Abstract
Articular cartilage defects heal poorly. Autologous Matrix-Induced Chondrogenesis (AMIC) is an innovative treatment for localized full-thickness cartilage defects combining the well-known microfracturing with collagen scaffold and fibrin glue. The purpose of this prospective study was to evaluate the medium-term results of this enhanced microfracture technique for the treatment of chondral lesions of the knee. Thirty-two chondral lesions in 27 patients were treated with AMIC. Within the context of clinical follow-up, these patients were evaluated for up to 5 years after the intervention. Five different scores (Meyer score, Tegner score, Lysholm score, ICRS score, Cincinatti score) as well as radiographs were used for outcome analysis. Articular resurfacing was assessed by magnetic resonance imaging (MRI). The average age of patients (11 females, 16 males; mean body mass index 26, range 20–32) was 37 years (range 16–50 years). The mean defect size of the chondral lesions was 4.2 cm2 (range 1.3–8.8 cm2). All defects were classified as grade IV according to the Outerbridge classification. The follow-up period was between 24 and 62 months with a mean of 37 months. Twenty out of 23 individuals (87%) questioned were subjectively highly satisfied with the results after surgery. Significant improvement (P < 0.05) of all scores was observed as early as 12 months after AMIC, and further increased values were notable up to 24 months postoperatively. MRI analysis showed moderate to complete filling with a normal to incidentally hyperintense signal in most cases. Results did not show a clinical impact of patient’s age at the time of operation, body mass index and number of previous operations (n.s.). In contrast, males showed significant higher values in the ICRS score compared to their female counterparts. AMIC is an effective and safe method of treating symptomatic full-thickness chondral defects of the knee in appropriately selected cases. However, further studies with long-term follow-up are needed to determine whether the grafted area will maintain structural and functional integrity over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The limited healing potential of articular cartilage is a well-known problem in orthopedic surgery [28]. Cartilage degeneration may be accompanied by pain, immobility, stiffness, loss of quality of life and can potentially lead to severe osteoarthritis in the long term. Recently, a variety of surgical techniques that aim for resurfacing and regenerating of the articular cartilage have evolved. Since the clinical introduction of ACI by Brittberg et al. [7], a variety of clinical studies have documented the clinical effectiveness of implanting autologous culture-expanded chondrocytes for the regeneration of cartilage [28]. For classical ACI, a periosteal flap or a collagen sheet is sutured to the surrounding healthy cartilage rim, creating a reservoir for the injection of the autologous chondrocyte cell suspension. The need for an intact cartilage rim limits the use of classical ACI to some regions of the knee [19]. Furthermore, the periosteal graft covering the defect gave rise to problems [30]. To overcome the intrinsic technical disadvantages of classical ACI, cartilage tissue engineered grafts were developed that use the regenerative potential of autologous chondrocytes with three-dimensional scaffolds to stabilize the graft. Meanwhile, clinical results show the safety and effectiveness of collagen-based autologous chondrocyte grafts for the repair of cartilage defects [2]. However, cutting and repeated manipulation of the seeded membrane may result in the loss of critical chondrocytes [35]. The limited number of autologous chondrocytes from spare cartilage may hardly be adequate for the high demand of cells to constitute engineered cartilage. Thus, the number of harvested cells has to be expanded in vitro before seeding. Chondrogenic cells that are in more abundant supply can be used for cartilage tissue engineering. Bone marrow-derived mesenchymal stem cells (MSCs) are more plentiful and can be induced to form chondrogenic cells in vitro [21]. In procedures that penetrate the subchondral bone, chondrogenic cells (MSCs) migrate in the fibrin network of the blood clot [9]. However, the fibrin clot is not mechanically stable to withstand the tangential forces [12]. An implanted exogenous scaffold (e.g. a collagen matrix) may improve the mechanical stability and durability for endogenous cells and may provide a proper stimulus for chondrogenic differentiation and cartilage regeneration. The AMIC procedure provides two major advantages; on the one hand, it is a one-step procedure with no need of cartilage harvesting potentially leading to donor site morbidity, and on the other hand, it is cost-effective with no need of in vitro cell expansion.
The aim of this prospective study was to evaluate the AMIC procedure using a collagen I/III scaffold for the treatment of focal cartilage defects of the knee. Our hypothesis is that AMIC proves suitable to treat cartilage defects, to achieve functionality and to confirm clinical improvement over a period of up to 5 years after operation in a prospective clinical trial.
