Abstract
Purpose
Osteochondral defects (i.e., defects which affect both the articular cartilage and underlying subchondral bone) are often associated with mechanical instability of the joint and therefore with the risk of inducing osteoarthritic degenerative changes. This review addresses the current surgical treatments and most promising tissue engineering approaches for articular cartilage and subchondral bone regeneration.
Methods
The capability to repair osteochondral or bone defects remains a challenging goal for surgeons and researchers. So far, most clinical approaches have been shown to have limited capacity to treat severe lesions. Current surgical repair strategies vary according to the nature and size of the lesion and the preference of the operating surgeon. Tissue engineering has emerged as a promising alternative strategy that essentially develops viable substitutes capable of repairing or regenerating the functions of damaged tissue.
Results
An overview of novel and most promising osteochondroconductive scaffolds, osteochondroinductive signals, osteochondrogenic precursor cells, and scaffold fixation approaches are presented addressing advantages, drawbacks, and future prospectives for osteochondral regenerative medicine.
Conclusion
Tissue engineering has emerged as an excellent approach for the repair and regeneration of damaged tissue, with the potential to circumvent all the limitations of autologous and allogeneic tissue repair.
Level of evidence
Systematic review, Level III.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Articular cartilage is a specialized tissue that plays a very important role in the natural joints [65], it increases joint congruence, it protects the subchondral bone from high stresses, and it reduces friction at the edge of long bones [91]. Cartilage response to injury does not follow the typical necrosis, inflammation, repair and scar tissue remodeling cascade of events, as it lacks vascularization. Cartilage lesions are therefore usually irretrievable, due to the avascular nature of this tissue and consequent lack of access to a pool of potential reparative cells and growth factors [94].
Osteochondral lesions, which involve both the articular cartilage and the subchondral bone, typically lead to the formation of fibrocartilage that has different biomechanical properties from the native hyaline cartilage and does not protect the subchondral bone from further degeneration [76]. This process involves both repaired and adjacent native tissues leading to the insurgence of severe pain, joint deformity, and loss of joint motion. Osteochondral defects typically require surgical procedures [83]. A brief description of the current surgical treatments used will be made, followed by an exhaustive overview of innovative tissue engineering approaches for the regeneration of osteochondral lesions.
Current surgical treatments
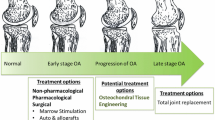
Currently, for the treatment of osteochondral lesions <2.5 cm2, mostly the techniques of Joint Debridement, Microfracture, and Mosaicplasty have been used, the choice of which to use depending on several factors, e.g., size of lesion, availability of particular treatment, the age and requirements of the patient [33, 46].
Joint Debridement eliminates debris from the joint space or joint surface only to alleviate pain [43, 87] and, in clinical, is usually associated with bone marrow stimulation technique, or microfracture. Bone marrow stimulation consists in penetration of the subchondral bone and release of progenitor cells from the bone marrow cavity into the defect. Such progenitor cells originate cells resembling morphologically the chondrocytes and that produce a fibrocartilaginous cartilage. Mosaicplasty, also called autologous osteochondral grafts, removes cylindrical plugs of hyaline cartilage with underlying subchondral bone from an unaffected area and implants them into the chondral defect, prepared with perpendicular edges of normal cartilage around, to create a “mosaic” pattern [34].
For osteochondral lesions >2.5 cm2, cells with chondrogenic potential and osteochondral allografts have been used. Autologous Chondrocytes Implantation (ACI) consists in the isolation of chondrocytes from a small cartilage piece harvested from a low-weight-bearing area of the knee joint, their expansion in vitro for 2–3 weeks and transplantation into the chondral defect and covering with a periosteal patch usually from the upper tibia surface. The introduction of matrix-associated ACI (M-ACI) is the first ever application of tissue engineering in orthopedic surgery; it minimizes donor site morbidity associated with periosteum harvesting and prevents cell de-differentiation [4, 31, 68]. Various scaffolds are available for clinical use, but there are only a few technical reports about arthroscopic M-ACI in the literature [54, 67, 95].
Recently, 53 patients with ostheochondral defects were treated with M-ACI and after 1 year, Magnetic Resonance Imaging (MRI) revealed a completely repaired defect with slight subchondral bone abnormality in 38 cases. A 5-year follow-up revealed a complete integration with the surrounding native cartilage with no detachment or bone marrow edema in 15 out of 17 patients [97].
Osteochondral allografts are often indicated as a rescue treatment in post-traumatic situations after periarticular fracture in lesions in which there is a significant bony deficit and also after failure of other techniques [48].
Each of the above-mentioned methods has shown to have limited success in particular focusing on long-term repair, and they are usually limited to small lesions. Currently, when articular cartilage is severely damaged and it is combined with lower limb malalignment, periarticular osteotomies are the most widely used treatment methods. If varus or valgus alignment occurs, the limb is realigned to transfer joint load away from the damaged cartilage surfaces. Procedures result in long-term pain relief but overloading of the unaffected compartments implies its progressive degeneration over several years leading to complete joint failure [9]. For severe degeneration of the joint, this is replaced by a total prosthetic joint typically made up of metallic alloy combined with polyethylene liner and cup, components that suffer high wear and corrosion and at the same time are still much stiffer than cartilage, so that shock absorption, lubrication, and deformation properties are lacking in the artificial joints. Detailed limitations of the current surgical strategies for osteochondral regeneration have been recently reviewed [29].
Tissue engineering in the orthopedic field
In the last few decades, tissue engineering has emerged as a promising alternative multi-disciplinary approach for the repair and regeneration of damaged tissue. Making joint of the latest developments in materials science, engineering, chemistry, and cell biology applied to medical science, it aims at restoring both function and mechanical properties of native articular cartilage and subchondral bone. Within osteochondral tissue engineering, different approaches have been followed but the best results will probably be achieved by the combination of 1. Osteochondroconductive scaffolds, 2. Osteochondroinductive signals, 3. Osteochondrogenic precursor cells, 4. Suitable fixation. A description of the most recent approaches in osteochondral tissue engineering for each topic will be presented.
