Abstract
Purpose
Removal of the meniscus leads to progressive degenerative arthritis of the knee on a long-term basis; therefore, meniscal allograft transplantation has been proposed as an alternative to meniscectomy. Preservation methods are required to build up operational stocks and to provide living grafts of a practical size at the right time for patients. Methods for meniscus preservation have been published, and relevant literature confirms that using standard cryopreservation, the chondrocyte survival in situ is inadequate and extremely variable and the cryoinjury mechanisms are not completely established. The aim of the present study is to further investigate possible cellular injury caused by cryopreservation by analysing apoptosis and ultrastructural damage to menisci.
Methods
Seven human menisci that were cryopreserved by standard method were used. All tissue samples were processed simultaneously for routine light microscopy, scanning and transmission electron microscopy as well as apoptosis assessment by the use of ISOL method.
Results
With respect to cellularity, significant differences (P < 0.05) between the fresh (14.6 ± 3.5) (mean ± SD) and cryopreserved menisci (9.2 ± 2.8) (mean ± SD) were observed. Apoptosis using ISOL method was observed in fibrochondrocytes of fresh and cryopreserved menisci. The quantitative analysis revealed significant differences (P < 0.05) between fresh meniscus samples, where the apoptotic index was 0.8 ± 2.3% (mean ± SD), and cryopreserved meniscus samples, where this index was 50 ± 18.1% (mean ± SD).
Conclusion
The results suggest that apoptosis occurs during meniscus cryopreservation. The major findings of this study are cellular damage in meniscus cryopreservation suggesting apoptosis-mediated cell loss. The findings reported herein encourage to further investigations in preservation procedures to enhance maximum long-term clinical survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The meniscus plays a crucial role in the biomechanical homeostasis of the knee joint. It functions to provide shock absorption, load bearing, load transmission, lubrication, and joint congruency [9, 17]. When the meniscus is completely lost, transplantation of a meniscus allograft has been a therapeutic option with favourable improvements in knee pain and functionality [12, 13, 15, 21].
Deep frozen, lyophilized (freeze-dried) have been published as preservation methods; however, clinical results using these preservation methods have been discouraging [18, 22]. Cryopreservation, which is usually accomplished with dimethyl sulphoxide o glycerol, at least partially preserves cell membrane integrity and donor chondrocyte viability [10, 14, 18, 19].
Advances in low-temperature biology have produced high-viability preservation for cells and tissues; however, the development of preservation methods is not straightforward, and methods that work for many cells in suspension and connective tissue do not work for certain cell types and tissue, including chondrocytes in intact articular cartilage [1, 13, 15, 20].
Maintaining living chondrocytes within the meniscus is required for success. Chondrocytes occupy only 5% of the structure, but are responsible for the maintenance of the extensive surrounding matrix that comprises a highly complex network of collagen fibrils, associated proteoglycans and other non-collagenous proteins. In a recent paper, the percentage of cell survival after cryopreservation has been established between 4 and 54% [7].
Instead of optimal tissue preservation, cumulative evidence suggests that cryopreserved menisci suffer variable tissue changes, metabolic damage and some loss of cell structural details [15, 16]. As a basis for future studies on menisci cryopreservation, we initiated the present study to evaluate apoptotic cells died on menisci obtained and cryopreserved under current standard protocols.
Apoptosis, or programmed cell death, is a gene-activated event that occurs as a normal consequence of development as well as a result of cellular stress [4]. Baust et al. [3] demonstrated that cell viability improves following inhibition of cryopreservation-induced apoptosis. Our group demonstrated in a previous paper apoptosis as a possible contributing factor in cryopreservation failure [23].
The aim of the present study has been to investigate possible cellular injury caused by cryopreservation by analysing apoptosis and ultrastructural damage to menisci on the basis of further studies of apoptosis inhibition.
Materials and methods
Seven fresh human lateral menisci were harvested in sterile conditions during total of seven consecutive knee-replacement procedures for degenerative arthritis (6 women and 1 man). The study group had a mean age of 71.4 years (age range 61–80 years). Informed consent was obtained from each donor according to Spanish legislative framework (RD 1301/2006).
Radiographic evaluation as well as clinical intraoperative assessments was performed in order to ascertain the quality of the tissue. Menisci with macroscopic degeneration or even minimal calcification were excluded from the study. Microbiological culture was also performed for each graft, and if positive, they were also excluded.
Transport from operating theatre to cryobiology unit was performed in cold saline to 4°C.
Each meniscus was divided in two parts: one of them was used for cryopreservation (cryopreserved menisci), and the other was immediately prepared for histological analysis (fresh menisci).
