Abstract
Different methods to reconstruct damaged posterolateral structures are available, but there has been little work studying their relative performance in combined PCL plus posterolateral corner (PLC) deficiency. We hypothesized that an ‘anatomic’ reconstruction with three graft bundles crossing the joint line would restore knee laxity closer to normal than a modified two-bundle Larson reconstruction. In a controlled laboratory study, the kinematics of cadaveric knees were measured electromagnetically with posterior drawer, external rotation, or varus rotation loads applied, with the knee at sequential stages: intact, PCL-deficient; PCL plus PLC-deficient; modified Larson reconstruction; anatomic PLC reconstruction. The graft bundles were tensioned sequentially to restore specific degrees of freedom to intact values of laxity at specific angles of knee flexion. A significant difference was not found between the two reconstructions. Both reconstructions restored external rotation and varus laxity to normal. Both restored posterior drawer to that caused by isolated PCL deficiency, but did not restore posterior laxity to normal. It was concluded that, with appropriate graft tensioning, both PLC reconstructions could restore both external rotation and varus laxity to normal, but not posterior drawer. The three-stranded anatomical reconstruction did not perform better than the modified two-strand Larson technique. Both of these isolated PLC reconstructions in knees with combined PCL plus PLC deficiency restored the knees to the laxity condition of an isolated PCL-deficiency, they could not reduce posterior drawer to normal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posterolateral corner (PLC) injuries are often associated with injuries to the PCL [7]. The complex anatomy of the posterolateral corner of the knee has been attributed to the differential growth of the fibula and the tibia [21]. Although the posterolateral corner has a complex structure, the principal contributors to the static stability of the knee are the fibular (lateral) collateral ligament, popliteus tendon and the popliteofibular ligament [8, 10, 14, 22, 26, 28, 30]. Due to the complex anatomy, various techniques of PLC reconstruction have been proposed, especially for the chronic injury. They can be broadly classified into non-anatomical reconstructions such as femoral osteotomy and advancement of the lax ligaments [11], biceps tendon tenodesis [4, 29] and Larson’s single femoral tunnel PLC reconstruction [15]. There has been a progression towards anatomical graft placement: Noyes and Barber-Westin [18] used two closely-spaced femoral tunnels, either side of the epicondyle, and later Bicos and Arciero [1] placed their tunnels anatomically at the femoral attachments of the lateral collateral ligament and the popliteus tendon. The anatomical reconstructions have included popliteal bypass procedures and combined popliteal bypass and popliteofibular ligament reconstruction [13, 24].
The surgical results of PLC reconstruction procedures have been highly variable. Reviewing the causes of failure of 57 PLC reconstructions Noyes et al. [19] concluded that the most common causes were non-anatomical reconstruction, failure to reconstruct other ligament injuries and untreated varus malalignment. LaPrade et al. [13] were of the opinion that an ‘anatomic’ reconstruction of the PLC is necessary to improve the clinical results of PLC reconstruction. They developed an anatomic method of reconstructing the static stabilizers of the PLC, but the “popliteofibular ligament” part of the anatomic PLC reconstruction they showed appeared to stabilise the tibiofibular joint, rather than passing from the femur to the fibula. Sekiya and Kurtz [24] described an anatomic PLC reconstruction with Achilles tendon allograft that reconstructed all the main static stabilizers of the PLC. In a cadaver study Cooley et al. [5] compared the biceps tendon tenodesis, isolated popliteal bypass and Larson’s PLC reconstruction; they found the Larson’s reconstruction was best. Larson’s PLC reconstruction aims to restore the functions of the popliteofibular ligament and the lateral collateral ligament; it is non-anatomical because the femoral insertion is at the lateral epicondyle and not at the anatomical insertion sites of these structures [5, 15]. Larson’s reconstruction is a widely-used used PLC reconstruction procedure [6, 12].
The aim of this study was to analyse the effect of a modified Larson PLC reconstruction and an anatomic PLC reconstruction in cadaver knees with combined PCL and PLC deficiency. We were interested in finding out if a PCL and PLC-deficient knee would revert to the laxity status of an isolated PCL-deficient knee by only reconstructing the PLC. We hypothesised that the anatomic reconstruction would be better than the modified Larson PLC reconstruction in controlling the laxity of a combined PCL and PLC-deficient knee.