Materials and methods
All patients participating in the present study were educated in detail about the surgical technique and all alternative procedures with their advantages and disadvantages, and all participants chose to undergo the index surgical procedure. All patients signed informed consent to participate in follow-up examinations including radiographs and magnetic resonance tomography. The study was performed in compliance with the ethical review board of the University of Luebeck, Germany.
The indications for the index procedure in this series were clinical symptomatic chondral lesions grades III-IV according to Outerbridge [29], defect sizes more than 1 cm2 and defects situated at the femoral condyle, the patella or the trochlea. The main exclusion criteria were advanced osteoarthritis, significant narrowing of the joint lines, underlying rheumatic disease, total meniscectomie, massive overweight (BMI > 35) and deviation of the mechanical axis to the affected compartment.
Radiographs were taken preoperatively and possible osteoarthritic degenerations were evaluated by two independent observers using the Kellgren-Lawrence scoring system [4]. The observers were blinded to the procedure. A Kellgren-Lawrence Score of greater than or equal to 2 is defined as osteoarthritis.
From 2003 to 2005, 27 patients with 32 chondral defects of the knee were treated with AMIC. By March 2008, all patients had reached a minimum follow-up of 2 years. Four patients gave consent to a clinical follow-up examination of 4 years. Clinical examinations were performed on a regular basis and supplemented with additional radiographs and magnetic resonance tomography. All patients were evaluated standardized using the Lysholm score, the Tegner score, the Meyer score, the ICRS Score and the Cincinnati score.
In general, the median age of patients (11 females, 16 males; mean body mass index 26, range 20–32) was 39 years (range 16–50 years). The mean defect size of the chondral lesions was 4.2 cm2 (range 1.3–8.8 cm2). All defects were classified as grade IV according to the Outerbridge classification [29]. The defects were situated on the medial femoral condyle (n = 7), on the lateral femoral condyle (n = 3), on the patella (n = 9), at the trochlea (n = 2) and on the femoral condyle and the patella (n = 6). The chondral lesions were of traumatic origin in 13 patients (44%), due to aseptic necrosis of the subchondral bone in one patient (3%) and idiopathic in 18 patients (53%). In 16 patients (57%), the right knee, and in 11 patients (43%), the left knee was treated. Previous surgical procedures (n = 59) were arthroscopies (n = 30), partial meniscectomies (n = 6), abrasion arthroplasty (n = 9), drilling or microfracture (n = 5) and shaving (n = 9). When performing the AMIC procedure, concomitant surgical procedures such as a patella realignment surgery (n = 2) and medial capsular shift (n = 1) were performed. Three patients (one with cartilage defects in both knees) had to be excluded from follow-up due to the exclusion criteria.
Surgical procedure
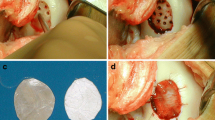
Degenerative and attached cartilage was completely removed during an arthroscopy. A minimal invasive portal was used to gain access to the cartilage defect. Perforations into the subchondral bone were made with a sharp canula every 5 mm. The microfractured defect was covered with a collagen I/III matrix of porcine origin (Geistlich Pharma AG, Wolhusen, Switzerland) that was prior trimmed to fit to the cartilage lesion by adaption to an appropriate template, which matches the size of the defect. Partial autologous fibrin glue (Baxter-Immuno, Heidelberg, Germany and autologous patient serum) was used regularly to fix the matrix. The knee joint was held in an extended position for 5 min before the joint was flexed ten times to test the stability and position of the matrix. Fig. 1 illustrates the steps of the AMIC procedure. The minimal invasive cut was closed in layers with standard techniques, and a drainage without suction was applied. The knee was immobilized for 7 days in extension followed by continuous passive motion for 6 weeks and nonweight bearing for 6 weeks.
Different scores formed the basis for evaluation of the therapeutic success: the Meyer score [2], the Tegner score [6], the Lysholm score [6], the Cincinnati score [38] and the ICRS score [18], representing the IKDC evaluation endorsed by the International Cartilage Repair Society (ICRS, www.cartilage.org). Postoperative findings that resulted from follow-up examinations conducted on a regularly basis every 12 months were documented and compared with the preoperative findings. Patients were informed to return to clinical follow-up after 12 months with current radiographs. At least 1 year after transplantation, repair and resurfacing of cartilage defects (n = 15) were evaluated with a state-of-the-art 1.5 Tesla MRI scanner (Siemens AG, Erlangen, Germany), and a modified MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) scoring system was applied [22]. According to the published classification system, marginal modifications were performed.