Osteochondroconductive scaffolds
Tissue engineering requires a tissue-conductive system in order to mimic the 3D environment of the extracellular matrix (ECM), provide structural support to the regenerated and surrounding tissues, and provide an increased surface area to volume ratio for cellular migration, adhesion, and differentiation [11]. Note that the cellular growth and subsequent tissue regeneration depend in part on the characteristics and porosity of the scaffold. It must be biodegradable and biocompatible, and it must present a suitable fixation to the defect site, facilitate cell attachment, regulate cell expression, and promote the supply of nutrients and growth factors [26]. In order to control and direct cell behavior, a well-defined biomimetic environment, which surrounds the cells and promotes specific cell interactions, is necessary. Scaffold properties depend primarily on the nature of the biomaterial and the fabrication process. The nature of the biomaterial has been the subject of extensive studies including different materials such as natural, synthetic materials, ceramics or composite of these compounds.
Natural materials provide a more physiological environment for cell adhesion and proliferation and may be further divided into protein-based matrices such as collagen and fibrin, and carbohydrate-based matrices such as alginate, agarose, chitosan, and hyaluronan [75–77].
Natural materials are biocompatible, biodegradable, and they have the ability to mimic certain aspects of native ECM, thus facilitating cell adhesion, migration, differentiation, and ECM deposition. However, natural materials have several disadvantages such as immunogenecity, difficulty in processing, and a potential risk of transmitting animal-originated pathogens [57]. Moreover, despite the biocompatibility, these materials are mechanically weak and undergo rapid degradation upon implantation if not cross-linked with appropriate chemical reagents [57].
Synthetic materials have been used extensively in tissue engineering both in vitro and in vivo due to their easy molding characteristics, relatively easy production and the ability to control dissolution and degradation [11]. The most popular biodegradable synthetic polymers include poly(α-hydroxy acids), especially poly(lactic acid) (PLA), poly(glycolic acid) (PGA) and their co-polymers (PLGA), poly(ε-caprolactone) (PCL), poly(propylene fumarate) (PPF), poly(dioxanone) (PDO) [65]. Although synthetic materials are biocompatible, they do not have natural sites for cell adhesion and these often need to be added. Further, their in vivo degradation by a hydrolytic reaction causes a local reduction in pH and possible inflammation response [26].
Ceramics, such as hydroxyapatite (HA) or other calcium phosphate ceramics (including tricalcium phosphate, TCP) or bioactive glasses are known to promote, when implanted, the formation of a bone-like apatite layer on their surfaces [7, 70]. They have been investigated extensively during the last decades and are widely used for bone replacement, due to their osteoconductivity and high biocompatibility, also associated with stem cells therapy [66].
The main purpose for osteochondral tissue engineering is to recreate a more biomimetic scaffold combining synthetic materials with cell-recognition sites of naturally derived materials [7, 69, 92]. In addition, looking to the architectures of native tissues, novel graded scaffolds represent the challenge for osteochondral defect treatment. In fact, bone and cartilage have complete different properties. For bone, mechanically stiff biomaterials with options for medium perfusion and vascularization are required to support cell expansion, as well as the production of bone matrix rich in type I collagen and HA. By contrast, native cartilage matrix consists of an avascular highly hydrated proteoglycan hydrogel embedded into a type II collagen network.
Although several studies focus on the design and optimization of a stratified osteochondral graft with biomimetic multi-tissue regions, only few show good results in experiments in vivo. Here, some encouraging examples are mentioned.
A multi-phased scaffold of agarose hydrogel and sintered microspheres of PLGA-bioactive glass composite has showed in vitro a controlled chondrocyte and osteoblast culture on each scaffold region, resulting in the formation of three distinct yet continuous regions of cartilage, calcified cartilage, and bone-like matrices [45]. Very recently, a poly vinyl alcohol/gelatin-nano-hydroxyapatite/polyamide6 (PVA-n-HA/PA6) bilayered scaffold seeded with induced bone marrow stem cells (BMSCs) showed ectopic neocartilage formation in the PVA layer and reconstitution of the subchondral bone, confined within the n-HA/PA6 layer, when implanted into the rabbit muscle pouch for up to 12 weeks [82]. A biphasic scaffold combining hyaluronic acid and atelocallagen for the chondral phase and combining HA and β-TCP for the osseous phase has proved to be effective for repairing osteochondral defects, when implanted in the knee joint of a porcine model [41]. Another biphasic scaffold based on collagen-glycosaminoglycan and nano calcium phosphate has been developed to mimic the composition and structure of articular cartilage on one side, subchondral bone on the other side and the continuous, gradual soft interface between these tissues. The different properties of the osseous and cartilaginous compartments seem promising but, as far as we know, this scaffold has not yet been tested in a biological system [36].
Very recently, a 3D biomimetic scaffold (MaioRegen, Fin-Ceramica Faenza S.p.A., Italy) was obtained by nucleating type I collagen fibrils with HA nanoparticles, in two configurations, bi- and tri-layered, to reproduce, respectively, chondral and osteochondral anatomy [52]. This scaffold has been tested in chondral defects and deep osteochondral defects made in the metacarpal bone of two adult horses and treated, respectively, with the chondral and osteochondral grafts. Results showed that the growth of trabecular bone in the osteochondral lesion was evident and newly formed fibrocartilaginous tissue was present [53]. The same scaffold has also been tested in the femoral condyles of sheeps and 6 months after surgery, histologic and gross evaluation of specimens showed good integration of the chondral surface and significantly better bone regeneration [51]. The same scaffold has been used already in an early stability clinical trial on 13 patients with 15 large degenerative chondral lesions: four at the medial femoral condyle, two at the lateral femoral condyles, five at the patellas, and four at the trochleas. The mean size of the defects was 2.8 cm2 and MRI evaluation at short-term follow-up has demonstrated good stability of the scaffold without any other fixation device and the histological analysis showed the formation of subchondral bone without the presence of biomaterial and the cartilage repair tissue appeared to be engaged in an ongoing maturation process [52]. Presently, an extensive clinical trial is ongoing involving eleven European centers and 150 patients (http://clinicaltrialsfeeds.org/clinical-trials/show/NCT01282034).