Method
Specimen preparation
Ten knees without evidence of previous injury or surgery were obtained from a tissue bank (IIAM, Jessup, PA, USA), following ethical committee approval. The experimental protocol was developed on two knees, leaving eight knees for which the results are presented. This eight had a mean age of 68 years (54–78); four were male, four female. They were stored at −20°C and were thawed overnight before use. The specimens were covered in wet tissue paper between the preparation and testing to prevent dehydration. The fibula was fixed to the tibia using two trans-cortical screws placed transversely distal to the head of the fibula to maintain the restraining effect of the interosseous membrane [23, 25]. A lateral para-patellar arthrotomy and posterior capsulotomy (for later PCL excision) and a lateral skin incision over the fibular head and the posterolateral structures (to cut and reconstruct them later) were made before starting the experiments; remaining soft tissues including the skin were left intact throughout. It was then confirmed that the knees did not have arthritic changes that would have affected the experiments. The proximal femur was potted in bone cement (PMMA). An aluminium intra-medullary rod was cemented into the tibial medullary canal; a pulley was fixed to this rod to apply internal and external rotational torque. A Steinman pin inserted across the proximal tibia near the joint line with a brass pulley system applied anterior and posterior drawer forces without impeding coupled tibial rotations (Fig. 1).
Motion analysis system
A custom made wooden kinematics rig was used in conjunction with the electro-magnetic tracking system “Nest of Birds” (Ascension technology, Burlington, VT, USA) for motion analysis. This provided 6 degrees of freedom data at up to 105 Hz for tibio-femoral motion and was accurate to ±0.06 mm in a 30 mm translation and ±0.4° in a 20° rotation [2]. Mounting blocks for the electromagnetic sensors were fixed securely to the tibia and femur; a transmitter was fixed on the wooden rig. In the extended knee, points on the proximal femur in the medullary canal and the medial and lateral femoral epicondyles were digitised to define the co-ordinate system. The proximal femur that had been potted in PMMA was fixed in the rig so that the epicondylar axis was aligned approximately to the rotational axis of the rig (Fig. 1). It was not necessary to align the flexion axis of the knee exactly to that of the test rig, because the motion sensors were mounted directly on the bones and so only relative motion between tibia and femur were calculated. The knee was flexed-extended by hand and data was recorded for the third extension motion. The position data was displayed in relation to the anterior drawer test with the ACL intact.
Testing protocol
A fixed protocol was followed for each knee (Table 1). The knee was first tested in the ‘intact’ condition (i.e. after the capsulotomies), then the PCL, including the meniscofemoral ligaments, was cut. In the last cutting step the posterolateral structures, i.e. lateral collateral ligament and the popliteus tendon at its femoral insertion were sectioned. A Modified Larson and an anatomic PLC reconstruction were performed in that order in the next two steps. At each step the knees were tested with the loads listed in Table 1. Kinematic data was recorded in the form of angle of flexion, anterior–posterior translation, internal–external rotation, varus–valgus rotation, distraction–compression and medial–lateral translation in the moving knee.
Modified Larson PLC reconstruction
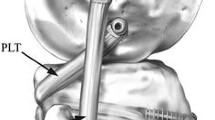
The modified Larson PLC reconstruction [5, 15] was performed with the tendon of the semitendinosus muscle (Fig. 2). The tendon graft was whip-stitched at both ends with Fiberwire® (Arthrex Inc, Naples, Florida, USA). A 5 mm tunnel was drilled through the widest part of the fibular head in an anterolateral to posteromedial direction. The tendon graft was passed through the tunnel so that the middle part of the graft was in the fibular tunnel. The tendon was fixed in the fibular head with an Arthrex 7 × 23 mm interference screw augmented with bone cement. Both the anterior and the posterior limbs of the graft were held together and the isometric point was located on the the femur while the knee was flexed and extended from 0 to 90°. This was on the point of the lateral epicondyle or at its posterior edge, so the tip of the epicondyle was always covered by the 5 mm graft tunnel entrance, which was slightly distal/anterior to the attachment of the collateral ligament. Two 5 mm tunnels were drilled from the isometric point, each exiting at a different point on the medial femoral cortex. The anterior and posterior limbs of the graft were passed through these tunnels and tensioned independently by means of custom-made adjustable screws, to which the leading sutures were tied, on the medial condyle. The anterior limb of the graft, which mimicked the lateral collateral ligament, was tensioned at 20° of knee flexion to match the laxity of the intact knee when it was subjected to a varus moment of 5 Nm at 20° knee flexion, within ±1°. The posterior limb of the graft, which is supposed to represent the popliteofibular ligament complex, was tensioned at 90° knee flexion to match the laxity of the intact knee at 90° knee flexion when it was subjected to an external rotation torque of 5 Nm, within ±1°.