Statistical analysis
Coherent data of ordinal scaled variables were tested using the Student’s t-test. Statistical significance was tested with the Wilcoxon test for related and nonrelated samples. Differences were considered significant at P < 0.05. All comparisons were performed between scorings at the individual points in time of the follow-up period against the preoperative scores.
Results
At the time of follow-up, all patients had reached a minimum of 2 years after the index procedure. Three patients had to be excluded from the study, because in 1 patient, a delayed diagnosis of rheumatoid arthritis (treated defects in both knees) was made, and 2 patients were referred for arthroplasty due to progressive osteoarthritis. The follow-up period was between 24 and 62 months with a mean of 37 months. For 4 patients, 4 or more years had elapsed since the operation at the time this study was completed. Postoperative complications occured in two individuals but were of no negative consequence after treatment (muscle vein thrombosis, effusion after tumbling).
Twenty out of 23 individuals (87%) questioned were subjectively highly satisfied with the results after surgery and assured that they would undergo the same procedure again if they were in the same situation as at that time. From their subjective point of view, patients stated overall improvement of knee function of an average 89% (range, 70–100%; ±10.7), on a scale with 0% being knee function not allowing one to participate in normal daily life activities and 100% representing a knee function that allowed the patient all activities, including sports without any limitation at the same level as before onset of pain and disability. Three patients did not subjectively benefit from surgery and were not satisfied with their outcome.
The mean Meyer score showed a significant increase from preoperative 9 (±3) up to 14 (±3) at 12 months and up to 16 (±3) at 24 months follow-up. In the follow-up after 36 months the mean value was 14 (±3). In comparison to the preoperative values, the increase in values was significant at 12–36 months follow-up.
The mean Lysholm score was preoperative 36 (±21). A significant improvement was seen in the follow-up at 12 months with 67 (±28) and at 24 months with 76 (±24). After that, values decreased at 36 months with 62 (±25) and at 48 months with 47 (±22). Fig. 2 summarizes the results of the Lysholm score.
The mean Tegner score was after 1 year 3.4, after 2 years 4.1 and after 3 years 4.0. In conclusion, scores up to the 36-month follow-up were significantly higher compared to the preoperative values. The decline in scores between the 24-month and 36-month follow-up was not statistically significant.
The mean preoperative ICRS score was 31 (±15). At the follow-up, a significant increase in mean values was noted on the one hand at 12 months (59, ±24) and on the other hand at 24 months (68, ±22). The mean ICRS score was 54 (±25) at 36 months and 37 (±4) at 48 months. Results of the ICRS score are summarized in Fig. 3.
Preoperative values evaluated by the Cincinnati score accounted for a mean of 46 (±18). In comparison with the preoperative scores, values improved at 12 months (66, ±23) and 24 months (74, ±23) significantly. No further increase was demonstrated at follow-up after 36 months (62, ±26) and 48 months (37, ±9). Results of the Cincinnati score are summarized in Fig. 4.
In order to determine the influence of patient age at the time of operation on the results, patients were divided into three subgroups: patients aged between 18 and 32 years, patients aged between 33 and 46 years and patients between 47 and 60 years. Looking at the score results, none of the scores showed a significant difference. In older patients, the body mass index was significantly lower compared to the young patients group (P = 0.04). These findings did not show an impact on the clinical follow-up scores.
The Lysholm score and the IKDC score showed both for cartilage defects situated at the patella and medial femur condyle a significant increase in values up to 24 months. Values at 36 months decline significantly in the group of defects at the patella, but not at the femoral condyle.
The defect size in the individual groups (group I: defect size >0–3 cm2, group II: defect size >3–6 cm2, group III: defect size >6–9 cm2) did not differ significantly in the scores. In contrast, 2 cases exceeding a defect size of more than 8 cm2 lead to greatly reduced score results.
In order to investigate whether the score results were dependent on the number of previous operations, the patients were divided into two subgroups (no previous operation and previous operation). The score values proved to be independent of whether or not the patients had previously been operated.
There were no significant differences between male and female patients calculated for the Meyer score, Tegner score and Lysholm score, although males did show a tendency of better score values compared to their female counterparts (n.s.). The ICRS score did show significant better results up to 36-month follow-up for males compared to their female counterparts (P < 0.003); no female patient had a follow-up longer than 36 months. Fig. 5 illustrates the gender-specific differences of the ICRS score.