Osteochondroinductive signals
Induction of osteochondral tissue formation refers to the capacity of many physiological stimuli to stimulate stem cells or immature bone/cartilage cells to grow and mature, forming healthy tissue. Most of these stimuli are protein molecules (e.g., growth factors and cytokines). Much interest has centered on a group of proteins called Bone Morphogenetic Proteins (BMPs), which have a powerful effect in stimulating new bone/cartilage formation, but they are not the only option for osteochondroinduction [71, 86]. Many other growth factors are now known to have specific effects on the cell growth, migration, and development. Some of the factors that are most likely to have clinical value in the future are as follows: Epidermal Growth Factor (EGF), Platelet-Derived Growth Factor (PDGF), Fibroblast Growth Factors (FGFs), Parathyroid Hormone-Related Peptide (PTHrp), Insulin-like Growth Factors (IGFs), Transforming Growth Factor-Beta (TGF-β), and Vascular Endothelial Growth Factors (VEGFs) [26, 47, 89]. Several of these are under active investigation, though none of these works have yet advanced to the point of offering these as routine treatment options to patients. As an example, a recent study has infused a composite scaffold of PCL and HA with TGF-β3 and transplanted into the rabbit condyle. 3–4 weeks after, all rabbits resumed weithbearing and locomotion. After 4 months, these scaffolds were fully covered with hyaline cartilage in the articular surface, with uniformly distributed chondrocytes in a matrix with collagen type II and aggrecan and recruited roughly 130% more cells into the regenerated articular cartilage than did scaffolds that had not been loaded with TGF-β3, which instead had only isolated cartilage formation, with reduced thickness and density [56].
The method by which a growth factor is released can have a significant effect on therapeutic efficacy because the dose and spatio-temporal release of such agents at the lesion site is crucial for achieving a successful outcome. Common growth factors delivery methods involve systemic administration or direct injection into the defect site close to or in direct contact to the scaffold [57]. However, as a result of the short half-life of many inductive proteins, this method requires very high doses for therapeutic effect and still may not permit the necessary concentration of the factor to be maintained for the appropriate period of time [38].
Different strategies have been developed to create a controlled release, even if success has been restricted by problems of dosage, lack of full activity of recombinant factors and the inability to sustain the presence of the factor for an appropriate length of time [73]. Moreover, spatially controlled delivery of growth factors in the scaffold is crucial for engineering composite tissue structures, such as osteochondral constructs [98]. One of the most common methods of creating controlled growth factors and/or drug delivery is to utilize the physical properties of the scaffold material to regulate the amount of factor released. The target growth factor is mixed with the scaffold precursors during fabrication. In such systems, the properties of the scaffold, such as pore size or cross-linking density, regulate release by diffusion. For instance, BMP-2 and TGF-β1-loaded PLGA microspheres were utilized with a gradient scaffold fabrication technology to produce microsphere-based scaffolds containing opposing gradients of these signals. This scaffold, seeded with stem cells, has shown good osteochondral tissue regeneration [19]. Hydrogel scaffolds, based on the polymer oligo(poly(ethylene glycol) fumarate), consisting of two layers: a bone-forming layer and a cartilage-forming layer loaded with TGF-β1 encapsulated into gelatin microparticles. This approach exerts some effect on cartilage quality in the defect area in a rabbit model and demonstrates the exciting potential of these polymer-based hydrogels as carriers of bioactive agents for tissue repair [37]. In another study, BMP-2 and IGF-I were incorporated in PLGA and silk fibroin microspheres and an arginate gel was fabricated with microspheres incorporated as gradient. Silk microspheres were more efficient in delivering BMP-2, probably due to sustained release of the growth factor. This microsphere/scaffold system presents a novel opportunity for the spatial control delivery of multiple growth factors in a 3D culture environment [98].
Localized gene-therapy approaches to the delivery of inductive factors may circumvent limitations of direct protein delivery. Delivery of genes encoding the inductive factors allows sustained and localized protein production and can be used for either short-term (e.g., transient transfection of cells) or long-term (e.g., retroviral transduction) delivery [13, 14, 63, 78]. In either case, gene delivery takes advantage of the protein synthesis machinery of the cells to produce the specific bioactive factor. However, the cell colonization of the scaffold remains a tight problem for tissue regeneration in large defect cases.
Recently, the usage of superparamagnetic nanoparticles (MNPs) for biological and medical purposes has been increasing and their biocompatibility is validated by several studies [44, 81, 90]. MNPs are unique in their reaction to magnetic force and these properties have been already used in the last decade for in vitro and in vivo applications (e.g., hyperthermia, contrast agent for MRI, magnetic drug delivery), whereas only few authors proposed approaches for tissue engineering [1, 2, 32]. Considering the employment of a magnetic field for targeted therapy, some limitations are evident in clinical application. In fact, since the magnetic gradient decreases with the distance to the target, the main limitation of magnetic drug delivery relates to the strength of the external field that can be applied to obtain the necessary magnetic gradient to control the residence time of MNPs in the desired area or which triggers the drug desorption [22, 39]. The limits inherent to the use of external magnetic fields can be circumvented by means of internal magnets located in the proximity of the target by minimally invasive surgery [79, 80]. Recently, very innovative tissue engineering approaches with pioneering magnetic scaffolds for osteochondral defect have been developed [8, 93]. Under an external magnetic field, the magnetic moment of the proposed scaffolds enables them with the fascinating possibility of being continuously controlled and reloaded from an external supervising center with tissue growth factors. The scaffolds will work like a fixed “station” that offers a long-living assistance to implanted tissue engineering constructs, providing a unique possibility to adjust the scaffold activity to the personal needs of the patient, overcoming the present difficulties of magnetic guiding. Nanomagnetic targeting represents a promising strategy to guide and accumulate growth factors, drug doses and cells in the scaffold with gradient, and reduce their loss and undesired side effects.