Anatomic PLC reconstruction
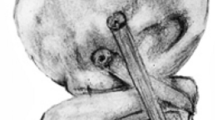
The anatomic PCL reconstruction was designed to reconstruct the lateral collateral ligament, popliteus tendon and the popliteofibular ligament complex (Fig. 3). The femoral tunnels from the previous reconstruction were obliterated with bone cement. The semitendinosus graft used in the previous reconstruction was reused. The fibular head tunnel of the graft was checked for loosening of the fixation, which was not disturbed. The tendon could be reused because the femoral fixation of the two ends had been via their leader sutures, so the graft itself had not been damaged. The anatomical attachments of the lateral collateral ligament and the popliteus tendon on the lateral femoral condyle were dissected and a 5 mm tunnel was made for the lateral collateral ligament, and a 7 mm tunnel for the popliteus complex, starting from their anatomical attachments and exiting on the medial cortex. The anterior part of the semitendinosus tendon graft was used to recreate the lateral collateral ligament and hence was inserted into the tunnel made at the attachment of the lateral collateral ligament on the lateral femoral condyle and tensioned to match the laxity of the intact knee when it was subjected to a varus moment of 5 Nm at 20° knee flexion, within ±1°. A 5 mm tibial tunnel was made from Gerdy’s tubercle to the posterolateral aspect of the lateral tibial condyle 10 mm below the joint line and adjacent to the groove for the popliteus tendon. A gracillis tendon graft was prepared and passed through this tunnel, then fixed at the anterior end with an 8 mm interference screw augmented with bone cement. The posterior limb of the semitendinosus and the gracillis grafts, representing the popliteofibular ligament complex and the popliteus tendon, respectively, were inserted into the tunnel made at the insertion of the popliteus tendon on the lateral femoral condyle. These two grafts were tensioned at 90° knee flexion to match the laxity of the intact knee at 90° knee flexion when it was subjected to an external rotation torque of 5Nm, within ±1°.
‘Anatomical’ three-strand reconstruction of the posterolateral corner of a left knee. The popliteal grafts pass deep to the lateral collateral graft and attach to the femur antero-distal to the lateral collateral ligament. As they route postero-distally, the popliteus graft passes deep to the capsular structures, en route to the posterolateral corner of the proximal tibia, while the politeofibular graft passes to the proximal-posterior styloid process of the fibula
Statistical analysis
The intact condition of each knee served as its own control; two-way repeated-measures ANOVAs with post-hoc Bonferroni tests were used to analyse the data; P < 0.05 was taken to be significant.
Results
Posterior drawer
With a posterior drawer force of 80 N the tibial posterior laxity of the intact knee ranged from 4 mm at 0° to 6 mm at 100° knee flexion (Fig. 4). The resection of the PCL increased this posterior laxity to 6 mm at 0° to 17 mm at 100°. Cutting the posterolateral structures resulted in a further increase of the posterior laxity, which then ranged from 10 mm at 0° to 26 mm at 100° knee flexion. There was a significant difference between the PCL cut and the combined PCL and PLC cut laxity at all angles of knee flexion (P < 0.05 at 10° and P < 0.01 from 20 to 100°) except near 0° (P > 0.05). Both of the posterolateral reconstructions restored the tibial posterior laxity so that it did not differ significantly (P > 0.05) from that in the isolated PCL-deficient state at all angles of knee flexion tested. A significant difference was not found between the posterior laxity after anatomic versus the modified Larson reconstructions at all angles of knee flexion (P > 0.05). The reduction in posterior laxity was not statistically significant (P > 0.05) for either of the reconstructions from 0 to 20° knee flexion. The reduction after the modified Larson reconstruction was significant from 30 to 90° (P < 0.05 to P < 0.01), and after anatomic reconstruction from 30 to 100° (P < 0.01 to P < 0.001).