Results of imaging diagnostics
The state of osteoarthritis was evaluated by the well-established Kellgren and Lawrence score, staging osteoarthritis from grade I to grade IV [4]. All patients returned for clinical follow-up with current radiographies (p.a. and lateral). Some radiographs were weight-bearing radiographies and others not. Because narrowing of the joint space is one of the major criteria of the Kellgren and Lawrence score, results were by implication not comparable and we did not perform statistical analysis. Irrespective of the joint space criterion, 3 of the follow-up radiographs showed signs of progressive osteoarthritis (osteophytes, subchondral sclerosis).
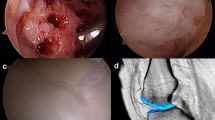
The majority of the patients (n = 15) treated with AMIC were examined by magnetic resonance imaging (MRI) at the end of their clinical follow-up investigation. A modified MOCART (Magnetic Resonance Observation of cartilage Repair Tissue) scoring system was applied, including seven variables to describe the morphology and signal intensity of the repair tissue [22]. Of all investigated features visible on MRI images, three items were predominant. Bone marrow lesions (BMLs, Fig. 6a) (n = 7), effusion (n = 8) and osseous hypertrophy underneath the repair tissue (n = 9) were found. The majority of patients (n = 10) showed a defect filling of more than 50% (Fig. 6b). In nine patients, a second cartilage defect was found within the same knee joint. None of the new defects was located at the same compartment as the repair tissue. Meniscal lesions (n = 4) and osteophytes (n = 3) could be found in some knees.
Discussion
The key finding in the present study was that an enhanced microfracture technique (AMIC) is well suited for the treatment of patients with focal cartilage defects. This is the first clinical study presenting mid-term results from autologous matrix-induced chondrogenesis (AMIC) of up to 60 months; thus, the required 24 months to obtain the final regenerate quality was fulfilled [3]. The status of the patient 2 years after cartilage repair is considered an important indicator for the future outcome [18].
In contrast to concurrent cartilage repair procedures, the AMIC procedure is easy to handle and can be done in a one-step surgery. No damage to healthy cartilage is carried out and, moreover, in vitro cultivation and differentiation of cells can be avoided using this enhanced microfracture technique. In previous studies, the hypothesis was verified that perforation of the subchondral bone plate gives rise to the stem cell pool of the bone marrow and leads to release of further marrow elements as growth factors and cytokines [17, 34]. We have presented strong evidence that bone marrow cells can be guided directly to a cartilage defect by a collagenous matrix and that MSCs can be isolated regularly from the matrix [17]. Therefore, this technique is less expensive, less time intensive and offers availability to all patients [1].
In cartilage repair procedures, usually the graft is secured to the surrounding native cartilage by sutures, which is a technically demanding and time-consuming procedure and may further damage the native tissue [10]. In a goat model, suturing of articular cartilage induced severe local damage, which was progressive and reminiscent of that associated with the early stages of osteoarthritis [14]. In the AMIC procedure, the matrix is fixed with fibrin glue. Fibrin glue can be used to adhere other engineered cartilage onto the recipient site, as a stand-alone scaffold or as a growth factor [10]. Its utility may be limited by its inferior mechanical properties, its inability to allow immigration of host cells and the possibility of evoking immune and inflammatory responses [13]. In this series, we used a semiautologous fibrin glue that offers superior properties compared to commercial fibrin glue, as we published before [13]. An implanted collagen scaffold seems to improve the mechanical stability of fibrin glue; we did not observe transplant loosening, debonding of the graft or ablation and in turn clinical complications and reoperations. Collagen scaffolds have been used in many studies that have loaded chondrocytes or MSCs to build cartilage in vitro or in vivo [2, 26, 33]. In a former microscopic study, we showed that cells grown on a collagen I/III membrane (Chondro-Gide®) form a multi-layered apical cell sheet with partially spindly, process-bearing cells and partially roundish, chondrocyte-like cells [13].
Well-established rating systems have been used in this study to summarize relevant outcome measures. Our data endorse the fact that the Lysholm score, rating mostly patients’ self reported criteria, leads to higher scores and categorical ratings. This may be based on the fact that this scale allows one to achieve a satisfactory score even when significant problems with the knee persist. In contrast, the IKDC guidelines for rating overall outcome are more stringent since the worst rating for any item within a group determines the overall group rating and the worst group rating determines the final IKDC rating [15]. In total, five different scores were enclosed in this study to allow a most objective view for outcome analysis.