Osteochondrogenic precursor cells
Since only living cells can make tissue, the success of any grafting procedure is dependent on having enough tissue-forming cells in the area. In some situations, the healthy tissues around the graft site will contain a sufficient number of tissue-forming cells. However, in many clinical settings, the number of bone/cartilage-forming cells in the surrounding tissues may be limited. Areas of scarring, previous surgery or infection, tissue gaps, and areas previously treated with radiation therapy are all likely to be deficient in tissue-forming cells.
ACI and M-ACI have been successfully clinically applied to treat focal post-traumatic lesions of the knee joint (see section “Current surgical treatments”). Despite promising clinical results, the use of chondrocytes may be troublesome due to the requirement of large numbers of chondrocytes to fill large defect volumes and dedifferentiation of cultured chondrocytes in vitro. Moreover, the harvest of cartilage for a cartilage biopsy includes two separate operations and presents limitations associated with donor site morbidity [68, 99].
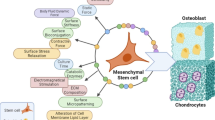
Enormous expectations are associated with stem cells with regard to cell therapy and tissue engineering. Mesenchymal stem cells (MSCs), defined as self-renewal, multi-potent progenitor cells with the capacity to differentiate into several distinct mesenchymal lineages, represent a promising cell source for treating osteochondral defects [3]. They are also known as marrow stromal cells or mesenchymal progenitor cells [12].
Numerous studies have been performed to investigate and improve the in vitro chondrogenesis process (by the use of various growth factors) and potential therapeutic use of MSCs [17, 59]. MSCs from bone marrow can be injected into a graft site or mixed with other components as a composite graft. However, there is limited evidence that scaffold techniques result in homogeneous distribution of cells [42]. Furthermore, the cell yield for bone marrow harvest is relatively small because only 10 to 25 ml of bone marrow can be obtained from humans [40]. A second source of autologous stem cells is the periosteum, but the harvest is invasive and moreover yields a paucity of cells [88].
MSCs derived from the Synovial Membrane (SM) are attracting considerable attention, since they apparently have great chondrogenic potential [16]. The SM is composed of a cellular lining layer adjacent to the joint cavity and a supportive layer that merges with the fibrous layer of the joint capsule. A small SM biopsy represents an easily accessible source of autologous MSCs in the context of an explorative or therapeutic arthroscopy. SM-derived cells from adult human donors of various ages can be expanded in vitro over at least 10 passages maintaining their multi-lineage differentiation potential. They may play a role in the regenerative response during arthritic diseases and are promising candidates for developing novel cell-based therapeutic approaches for post-natal skeletal tissue repair. In fact, when grown in monolayers, the synovial cells exhibited a fibroblast-like phenotype [96], but when cultured in a 3D alginate system, they underwent an immediate chondrogenic switch, even in the absence of growth factors [55]. A very recent study has shown the potential use of SM-derived cells as a cell source for cartilage tissue engineering. Cells were seeded into clinically relevant agarose hydrogel scaffolds, and they received TGF-β3 for the first 21 days. The constructs exhibited good mechanical and biochemical properties comparable to native tissue [85].
An alternative source of stem cells is adipose tissue. The adipose tissue contains a large number of stromal stem cells and is an abundant, easily accessible and reproducible cell source for musculo-skeletal regenerative medicine applications [27, 58]. Adipose stem cells (ASCs) have been reported to differentiate along different lineages, including bone, cartilage, fat, muscle, and nerve [84, 101]. Moreover, ASCs have been shown to be immunoprivileged [30] and appear to be more genetically stable in long-term culture compared to BMSCs [15]. Although ASCs are widely used as seeding cells for osteochondral tissue engineering studies [21, 35, 74], several challenges remain before ASCs can be used in everyday clinical practice. Indeed, at present, chondrogenesis and osteogenesis occur slower respect to adipogenesis and also the cell yields are still low, despite these differentiation protocols including several factors similar to the adipogenesis protocols [60]. Any improvement in these differentiation timeframes will make ASCs more attractive for tissue engineering purposes.
The Umbilical Cord Blood (UCB) has been used as a further alternative source of MSCs [64]. The blood remaining in the umbilical vein following birth contains a rich source of hematopoietic stem and progenitor cells and has been used successfully as an alternative allogeneic donor source to treat a variety of pediatric genetic, hematologic, immunologic, and oncologic disorders [10, 28]. Moreover, UCB-derived stem cells showed to have more chondrogenic potential in vitro than the bone marrow MSC based [6]. Cultured in a defined chondrogenic medium supplemented with TGF-β, UCB-derived stem cells present expression of genes typical of cartilage and production of cartilaginous ECM after 2–3 weeks, so that they are becoming an attractive cell source also for cartilage tissue engineering [25]. In addition, UCB-derived stem cells form cartilage and/or bone tissue when combined with different material. Human UCB cells-loaded calcium phosphate ceramic cylinders were implanted in a critical size bone defect in rats. After 12 weeks, a clear bony reconstitution was observed [50]. The same authors proved also the chondrogenic potential of UCB cells by seeding them into gelatin sponges and implanting them in nude mice; after 3 weeks, the implanted cells demonstrated strong chondrogenic differentiation [50]. In another study, UCB-derived cells were seeded onto PGA scaffolds and maintained in a rotating bioreactor with serum-free medium supplemented with TGF-β1. After 12 weeks, the constructs exhibited chondrogenic differentiation displaying histological and functional properties of native cartilage tissue [24].