Coupled external rotation with posterior drawer
A posterior drawer of 80 N to the intact knee resulted in approximately 7° of coupled external rotation from 20° to 100° knee flexion (Fig. 5). Cutting the PCL reduced the coupled external rotation in response to the posterior drawer, which then ranged from 6° to 1° rotation between 10° and 100° knee flexion. Cutting the PCL and PLC resulted in a significant increase in coupled external rotation from 20° (P < 0.05) to 100° (P < 0.001). A significant difference was not found between either of the reconstructions and the isolated PCL deficient condition (P > 0.05 for both), nor was a significant difference found between the reconstructions (P > 0.05) at any angle of knee flexion. Although not statistically significant, the anatomic PLC reconstruction induced coupled rotation more like an isolated PCL deficient knee than the modified Larson reconstruction, as knee flexion increased. Figure 5 shows, via the SD bars, that the coupled rotation behaviour was variable between knees.
External rotation laxity
An external rotation torque of 5 Nm resulted in approximately 15° external rotation laxity in the intact knee (Fig. 6). Cutting the PCL did not result in a measurable increase in the external rotation laxity. Cutting the posterolateral structures resulted in a significant increase in the external rotation laxity, which then ranged from 27° at 10° flexion (P < 0.01) to 37° at 100° flexion (P < 0.001). A significant difference was not found between either of the two PLC reconstructions and the isolated PCL-deficient knee, nor, therefore, the intact knee, throughout the range of knee motion, nor was a significant difference found between the two reconstructions at any angle of knee flexion. Both reconstructions reduced the external rotation significantly, compared to the PCL and PLC deficient-laxity: P < 0.05 at 10° flexion to P < 0.001 at 100° for the anatomic; P< 0.01 at 0° flexion to P < 0.001 at 100° for the modified Larson.
Varus laxity
A 5Nm varus moment resulted in an average tibial varus rotation of approximately 4° in an intact knee from 10° to 100° flexion (Fig. 7). Isolated PCL resection did not increase the varus laxity measurably. Cutting the PCL and the PLC resulted in a significant increase in the varus laxity from 10° to 100° (P < 0.001). Both the modified Larson as well as the anatomic reconstruction restored the knee to the isolated PCL deficient condition (P > 0.05) and, hence, also restored the knee to the intact knee condition, from 0° to 100° flexion. A significant difference was not found between the reconstructions at any angle of knee flexion (P > 0.05). Both the reconstructions were significantly different from the combined PCL and PLC-deficient knee throughout the arc of knee motion except at 0° (P > 0.05 at 0°; P < 0.001 at 20°–100° flexion).
Discussion
Both the modified Larson and the anatomic PLC reconstructions restored the laxity of the combined PCL and PLC-deficient knees so that they did not differ significantly from an isolated PCL deficient state: the posterior drawer remained abnormal, but both the external rotation and varus laxities were restored so that they did not differ significantly from normal across the arc of knee flexion examined. The performance of the two reconstructions did not differ significantly in any respect, hence these results do not support the hypothesis that the anatomic PLC reconstruction would be better than the modified Larson PLC reconstruction. However, although both reconstructions reduced posterior laxity significantly, neither of them reduced it below that seen in isolated PCL deficiency.
