis 67:1034–1036
Sekiya JK, Haemmerle MJ, Stabile KJ, Vogrin TM, Harner CD (2005) Biomechanical analysis of a combined double-bundle posterior cruciate ligament and posterolateral corner reconstruction. Am J Sports Med 33:360–369
Shelbourne KD, Davis TJ, Patel DV (1999) The natural history of acute, isolated, nonoperatively treated posterior cruciate ligament injuries. A prospective study. Am J Sports Med 27:276–283
Shi SY, Ying XZ, Zheng Q, Cao GP (2009) Isometric reconstruction of the posterolateral corner of the knee. Acta Orthop Belg 75:504–511
Shino K, Horibe S, Nakata K, Hamada M, Nakamura N (1995) Conservative treatment of isolate injuries of the posterior cruciate ligament in athletes. J Bone Joint Surg [Br] 77:895–900
Skyhar M, Warren R, Ortiz G, Schwartz E, Otis JC (1993) The effects of the posterior cruciate ligament and posterolateral complex on the articular contact pressures within the knee. J Bone Joint Surg [Am] 75:694–699
Strauss EJ, Ishak C, Inzerillo C, Walsh M, Yildirim G, Walker P, Jazrawi L, Rosen J (2007) Effect of tibial positioning on the diagnosis of posterolateral rotatory instability in the posterior cruciate ligament-deficient knee. Br J Sports Med 41:481–485
Sugita T, Amis AA (2001) Anatomic and biomechanical study of the lateral collateral and popliteofibular ligaments. Am J Sports Med 29:466–472
Wajsfisz A, Christel P, Djian P (2010) Does combined posterior cruciate ligament and posterolateral corner reconstruction for chronic posterior and posterolateral instability restore normal knee function? Orthop Traumatol Surg Res 96:394–399
Wang CJ, Chen HS, Huang TW, Yuan LJ (2002) Outcome of surgical reconstruction for posterior cruciate and posterolateral instabilities of the knee. Injury 33:815–821
Zehms CT, Whiddon DR, Miller MD, Quinby JS, Montgomery SL, Campbell RB, Sekiya JK (2008) Comparison of a double bundle arthroscopic inlay and open inlay posterior cruciate ligament reconstruction using clinically relevant tools: a cadaveric study. Arthroscopy 24:472–480
Zhang H, Hong L, Wang XS, Zhang J, Geng XS, Liu X, Feng H (2010) Single-bundle posterior cruciate ligament reconstruction and mini-open popliteofibular ligament reconstruction in knees with severe posterior and posterolateral rotation instability: clinical results of minimum 2-year follow-up. Arthroscopy 26:508–514
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zorzi, C., Alam, M., Iacono, V. et al. Combined PCL and PLC reconstruction in chronic posterolateral instability. Knee Surg Sports Traumatol Arthrosc 21, 1036–1042 (2013). https://doi.org/10.1007/s00167-011-1771-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-011-1771-y