In the current study, reporting mid-term results, patient satisfaction was found somewhat less compared to other publications. Authors reporting results after ACI claimed patient satisfaction of up to 95% after 2 years [8]. Repeat operations using the ACI procedure were mainly associated with the periosteal flap. This disadvantage of the original ACI technique cannot occur using the AMIC procedure. Mid-term, all ACI studies showed significant improvement in each of the different scoring methods employed [2]. Our results are comparable and indicate a significant improvement in four different scoring systems after a follow-up of up to 36 months after AMIC. Unfortunately, no other AMIC studies with mid-term results have been published to date.
Bone marrow stimulation techniques produced similar results in comparison with autologous chondrocyte implantation [16]. A current systematic analysis of the existing clinical literature of microfracture in the knee revealed that this technique effectively improved knee function in all studies during the first 24 months after microfracture, but the reports on durability of the initial functional improvement were conflicting [25]. Several factors were identified that affected clinical outcome. Shortcomings of the technique include limited hyaline repair tissue, variable repair cartilage volume and possible functional deterioration. As we did observe in the current study, some scores do not improve after a follow-up of 24 months as it has been reported for microfracturing [25]. In contrast, score results remain stable up to 60 months after AMIC, while they further decline after microfracture. It may be hypothesized that the durability of repair tissue is higher after AMIC compared to microfracture, leading to improved mid- and long-term results. Comparative studies to elucidate this fact are on their way.
We acknowledge that the presented patient population is heterogenous, which reflects the situation of patients with an indication for cartilage repair surgery. It is common that a patient population with cartilage defects of the knee may present with more than one isolated underlying pathology and thus needs more than one singular surgical procedure to address of all them. In our series concomitant surgical procedures were performed in three cases. Previous surgical procedures did not prove to negatively influence the follow-up results in our series. This is in contrast with the literature, reporting defects that had prior treatment affecting the subchondral bone failed at a rate 3 times that of nontreated defects [24].
After the AMIC technique, 2 out of 27 patients were subjected to revision surgery due to symptoms like grinding, catching, pain or swelling. This re-intervention rate is at first glance relatively high and may be related to this challenging patient cohort. This is in concordance with other studies reporting rates of revision surgery of between 0% [18] and 25% [23]. Neither of the 2 patients reached 50 points in the Lysholm score or 40 points in the ICRS score, indicating that there might be a threshold for long-term graft survival and successful tissue regeneration, as prior published [18]. Our data strengthen the fact that even if a graft regeneration takes a long time (2–3 years after surgery), a continuous improvement should be detected 12 months after surgery. In this context, the lack of clinical improvement, combined with insufficient MRI results, may be signs of long-term graft failure.
Unlike described in the literature, we could not show a correlation between clinical results and number of previous surgeries at the time of operation in general. This is in accordance with published data concerning mid-term results after MACT [2]. A current study shows contrary to the current data, that defects which had prior treatment affecting the subchondral bone failed at a rate 3 times that of nontreated defects [24]. The data demonstrate that marrow stimulation techniques have a strong negative effect on subsequent cartilage repair with autologous chondrocyte implantation and, therefore, should be used judiciously in larger cartilage defects that could require future treatment with autologous chondrocyte implantation. One have to regret that two patients with a defect size more than 8 cm2 did not benefit from the enhanced microfracture procedure. In consequence of this fact, we do not recommend the AMIC technique in cartilage defects more than 8 cm2.
Like described in the literature, we could not show a correlation between clinical results and patient age. Results after ACI showed a failure rate in older patients that is comparable with rates reported in younger patient groups [31].
In the current series, cartilage repair seems to be more efficient in males compared to their female counterparts. Little is known about gender-specific differences in cartilage repair. Further studies should elucidate this fact for a better understanding of gender-related dimorphism in knee pathology and improvement of related surgical treatments. In other fields, a gender-specific research is already on its way [5, 32].
Actual results strengthen the fact that clinical results and the incidence of complications is influenced by defect location. As it has been published before, there was a tendency for better clinical results at the femoral condyle compared to the patella [20]. Concerning the ACI, an increased rate of hypertrophy was found for patellar defects, but no correlation was found for the occurrence of delamination, insufficient regeneration and disturbed fusion [27]. Thus, therapeutic concepts had to be developed to prevent these typical complications of cartilage defect therapy at the patella. In the current series, no hypertrophy of repair tissue was seen in MRI follow-up studies at the patella. In conclusion, it may be hypothesized that different defect localizations in the knee need different cartilage repair procedures to improve functional results after cartilage repair surgery.