Suitable fixation
Failure to regenerate the intricate tissue-to-tissue interface has been reported to compromise graft stability and long-term clinical outcome, consequently, the biological fixation or integrative repair of tissues remain a significant clinical challenge [23, 61, 62]. The limited number of publications regarding scaffold fixation is an indication of the lack of attention in this area even if this is a critical step in tissue regeneration.
A significant barrier to clinical translation is how to achieve biological fixation, functional integration, and how to prevent micromotion at the interfaces of the tissue-engineered orthopedic grafts, be it bone, ligaments or cartilage, either with each other and/or with the host environment [72].
At present, in the treatment of small osteochondral lesions, most surgeons do not use any fixation system, the stability of the scaffold being granted only by the fibrin clot and by the congruency between the prepared lesion site and the geometry of the scaffold or they use biocompatible glues (e.g., fibrin glue) as an adjuvant treatment to enhance the scaffold fixation. Recently, several studies have shown that biocompatible glues do not improve the fixation compared with the press-fit technique only [5, 20, 49]. Fibrin glue preserves the scaffold integrity compared with other more invasive fixation methods but shows less adhesion of the scaffold to the cartilage or chondral bone [5].
Suturing of cartilage grafts may cause difficulty in fixation and cartilage may shift and the operation procedure becomes longer. Multiple suture may cause fracture of the cartilage and may lead to resorption in postoperative period [18].
The pin fixation and the transosseous fixation technique have been proposed as alternatives to the conventional suture method [100]. They have been shown significantly higher ultimate failure load, yield load and stiffness than the classic suture technique for the fixation of bioabsorbable scaffolds. At the same time, the results have shown that the material properties of the scaffold have a strong influence on the biomechanical tests; therefore, the results cannot be transferred to the fixation of other matrices.
A different approach using Butyl 2-Cyanoacrylate as cartilage adhesive was proposed to facilitate matrix placement because it bonds strongly and immobilizes easily cartilage grafts [18]. Although it should be easy to apply, biodegradable and should establish a strong and flexible band, this technique is not diffused in clinical applications.
Interdisciplinary approaches that involve materials research and surgical technique development are mandatory to lead to optimal scaffold fixation with limited damage to the scaffolds.
An innovative fixation approach based on magnetic forces firmly holding the scaffold in the implanted position is coming out from a new project conception. The fascinating possibility to achieve efficient scaffold fixation via magnetic forces may provide a very elegant and simple solution to the problems of fixation that many devices present. To reach this aim, magnetic scaffolds are developed and under investigation to verify biocompatibility both in vitro and in vivo [8, 93]. These scaffolds should be enabled by a magnetization, sufficient to activate an attractive force toward selected fixating magnetic objects.
Conclusion
Tissue engineering aims to recreate the native environment to promote the appropriate cell behavior for tissue regeneration. The design of tissue engineering strategies for osteochondral lesions is in increasingly expansion but so far few of the approaches developed was effectively brought to the clinic due to the complexity of the tissue to be regenerated.
Having in mind the challenge to treat ever bigger and complex defects, it is mandatory to be able to control the 3D variable geometry of the articular surface, considering the proportions between bone and cartilage thickness and variation in the curvature radius of the articular surface but also the micro and nanoscale arrangement of cells and ECM within the tissue. In the future, multi-disciplinary osteochondral tissue engineering technology should provide the means to mimic the complex cascade of tissue regeneration in each region of the joint. The development of osteochondroconductive scaffolds, able to be fixed in order to avoid micromotion at the graft-tissue interfaces, together with osteochondroinductive signals and osteochondrogenic precursor cells are the main promising peculiarities necessary to restore function and mechanical features of the osteochondral tissue.
References
Amirfazli A (2007) Nanomedicine: magnetic nanoparticles hit the target. Nat Nanotechnol 2(8):467–468
Arruebo M, Fernandez Pacheco R, Ibarra MR, Santamaria J (2007) Magnetic nanoparticles for drug delivery. Nano Today 2:22–32
Barry FP, Murphy JM (2004) Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36(4):568–584
Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, Bentley G (2005) Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br 87(5):640–645
Bekkers JE, Tsuchida AI, Malda J, Creemers LB, Castelein RJ, Saris DB, Dhert WJ (2010) Quality of scaffold fixation in a human cadaver knee model. Osteoarthritis Cartilage 18(2):266–272
Berg L, Koch T, Heerkens T, Bessonov K, Thomsen P, Betts D (2009) Chondrogenic potential of mesenchymal stromal cells derived from equine bone marrow and umbilical cord blood. Vet Comp Orthop Traumatol 22(5):363–370
Bernhardt A, Lode A, Boxberger S, Pompe W, Gelinsky M (2008) Mineralised collagen—an artificial, extracellular bone matrix—improves osteogenic differentiation of bone marrow stromal cells. J Mater Sci Med 19(1):269–275