Cryopreservation and thawing protocol
Pieces of one square centimetre from the bodies of each single meniscus were divided into sections, transferred to TC-199 medium containing 10% dimethyl sulphoxide and 20% human serum albumin, and frozen at a controlled rate of −1°C per min until −80°C and then −5°C per min until −120°C. Tissue was stored in liquid nitrogen vapour (−156°C) until use.
Tissue to be examined was rapidly thawed in a waterbath at 37°C, and the cryoprotectant was removed by sequential dilution in cold saline solutions (4°C) for at least three times.
After thawing, cryopreserved menisci were also prepared for histological analysis.
Histological analysis
Samples of both menisci, fresh and cryopreserved, were taken for light and electron microscopic analyses.
For light microscopy, samples were fixed in 10% neutral-buffered formalin and paraffin-embedded. Four consecutive sections of 6 μm thickness were obtained from each block. One of these sections was stained with haematoxylin–eosin, another with Masson′s trichrome stain, and the remaining sections were used for apoptosis by using the ISOL assay technique.
Samples to be examined by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were fixed by immersion in 2.5% glutaraldehyde. The tissues were washed in 0.1 M sodium cacodylate and dehydrated through a series of 10-min immersion in 30, 50, 70, 95 and 100% acetone at room temperature. For TEM, once rinsed, the tissue was fixed in 1% osmium tetroxide and embedded in Araldite. Ultrathin sections (50 nm thickness) were stained with uranyl acetate and lead citrate and viewed with a Philips CM-10 microscope. For SEM, the tissue was coated with gold using the critical point method and examined in a Jeol JSM-6300 scanning electron microscope.
Apoptosis assessment
After deparaffinization and rehydratation, meniscus sections were initially incubated with 3% hydrogen peroxide to inhibit endogenous peroxidise activity, followed by pretreatment with 150 μl proteinase K in phosphate-buffered solution for 15 min. After incubation with equilibration buffer, the sections were incubated with a 60-μl solution of T4 DNA ligase (6 μl) and biotinylated oligo A (54 μl) in a humidified chamber for 14 h at 20°C. The sections were then incubated with 60 μl streptavidin-peroxidase conjugate for 30 min followed by staining with 150 μl diaminobenzidine for 10 min at room temperature. Finally, tissue sections were counterstained in 0.5% methyl green for 10 min at room temperature.
Quantitative study
The criterion to establish an accurate quantification was to evaluate two sections per meniscus in both groups (fresh menisci and cryopreserved menisci). In each section, five areas (110 μm2 each) stained with ISOL technique and randomly selected were analysed; hereby, a total area of 1,100 μm2 (550 μm2/section) was checked.
The areas were captured digitally at a magnification of ×400 with a Sony Exwaved HAD camera mounted on a Nikon Eclipse E1000 microscope. We performed a semiautomated cell counting using an image-analysis program (Image-Pro Plus, Media Cybernetics, Version 6, 2006). In each area, the cellularity (total number of fibrochondrocytes: non-apoptotic + apoptotic) was evaluated. Non-apoptotic fibrochondrocytes were considered when the nucleus showed a green colour; apoptotic fibrochondrocytes (ISOL-positive) were considered when the nucleus showed a brown colour.
Cellularity and apoptotic fibrochondrocytes number were expressed as mean ± standard deviation (SD) in a 110-μm2 area. Both parameters were calculated from the average of 10 areas analysed in every meniscus; later was obtained the mean ± standard deviation (SD) of seven menisci analysed in every group was obtained later.
The number of apoptotic fibrochondrocytes was divided by the total number of fibrochondrocytes to calculate the apoptotic index; this index was expressed as the mean percentage ± standard deviation (SD) for a total area of 1,100 μm2 in every group. This parameter was calculated from the sum of the total fibrochondrocytes counted in the ten areas analysed in every meniscus; later was obtained the mean ± standard deviation (SD) of seven menisci analysed in every group. The same procedure was realized for apoptotic fibrochondrocytes.
Quantification was performed in a blinded fashion by an expertise (JP).
Statistical analysis
All data were expressed as the mean ± standard deviation (SD). After testing the normal distribution, differences were analysed with Wilcoxon signed-rank test. P value <0.05 was considered significant.
Results
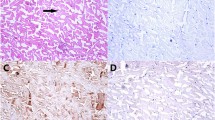
In fresh menisci, the typical histological features were evident in light microscopy by a homogeneous matrix and regular dispersion of cells; however, menisci that had been subjected to standard cryopreservation revealed empty lacunae (Fig. 1).
a Fresh meniscus; fibrochondrocytes in lacunae are either randomly arranged or disposed in longitudinal rows between bundles of dense collagen fibres. b Cryopreserved meniscus; lacunae containing degenerative fibrochondrocytes with atrophic nuclei and some lacunae that are devoid of a cell (arrow) are observed. Masson’s trichrome, 40×
The morphology of the cells, filling their lacunae, was round with round nuclei, whereas in cryopreserved menisci, the cells showed cytoplasmic absence and abnormalities in their nuclei (Fig. 2). With respect to cellularity, we observed significant differences (P < 0.05) between the fresh (14.6 ± 3.5) (mean ± SD) and cryopreserved menisci (9.2 ± 2.8) (Fig. 3).