We believe that it is logical to tension the individual grafts of a complex reconstruction so that their behaviour duplicates their principal role in stabilising the knee. Therefore, we modified Larson’s PLC reconstruction [5, 15] by tensioning the anterior limb of the graft to control varus laxity at 20° of knee flexion and the posterior limb to control external rotation at 90° of knee flexion. That method was based on the findings of Sugita and Amis [27], who found that the lateral collateral ligament became slack and poorly oriented to resist tibial external rotation as the knee flexion increased, while it is a primary restraint to varus laxity when the knee is at or near to extension [9]. On the other hand, the popliteofibular ligament complex remained tight and well aligned to resist tibial external rotation throughout the arc of knee flexion [27]. The popliteofibular ligament complex is an important restraint to tibial external rotation, especially in deep flexion. The anatomic reconstruction that we have described is similar to the PLC reconstruction by Seikya and Kurtz [24]. We once again tensioned the lateral collateral ligament graft to control varus laxity at 20° knee flexion and the combined popliteus bypass graft and the popliteofibular ligament graft to control external rotation at 90° knee flexion. The reader should note that tensing a graft to restore laxity to normal at one specific angle of knee flexion does not imply that normal laxity will be restored at other angles. That is because the graft tunnels may not be placed to reproduce a physiological pattern of length changes or constraint with knee flexion. The reconstructions in this paper did manage to restore the laxities examined so that they did not differ significantly from normal, across the arc of knee flexion examined.
Different PLC reconstructions have been described, that have used a variety of grafts, attachment points and tensioning protocols. Larson et al. [15] found the fibular head to be isometric to the femoral lateral epicondyle, so they recommended using a graft passed through the fibular head and inserting into the lateral epicondyle. Cooley et al. [5] compared Larson’s reconstruction with biceps tenodesis and the popliteal bypass procedure. They found that Larson’s procedure was the best amongst the three in restoring the tibial posterior translation and controlling external rotational laxity. Wascher et al. [29] in a cadaver study on isolated PLC injury found that the biceps tenodesis overconstrained tibial external rotation throughout the arc of knee flexion. Markolf et al. [16] also found that their reconstructions overconstrained tibial external rotation, especially when the knee was flexed, even with only 10 N graft tension. However, in a clinical setting the quality of structures available for tenodesis is questionable, so a more complex reconstruction may be appropriate. Suda et al. [26] recommended reconstruction of the lateral collateral ligament and the PCL and addition of a popliteal tendon reconstruction if necessary in combined PCL and PLC injury. LaPrade et al. [13] described an ‘anatomic’ PLC reconstruction, but the ‘popliteofibular ligament reconstruction’ described by them only stabilised the tibiofibular joint and did not pass from the femur to the fibula. Although they tensioned their lateral collateral ligament graft at 30° knee flexion, there was a significant difference in varus laxity between the intact and the PLC-reconstructed knee at 30°. In common with this study, the popliteus tendon reconstruction was a passive tendon graft, lacking the contractile property of the popliteus muscle; the effect of this on the knee is not known.
The main limitations of our study are the use of elderly cadaver knees; findings are only valid at time zero and only passive stabilising structures were evaluated. The effect of dynamic stabilising structures and biological effects of graft healing on the laxity of the knee cannot be accounted-for easily in cadaver experiments. Some tissues may have different properties in patients with sports injuries, who are younger than the specimens used in this study. Despite these limitations, the results of this study do fit well with other published works cited above. We also did not measure the tensions applied to the grafts, or caused in them when the knees were loaded. That would have given useful information, such as whether the simpler reconstruction required higher graft tension in order to have the same mechanical ability as the more complex one. However, the principle used in this work was that of laxity matching. Given that knees have a wide range of laxity when intact, it was not felt to be appropriate to quote ‘the tension that was needed to restore normal laxity’ in case that were misused as a guide to the correct tension to be applied to a specific knee at surgery. The laxity-matching method avoided the overconstraint which may result from application of a standard graft tension in all knees [16]. A further point is that the orientations of the PLC structures are variable, depending on the position of the fibula around the tibial plateau [27]; some knees might require a higher graft tension in order to control external rotation laxity.