Bock N, Riminucci A, Dionigi C, Russo A, Tampieri A, Landi E, Goranov VA, Marcacci M, Dediu V (2010) A novel route in bone tissue engineering: magnetic biomimetic scaffolds. Acta Biomater 6(3):786–796
Bonnin M, Chambat P (2004) Current status of valgus angle, tibial head closing wedge osteotomy in media gonarthrosis. Orthopade 33(2):135–142. doi:10.1007/s00132-003-0586-z
Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse EA (1989) Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA 86(10):3828–3832
Capito RM, Spector M (2003) Scaffold-based articular cartilage repair. IEEE Eng Med Biol Mag 22(5):42–50
Caplan AI (2009) Why are MSCs therapeutic? New data: new insight. J Pathol 217(2):318–324
Chen HC, Chang YH, Chuang CK, Lin CY, Sung LY, Wang YH, Hu YC (2009) The repair of osteochondral defects using baculovirus-mediated gene transfer with de-differentiated chondrocytes in bioreactor culture. Biomaterials 30(4):674–681
Cucchiarini M, Madry H (2005) Gene therapy for cartilage defects. J Gene Med 7(12):1495–1509
Dahl JA, Duggal S, Coulston N, Millar D, Melki J, Shahdadfar A, Brinchmann JE, Collas P (2008) Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. Int J Dev Biol 52(8):1033–1042
De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP (2001) Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 44(8):1928–1942
Diduch DR, Jordan LC, Mierisch CM, Balian G (2000) Marrow stromal cells embedded in alginate for repair of osteochondral defects. Arthroscopy 16(6):571–577
Doner F, Sari I, Ozturk A, Karasen RM, Bitiren M, Sutbeyaz Y (1996) The auricular cartilage graft fixation with Butyl 2-Cyanocrylate. Turk J Med Sci 28:285–290
Dormer NH, Singh M, Wang L, Berkland CJ, Detamore MS (2010) Osteochondral interface tissue engineering using macroscopic gradients of bioactive signals. Ann Biomed Eng 38(6):2167–2182
Efe T, Fuglein A, Heyse TJ, Stein T, Timmesfeld N, Fuchs-Winkelmann S, Schmitt J, Paletta JR, Schofer MD (2011) Fibrin glue does not improve the fixation of press-fitted cell-free collagen gel plugs in an ex vivo cartilage repair model. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-011-1571-4
Erisken C, Kalyon D, Wang H, Ornek C, Xu J (2011) Osteochondral tissue formation through adipose-derived stromal cell differentiation on biomimetic polycaprolactone nanofibrous scaffolds with graded insulin and beta-glycerol phosphate concentrations. Tissue Eng Part A 17(9–10):1239–1252
Foy SP, Manthe RL, Foy ST, Dimitrijevic S, Krishnamurthy N, Labhasetwar V (2010) Optical imaging and magnetic field targeting of magnetic nanoparticles in tumors. ACS Nano 4(9):5217–5224
Friedman MJ, Sherman OH, Fox JM, Del Pizzo W, Snyder SJ, Ferkel RJ (1985) Autogeneic anterior cruciate ligament (ACL) anterior reconstruction of the knee. A review. Clin Orthop Relat Res (196):9–14
Fuchs JR, Hannouche D, Terada S, Zand S, Vacanti JP, Fauza DO (2005) Cartilage engineering from ovine umbilical cord blood mesenchymal progenitor cells. Stem Cells 23(7):958–964
Gao J, Yao JQ, Caplan AI (2007) Stem cells for tissue engineering of articular cartilage. Proc Inst Mech Eng H 221(5):441–450
Getgood A, Brooks R, Fortier L, Rushton N (2009) Articular cartilage tissue engineering: today’s research, tomorrow’s practice? J Bone Joint Surg Br 91(5):565–576
Gimble JM, Grayson W, Guilak F, Lopez MJ, Vunjak-Novakovic G (2011) Adipose tissue as a stem cell source for musculoskeletal regeneration. Front Biosci (Schol Ed) 3:69–81
Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, Ortega J, Souillet G, Ferreira E, Laporte JP, Fernandez M, Chastang C (1997) Outcome of cord-blood transplantation from related and unrelated donors. Eurocord transplant group and the European blood and marrow transplantation group. N Engl J Med 337(6):373–381
Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, Kon E (2010) The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc 18(4):434–447
Gonzalez-Rey E, Gonzalez MA, Varela N, O’Valle F, Hernandez-Cortes P, Rico L, Buscher D, Delgado M (2010) Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Anna Rheum Dis 69(1):241–248
Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A (2006) A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. Knee 13(3):203–210
Gould P (2006) Nanomagnetism shows in vivo potential. Nano Today 1:34–39
Hangody L (2003) The mosaicplasty technique for osteochondral lesions of the talus. Foot Ankle Clin 8(2):259–273
Hangody L, Kish G, Karpati Z, Szerb I, Udvarhelyi I (1997) Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Traumatol Arthrosc 5(4):262–267
Hao W, Dong J, Jiang M, Wu J, Cui F, Zhou D (2010) Enhanced bone formation in large segmental radial defects by combining adipose-derived stem cells expressing bone morphogenetic protein 2 with nHA/RHLC/PLA scaffold. Int Orthop 34(8):1341–1349
Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ (2010) Design of a multiphase osteochondral scaffold III: fabrication of layered scaffolds with continuous interfaces. J Biomed Mater Res A 92(3):1078–1093
Holland TA, Bodde EW, Baggett LS, Tabata Y, Mikos AG, Jansen JA (2005) Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo (poly (ethylene glycol) fumarate) hydrogel scaffolds. J Biomed Mater Res A 75(1):156–167
Hsiong SX, Mooney DJ (2006) Regeneration of vascularized bone. Periodontol 2000 41:109–122
Hua MY, Yang HW, Chuang CK, Tsai RY, Chen WJ, Chuang KL, Chang YH, Chuang HC, Pang ST (2010) Magnetic-nanoparticle-modified paclitaxel for targeted therapy for prostate cancer. Biomaterials 31(28):7355–7363
Huang JI, Beanes SR, Zhu M, Lorenz HP, Hedrick MH, Benhaim P (2002) Rat extramedullary adipose tissue as a source of osteochondrogenic progenitor cells. Plast Reconstructr Surg 109(3):1033–1041; discussion 1042–1033