Apoptosis using ISOL method was observed in fibrochondrocytes of fresh and cryopreserved menisci (Fig. 4), although the quantitative analysis revealed significant differences (P < 0.05) between cryopreserved menisci (4.5 ± 1.8) and fresh menisci (0.1 ± 0.4) (Fig. 3). The mean of apoptotic index in cryopreserved menisci (50 ± 18.1%) was significantly higher (P < 0.05) than that in the fresh menisci (0.8 ± 2.3%).
Identification of apoptotic fibrochondrocytes (arrows) by ISOL method and counterstained with methyl green. a Fresh meniscus; apoptotic cells were not observed. b Cryopreserved meniscus; in non-apoptotic cells, the nuclei are green, whereas apoptotic cells are identified by brown staining (arrows). 40×
SEM showed differences in cell morphology between the fresh and cryopreserved menisci. Normal fibrochondrocyte showed a spherical morphology, whereas in the cryopreserved menisci, the fibrochondrocytes had lost their spherical morphology and have irregular surface with multiple blebs (Fig. 5).
TEM showed that the fibrochondrocytes in the fresh menisci appeared to be normal (Fig. 6a). On the contrary, in cryopreserved menisci, although occasional cells with necrotic changes were observed, the majority of the fibrochondrocytes had undergone apoptosis, with peripheral segregation and aggregation of chromatin into dense areas along the nuclear membrane, swellings and dilatations of cell organelles (Figs. 6b).
Transmission electron micrographs showing: a fibrochondrocyte characterized by a normal appearance of the nucleus, cytoplasm and cell membrane in fresh meniscus; b fibrochondrocytes from cryopreserved meniscus undergoing apoptosis can be observed in more advanced apoptotic morphological changes with fragmented nuclei, dilated organelles and apoptotic bodies
Discussion
The most important finding of the present study was to show a significant apoptotic cell death in menisci cryopreservation.
Although previous work has demonstrated that cryopreservation does not alter the meniscal structure, the reported percentage of cell survival after cryopreservation ranges from 4 to 54% [7].
After the completion of skeletal growth, chondrocytes continue to play a vital role in maintaining the biophysical properties of the tissue through remodelling and biosynthesis of matrix components. Little or no cell division is observed in mature cartilage, so it is reasonable to assume that the fraction of live chondrocytes in meniscus implants for success should be high [15]. Many papers support the notion that function of transplanted grafts is proportional to their content of living cells [7, 11, 21, 23].
Pegg et al. [16] have demonstrated that the crystallization of ice is directly responsible for poor recovery. Their observations show that the ice propagates through the matrix nucleating each chondron that it encounters. They suggest that one practical way forward is to avoid the formation of ice altogether, that is, to vitrify the system.
Our results revealed apoptosis in cryopreserved human menisci after thawing. Apoptosis has been demonstrated by Hilbert et al. [8] in explanted valves, suggesting the contribution of multiple factors, including immunological and chemical injury, hypoxia during valve processing, and reperfusion injury at the time of implantation. Regulation of apoptosis could be essential for the improvement of cryopreservation outcome [3].
However, the present study has certain limitations that need to be taken into account when considering its contribution. We have used menisci from patients affected with osteoarthritis, so a hypothetical cellular vulnerability could be developed by senescence.
Many articles have reported ISOL technique as one of the most specific for apoptotic cell characterization, directly comparable to DNA denaturing analysis. [2, 5, 6].
The cryopreservation-dependent signalling that induces apoptosis is unknown, although it is apparent that the activation of the apoptotic mechanism within the cells is a direct result of stress experienced during the freeze–thaw process. One possible route might be the physical stress that is exerted on a cell membrane as a consequence of the ‘shrinkage and swelling’ of a cell during the freeze–thaw process [5, 6].
As a mechanism of cell loss, cryopreservation-induced apoptosis could be inhibited by the use of different additive substances. Activation of caspases is one of the crucial steps in the execution of programmed cell death and is often considered to be ‘point of no return’ in apoptotic pathways. This activation results in the initiation of a cascade of endogenous enzymes that lead to the cleavage of intracellular proteins and nuclear DNA and the formation of apoptotic bodies. The initiation of apoptosis can be inhibited through inhibition of caspase activity, and this has been demonstrated to be beneficial to the cryosurvival of mammalian cells [5].