This experiment found that both the modified Larson and the anatomic reconstructions were effective, restoring the tibial posterior translation so that it did not differ significantly from a knee with an isolated PCL deficiency, restoring the external rotation and varus laxities of a combined PCL and PLC-deficient knee so that they did not differ significantly from normal. The authors believe that the success of these reconstructions has resulted from the use of graft tensioning protocols that restored specific aspects of the knee laxity to normal. However, an isolated PLC reconstruction cannot efficiently control tibial posterior drawer, for which the PCL is the primary restraint [3]. In theory, an isolated PLC reconstruction should be able to restore a combined PCL and PLC-deficient knee to an isolated PCL-deficient condition, so that the remaining PCL deficiency might then be managed conservatively. However, Noyes et al. [19] reported that such a reconstruction is doomed to fail. We believe that this is because, in the absence of the primary restraint to posterior drawer, the PLC reconstruction will be subjected to excessive loads and so liable to stretch-out.
In conclusion: this study has provided evidence on the roles of PLC reconstructions in knees with combined PCL plus PLC injuries. It did not find any difference in the ability of the two reconstruction methods tested, the two-stranded modified Larson reconstruction and the three-stranded ‘anatomic’ reconstruction, to control the laxity in knees with combined PCL plus PLC deficiency. Both methods could restore both the external rotation and varus laxities to normal, and reduced the posterior translation to that found in an isolated PCL deficiency. Therefore, we did not find biomechanical evidence to support the use of the more complex reconstruction method. This biomechanical finding in the laboratory must be interpreted with caution in the light of other clinical considerations, particularly whether the reconstructions will be able to withstand cyclic loading without stretching-out, which might relate to both the forces imposed during rehabilitation and the weakening of the graft that accompanies tissue remodelling. In the absence of relevant data, these considerations might lead a surgeon to prefer to use a more complex reconstruction than we have shown to be necessary, in order to add more graft material and fixation points. However, it should be noted that the semitendinosus graft used has a mean tensile strength of 1,216 N [20], which is stronger than the anatomical structures being reconstructed: the lateral collateral ligament 309 N [27] or 750 N [17]; the popliteofibular complex 186 N [27] or 425 N [17]. Further work with cyclic loading would help to address concerns about stretching-out.
References
Bicos J, Arciero RA (2006) Novel approach for reconstruction of the posterolateral corner using a free tendon graft technique. Sports Med Arthrosc Rev 14:28–36
Bull AM, Berkshire FH, Amis AA (1998) Accuracy of an electromagnetic measurement device and application to the measurement and description of knee joint motion. Proc Inst Mech Eng [Part H] Eng Med 212:347–355
Butler DL, Noyes FR, Grood ES (1980) Ligamentous restraints to anterior–posterior drawer in the human knee. A biomechanical study. J Bone Jt Surg 62 Am:259–270
Clancy WGJ (1988) Repair and reconstruction of posterior cruciate ligament. In: Chapman MW (ed) Operative orthopaedics. Lippincott, Philadelphia, pp 1651–1665
Cooley VJ, Harrington RM, Larson RV (1996) Effect of lateral ligament reconstruction on intra-articular posterior cruciate ligament graft force and knee motion. University of Washington research report, pp 37–41
Davies H, Unwin A, Aichroth P (2004) The posterolateral corner of the knee. Anatomy, biomechanics and management of injuries. Injury 35:68–75
Fanelli GC, Edson CJ (1995) Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy 11:526–529
Gollehon DL, Torzilli PA, Warren RF (1987) The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J Bone Joint Surg [Am] 69:233–242
Grood ES, Noyes FR, Butler DL, Santay WJ (1981) Ligamentous and capsular restraints preventing straight medial and lateral laxity in intact human cadaver knees. J Bone Jt Surg [Am] 63:1257–1269