Im GI, Ahn JH, Kim SY, Choi BS, Lee SW (2010) A hyaluronate-atelocollagen/beta-tricalcium phosphate-hydroxyapatite biphasic scaffold for the repair of osteochondral defects: a porcine study. Tissue Eng Part A 16(4):1189–1200
Iwasa J, Engebretsen L, Shima Y, Ochi M (2009) Clinical application of scaffolds for cartilage tissue engineering. Knee Surg Sports Traumatol Arthrosc 17(6):561–577
Jackson RW, Dieterichs C (2003) The results of arthroscopic lavage and debridement of osteoarthritic knees based on the severity of degeneration: a 4- to 6-year symptomatic follow-up. Arthroscopy 19(1):13–20
Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V (2008) Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm 5(2):316–327
Jiang J, Tang A, Ateshian GA, Guo XE, Hung CT, Lu HH (2010) Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Ann Biomed Eng 38(6):2183–2196
Kalson NS, Gikas PD, Briggs TW (2010) Current strategies for knee cartilage repair. Int J Clin Pract 64(10):1444–1452
Kieswetter K, Schwartz Z, Alderete M, Dean DD, Boyan BD (1997) Platelet derived growth factor stimulates chondrocyte proliferation but prevents endochondral maturation. Endocrine 6(3):257–264
Kim HT, Teng MS, Dang AC (2008) Chondrocyte apoptosis: implications for osteochondral allograft transplantation. Clin Orthop Relat Res 466(8):1819–1825
Knecht S, Erggelet C, Endres M, Sittinger M, Kaps C, Stussi E (2007) Mechanical testing of fixation techniques for scaffold-based tissue-engineered grafts. J Biomed Mater Res B Appl Biomater 83(1):50–57
Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, Greschat S, Knipper A, Bender J, Degistirici O, Gao J, Caplan AI, Colletti EJ, Almeida-Porada G, Muller HW, Zanjani E, Wernet P (2004) A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med 200(2):123–135
Kon E, Delcogliano M, Filardo G, Fini M, Giavaresi G, Francioli S, Martin I, Pressato D, Arcangeli E, Quarto R, Sandri M, Marcacci M (2010) Orderly osteochondral regeneration in a sheep model using a novel nano-composite multilayered biomaterial. J Orthop Res 28(1):116–124
Kon E, Delcogliano M, Filardo G, Pressato D, Busacca M, Grigolo B, Desando G, Marcacci M (2010) A novel nano-composite multi-layered biomaterial for treatment of osteochondral lesions: technique note and an early stability pilot clinical trial. Injury 41(7):693–701
Kon E, Mutini A, Arcangeli E, Delcogliano M, Filardo G, Nicoli Aldini N, Pressato D, Quarto R, Zaffagnini S, Marcacci M (2010) Novel nanostructured scaffold for osteochondral regeneration: pilot study in horses. Tissue Eng Regen Med 4:300–308
Kon E, Verdonk P, Condello V, Delcogliano M, Dhollander A, Filardo G, Pignotti E, Marcacci M (2009) Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: systematic clinical data review and study quality analysis. Am J Sports Med 37(1):156S–166S
Kurth T, Hedbom E, Shintani N, Sugimoto M, Chen FH, Haspl M, Martinovic S, Hunziker EB (2007) Chondrogenic potential of human synovial mesenchymal stem cells in alginate. Osteoarthr Cartil 15(10):1178–1189
Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ (2010) Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet 376:440–448
Lee SH, Shin H (2007) Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev 59(4–5):339–359
Lindroos B, Suuronen R, Miettinen S (2010) The potential of adipose stem cells in regenerative medicine. Stem Cell Rev 7(2):269–291
Liu Y, Shu XZ, Prestwich GD (2006) Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng 12(12):3405–3416
Locke M, Windsor J, Dunbar PR (2009) Human adipose-derived stem cells: isolation, characterization and applications in surgery. ANZ J Surg 79(4):235–244
Lu HH, Jiang J (2006) Interface tissue engineering and the formulation of multiple-tissue systems. Adv Biochem Eng Biotechnol 102:91–111
Lu HH, Subramony SD, Boushell MK, Zhang X (2010) Tissue engineering strategies for the regeneration of orthopedic interfaces. Ann Biomed Eng 38(6):2142–2154
Madry H, Orth P, Kaul G, Zurakowski D, Menger MD, Kohn D, Cucchiarini M (2010) Acceleration of articular cartilage repair by combined gene transfer of human insulin-like growth factor I and fibroblast growth factor-2 in vivo. Arch Orthop Trauma Surg 130(10):1311–1322
Malgieri A, Kantzari E, Patrizi MP, Gambardella S (2010) Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med 3(4):248–269
Mano JF, Reis RL (2007) Osteochondral defects: present situation and tissue engineering approaches. J Tissue Eng Regen Med 1(4):261–273
Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, Mastrogiacomo M, Cancedda R (2007) Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng 13(5):947–955
Marcacci M, Zaffagnini S, Kon E, Visani A, Iacono F, Loreti I (2002) Arthroscopic autologous chondrocyte transplantation: technical note. Knee Surg Sports Traumatol Arthrosc 10(3):154–159
Marlovits S, Zeller P, Singer P, Resinger C, Vecsei V (2006) Cartilage repair: generations of autologous chondrocyte transplantation. Eur J Radiol 57(1):24–31
Martin I, Miot S, Barbero A, Jakob M, Wendt D (2007) Osteochondral tissue engineering. J Biomech 40(4):750–765
Mastrogiacomo M, Muraglia A, Komlev V, Peyrin F, Rustichelli F, Crovace A, Cancedda R (2005) Tissue engineering of bone: search for a better scaffold. Orthod Craniofac Res 8(4):277–284
Miljkovic ND, Cooper GM, Marra KG (2008) Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthr Cartil 16(10):1121–1130
Moffat KL, Wang IN, Rodeo SA, Lu HH (2009) Orthopedic interface tissue engineering for the biological fixation of soft tissue grafts. Clin Sports Med 28(1):157–176