In addition to Pegg’s observations, our results herein encourage further investigation into meniscus preservation procedures to enhance maximum long-term clinical survival. In future, studies may be designed to address the exact time of apoptosis initiation and to understand which of the different pathways (intrinsic, extrinsic and apoptotic-inducing factors) is exactly responsible for chondrocyte cell death in meniscus.
Systematic investigation of possible contribution’s factors that promote or inhibit apoptosis will be necessary in order to improve current techniques in meniscus cryopreservation.
Conclusion
This study has demonstrated a significant degree of apoptotic cell death in meniscus cryopreservation. A combined ISOL assay and histological examination offers a suitable method for the detection of apoptosis in this tissue.
References
Acosta CA, Izal I, Ripalda P et al (2007) Cell viability and protein composition in cryopreserved cartilage. Clin Orthop Related Res 460:234–239
Alexis C, Dang MD, Hubert T et al (2009) Chondrocyte apoptosis after simulated intraarticular fracture. Clin Orthop Relat Res 467:1877–1884
Baust JM, van Buskirk R, Baust JG (2000) Cell viability improves following inhibition of cryopreservation induced apoptosis. In vitro Cell Dev Biol 36:262–270
Cummings MC, Winterford CM, Walker NI (1997) Apoptosis. Am J Surg Pathol 21:88–101
Didenko VV, Hornsby PJ (1996) Presence of double-strand breaks with single-base 3′ overhangs in cells undergoing apoptosis but not necrosis. J Cell Biol 135:1369–1376
Frankfurt OS, Krishen A (2001) Identification of apoptotic cells by formamide-induced DNA denaturation in condensed chromatin. J Histochem Cytochem 49:369–378
Gelber PE, Gonzalez G, Torres R et al (2009) Cryopreservation does not alter the ultrastructure of the meniscus. Knee Surg Sports Traumatol Arthrosc 17:639–644
Hilbert SL, Luna RE, Zhang J et al (1999) Allograft heart valves: the role of apoptosis-mediated cell loss. J Thorac Cardiovasc Surg 117:454–462
Hommen JP, Applegate GR, del Pizzo W (2007) Meniscus allograft transplantation: ten year results of cryopreserved allografts. Arthroscopy 23:388–393
Lewis PB, Williams JM, Hallab N et al (2008) Multiple freeze-thaw cycled meniscal allograft tissue: a biomechanical, biochemical, and histologic analysis. J Orthop Res 49–55
Malinin TJ, Wagner JL, Piña JC et al (1985) Hypothermic storage and cryopreservation of cartilage. Clin Orthop Rel Res 197:15
McDermott ID (2010) What tissue bankers should know about the use of allograft meniscus in orthopaedics. Cell Tissue Bank 11:75–85
Noyes FR, Barber-Westin SD, Rankin M (2004) Meniscus transplantation in symptomatic patients less than fifty years old. J Bone Joint Surg Am 86:1392–1404
Pegg DE, Wusteman MC, Wang L (2006) Cryopreservation of articular cartilage. Part 1: conventional cryopreservation methods. Cryobiology 52:335–346
Pegg DE, Wang L, Vaughan D, Hunt C (2006) Cryopreservation of articular cartilage. Part 2: mechanisms of cryoinjury. Cryobiology 52:347–359
Pegg DE, Wang L, Vaughan D (2006) Cryopreservation of articular cartilage. Part 3: the liquidus-tracking method. Cryobiology 52:360–368
Peters G, Wirth CJ (2003) The current state of meniscal allograft transplantation and replacement. Knee 10:19–31
Rendal ME, Maneiro E, Rodriguez M et al (2001) Effect of cryopreservation on human articular chondrocyte viability, proliferation and collagen expression. Cryobiology 41:2–10
Rijk PC (2004) Meniscal allograft transplantation—Part I: background, results, graft selection and preservation, and surgical considerations. Arthroscopy 20:728–743
Schachar NS, Novak K, Hurting MB et al (1999) Transplantation of cryopreserved osteochondral dowel allografts for repair of focal articular defects in an ovine model. J Orthop Res 17:909–920
Stone KR, Adelson WS, Pelsis JR et al (2010) Long-term survival of consurrent meniscus allograft transplantation and repair of the articular cartilage: a prospective 2- to 12-year follow-up report. J Bone Joint Surg Br 92:941–948
Tomford WW, Springfield DS, Mankin HJ (1992) Fresh and frozen articular cartilage allografts. Orthopedics 15:1183–1188
Villalba R, Peña J, Luque E et al (2001) Characterization of ultrastructural damage of valves cryopreserved under standard conditions. Cryobiology 43:81–84
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Villalba, R., Peña, J., Navarro, P. et al. Cryopreservation increases apoptosis in human menisci. Knee Surg Sports Traumatol Arthrosc 20, 298–303 (2012). https://doi.org/10.1007/s00167-011-1622-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-011-1622-x