Grood ES, Stowers SF, Noyes FR (1988) Limits of movement in the human knee: effect of sectioning the posterior cruciate ligament and posterolateral structures. J Bone Joint Surg [Am] 70:88–97
Hughston JC, Jacobson KE (1985) Chronic posterolateral rotatory instability of the knee. J Bone Joint Surg [Am] 67:351–359
Khanduja V, Somayaji HS, Harnett P, Utukuri M, Dowd GSE (2006) Combined reconstruction of chronic posterior cruciate ligament and posterolateral corner deficiency: a two to nine year follow up study. J Bone Joint Surg [Br] 88:1169–1172
LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A (2004) An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med 32:1405–1414
LaPrade RF, Tso A, Wentorf FA (2004) Force measurements on the fibular collateral ligament, popliteofibular ligament, and popliteus tendon to applied loads. Am J Sports Med 32:1695–1701
Larson RV, Sidles JA, Beals CT (1996) Isometry of lateral collateral and popliteofibular ligaments and a technique for reconstruction. University of Washington research report, pp 42–44
Markolf KL, Graves BR, Sigward SM, Jackson SR, McAllister DR (2007) Effects of posterolateral reconstructions on external tibial rotation and forces in a posterior cruciate ligament graft. J Bone Jt Surg [Am] 89:2351–2358
Maynard MJ, Deng XH, Wickiewicz TL, Warren RF (1996) The popliteofibular ligament, rediscovery of a key element in posterolateral stability. Am J Sports Med 24:311–316
Noyes FR, Barber-Westin SD (1995) Surgical reconstruction of severe posterolateral corner injuries of the knee using allograft tissues. Am J Sports Med 23:2–12
Noyes FR, Barber-Westin SD, Albright JC (2006) An analysis of the causes of failure in 57 consecutive posterolateral operative procedures. Am J Sports Med 34:1419–1430
Noyes FR, Butler DL, Grood ES, Zernicke RF, Hefzy MS (1984) Biomechanical analysis of human ligament grafts used in knee ligament repairs and reconstructions. J Bone Jt Surg [Am] 66:344–352
Oransky M, Canero G, Maiotti M (1989) Embryonic development of the posterolateral structures of the knee. Anat Rec 225:347–354
Pasque C, Noyes FR, Gibbons M, Levy M, Grood E (2003) The role of the popliteofibular ligament and the tendon of popliteus in providing stability in the human knee. J Bone Joint Surg [Br] 85:292–298
Race A, Amis AA (1998) PCL reconstruction—in vitro biomechanical comparison of “isometric” versus single and double bundled “anatomic” grafts. J Bone Joint Surg [Br] 80:173–179
Sekiya JK, Kurtz CA (2005) Posterolateral corner reconstruction of the knee: surgical technique utilizing a bifid Achilles tendon allograft and a double femoral tunnel. Arthroscopy 21:1400
Sekiya JK, Haemmerle MJ, Stabile KJ, Vogrin TM, Harner CD (2005) Biomechanical analysis of a combined double-bundle posterior cruciate ligament and posterolateral corner reconstruction. Am J Sports Med 33:360–369
Suda Y, Seedhom BB, Matsumoto H, Otani T (2000) Reconstructive treatment of posterolateral rotatory instability of the knee: a biomechanical study. Am J Knee Surg 13:110–116
Sugita T, Amis AA (2001) Anatomic and biomechanical study of the lateral collateral and popliteofibular ligaments. Am J Sports Med 29:466–472
Veltri DM, Deng XH, Torzilli PA, Maynard MJ, Warren RF (1996) The role of the popliteofibular ligament in stability of the human knee. A biomechanical study. Am J Sports Med 24:19–27
Wascher DC, Grauer JD, Markoff KL (1993) Biceps tendon tenodesis for posterolateral instability of the knee. An in vitro study. Am J Sports Med 21:400–406
Watanabe Y, Moriya H, Takahashi K, Yamagata M, Sonoda M, Shimada Y, Tamaki T (1993) Functional anatomy of the posterolateral structures of the knee. Arthroscopy 9:57–62
Acknowledgments
The running costs and surgical materials used in this work were provided to Imperial College by the Arthrex and Smith & Nephew (Endoscopy) companies. The electromagnetic tracking equipment was provided by a grant from the Arthritis Research Campaign.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Apsingi, S., Nguyen, T., Bull, A.M.J. et al. A comparison of modified Larson and ‘anatomic’ posterolateral corner reconstructions in knees with combined PCL and posterolateral corner deficiency. Knee Surg Sports Traumatol Arthrosc 17, 305–312 (2009). https://doi.org/10.1007/s00167-008-0696-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-008-0696-6