Nandi SK, Roy S, Mukherjee P, Kundu B, De DK, Basu D (2010) Orthopaedic applications of bone graft and graft substitutes: a review. Indian J Med Res 132:15–30
Natesan S, Baer DG, Walters TJ, Babu M, Christy RJ (2010) Adipose-derived stem cell delivery into collagen gels using chitosan microspheres. Tissue Eng Part A 16(4):1369–1384
Ng KW, Wang CC, Mauck RL, Kelly TA, Chahine NO, Costa KD, Ateshian GA, Hung CT (2005) A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs. J Orthop Res 23(1):134–141
Ochi M, Uchio Y, Tobita M, Kuriwaka M (2001) Current concepts in tissue engineering technique for repair of cartilage defect. Artif Organs 25(3):172–179
Pabbruwe MB, Esfandiari E, Kafienah W, Tarlton JF, Hollander AP (2009) Induction of cartilage integration by a chondrocyte/collagen-scaffold implant. Biomaterials 30(26):4277–4286
Partridge KA, Oreffo RO (2004) Gene delivery in bone tissue engineering: progress and prospects using viral and nonviral strategies. Tissue Eng 10(1–2):295–307
Phillips MA, Gran ML, Peppas NA (2010) Targeted nanodelivery of drugs and diagnostics. Nano Today 5:143–159
Polyak B, Fishbein I, Chorny M, Alferiev I, Williams D, Yellen B, Friedman G, Levy RJ (2008) High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc Natl Acad Sci USA 105:698–703
Prijic S, Scancar J, Romih R, Cemazar M, Bregar VB, Znidarsic A, Sersa G (2010) Increased cellular uptake of biocompatible superparamagnetic iron oxide nanoparticles into malignant cells by an external magnetic field. J Membr Biol 236(1):167–179
Qu D, Li J, Li Y, Khadka A, Zuo Y, Wang H, Liu Y, Cheng L (2011) Ectopic osteochondral formation of biomimetic porous PVA-n-HA/PA6 bilayered scaffold and BMSCs construct in rabbit. J Biomed Mater Res B Appl Biomater 96(1):9–15
Redman SN, Oldfield SF, Archer CW (2005) Current strategies for articular cartilage repair. Eur Cells Mater 9:23–32; discussion 23–32
Rodriguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ (2006) Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci USA 103(32):12167–12172
Sampat SR, O’Connell GD, Fong JV, Alegre-Aguaron E, Ateshian GA, Hung CT (2011) Growth factor priming of synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. doi:10.1089/ten.TEA.2011.0155
Sekiya I, Colter DC, Prockop DJ (2001) BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem Biophys Res Commun 284(2):411–418
Shannon FJ, Devitt AT, Poynton AR, Fitzpatrick P, Walsh MG (2001) Short-term benefit of arthroscopic washout in degenerative arthritis of the knee. Int Orthop 25(4):242–245
Solchaga LA, Cassiede P, Caplan AI (1998) Different response to osteo-inductive agents in bone marrow- and periosteum-derived cell preparations. Acta Orthop Scand 69(4):426–432
Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF (2005) FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol 203(2):398–409
Sun C, Du K, Fang C, Bhattarai N, Veiseh O, Kievit F, Stephen Z, Lee D, Ellenbogen RG, Ratner B, Zhang M (2010) PEG-mediated synthesis of highly dispersive multifunctional superparamagnetic nanoparticles: their physicochemical properties and function in vivo. ACS Nano 4(4):2402–2410
Swieszkowski W, Tuan BH, Kurzydlowski KJ, Hutmacher DW (2007) Repair and regeneration of osteochondral defects in the articular joints. Biomol Eng 24(5):489–495
Tampieri A, Celotti G, Landi E, Sandri M, Roveri N, Falini G (2003) Biologically inspired synthesis of bone-like composite: self-assembled collagen fibers/hydroxyapatite nanocrystals. J Biomed Mater Res A 67(2):618–625
Tampieri A, Landi E, Valentini F, Sandri M, D’Alessandro T, Dediu V, Marcacci M (2011) A conceptually new type of bio-hybrid scaffold for bone regeneration. Nanotechnology 22(1):015104
Temenoff JS, Mikos AG (2000) Review: tissue engineering for regeneration of articular cartilage. Biomaterials 21(5):431–440
Tognana E, Borrione A, De Luca C, Pavesio A (2007) Hyalograft® C: hyaluronan-based scaffolds in tissue-engineered cartilage. Cells Tissues Organs 186(2):97–103
Vandenabeele F, De Bari C, Moreels M, Lambrichts I, Dell’Accio F, Lippens PL, Luyten FP (2003) Morphological and immunocytochemical characterization of cultured fibroblast-like cells derived from adult human synovial membrane. Arch Histol Cytol 66(2):145–153
Ventura A, Memeo A, Borgo E, Terzaghi C, Legnani C, Albisetti W (2011) Repair of osteochondral lesions in the knee by chondrocyte implantation using the MACI((R)) technique. Knee Surg Sports Traumatol Arthrosc. doi: 10.1007/s00167-011-1575-0
Wang X, Wenk E, Zhang X, Meinel L, Vunjak-Novakovic G, Kaplan DL (2009) Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Release 134(2):81–90
Whittaker JP, Smith G, Makwana N, Roberts S, Harrison PE, Laing P, Richardson JB (2005) Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br 87(2):179–183
Zelle S, Zantop T, Schanz S, Petersen W (2007) Arthroscopic techniques for the fixation of a three-dimensional scaffold for autologous chondrocyte transplantation: structural properties in an in vitro model. Arthroscopy 23(10):1073–1078
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7(2):211–228
Acknowledgments
We are very grateful to Prof. M. Marcacci (Rizzoli Orthopaedic Institute) for valuable discussion and constructive criticism. The authors acknowledge the financial support from European Union project “MAGISTER” NMP3-LA-2008-214685.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panseri, S., Russo, A., Cunha, C. et al. Osteochondral tissue engineering approaches for articular cartilage and subchondral bone regeneration. Knee Surg Sports Traumatol Arthrosc 20, 1182–1191 (2012). https://doi.org/10.1007/s00167-011-1655-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-011-1655-1