In general, for measuring and evaluating the clinical outcome of a given treatment strategy, patient’s satisfaction and improvement are most important and are best assessed by well-established clinical outcome scores. From the scientific point of view, additional detailed questions arise regarding measurable parameters like morphology and quality of the formed repair tissue as well as defect filling and graft integration. These issues can be addressed, for example, by noninvasive MRI techniques. MOCART-based scoring systems are well established to allow a practical and morphological evaluation of cartilage repair tissue [11]. Whereas Trattnig et al. [36] report a score of 73 in patients 12 months after a comparable cartilage repair procedure in the knee, Welsch et al. [37] depict scores of 73 for the patella and 72 for the medial femoral condyle in a cross-sectional evaluation with a mean of 29 months after MACT. A head-to-head record with our results is not valid, as we used a modified MOCART score based on the fact, that perforation of the subchondral lamina is part of the AMIC technique and thus its integrity could not be evaluated as it is suggested in the MOCART score. In our series, the majority of chondral repair tissue filled the defect depth sufficiently, constituted a smooth surface and was isointense to the surrounding cartilage.
There are two limitations that need to be acknowledged and addressed regarding the present study. The first limitation concerns the heterogenous patient population, which reflects the situation of patients with an indication for cartilage repair surgery. The second limitation has to do with the extent to which the findings can be generalized beyond the cases studied. The number of cases is too limited for broad generalization. However, these limitations can be seen as fruitful avenues for future research under the same theme.
Conclusion
This case series study shows promising results after the AMIC procedure for the treatment of focal cartilage defects of the knee. Clinical evaluation of up to 60 months after implantation revealed an improvement of the patient′s condition as documented by reliable clinical outcome scores as well as articular resurfacing as assessed by MRI. The good clinical results found 1 year after AMIC lasted for mid-term, although a nonsignificant decline in functional scores was obvious beyond a follow-up of 36 months.
References
Behrens P (2005) Matrixgekoppelte Mikrofrakturierung. Arthroskopie 18:193–197
Behrens P, Bitter T, Kurz B, Russlies M (2006) Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)–5-year follow-up. Knee 13:194–202
Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, Skinner JA, Pringle J (2003) A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br 85:223–230
Bliddal H, Boesen M, Christensen R, Kubassova O, Torp-Pedersen S (2008) Imaging as a follow-up tool in clinical trials and clinical practice. Best Pract Res Clin Rheumatol 22:1109–1126
Boyer KA, Beaupre GS, Andriacchi TP (2008) Gender differences exist in the hip joint moments of healthy older walkers. J Biomech 41:3360–3365
Briggs KK, Steadman JR, Hay CJ, Hines SL (2009) Lysholm score and tegner activity level in individuals with normal knees. Am J Sports Med 37:898–901
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Browne JE, Anderson AF, Arciero R, Mandelbaum B, Moseley JB Jr, Micheli LJ, Fu F, Erggelet C (2005) Clinical outcome of autologous chondrocyte implantation at 5 years in US subjects. Clin Orthop Relat Res 436:237–245
Cerynik DL, Lewullis GE, Joves BC, Palmer MP, Tom JA (2009) Outcomes of microfracture in professional basketball players. Knee Surg Sports Traumatol Arthrosc 17:1135–1139
Chiang H, Jiang CC (2009) Repair of articular cartilage defects: review and perspectives. J Formos Med Assoc 108:87–101
Dhollander AA, Huysse WC, Verdonk PC, Verstraete KL, Verdonk R, Verbruggen G, Almqvist KF (2009) MRI evaluation of a new scaffold-based allogenic chondrocyte implantation for cartilage repair. Eur J Radiol [Epub ahead of print]
Dorotka R, Windberger U, Macfelda K, Bindreiter U, Toma C, Nehrer S (2005) Repair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrix. Biomaterials 26:3617–3629
Gille J, Meisner U, Ehlers EM, Muller A, Russlies M, Behrens P (2005) Migration pattern, morphology and viability of cells suspended in or sealed with fibrin glue: a histomorphologic study. Tissue Cell 37:339–348
Hunziker EB, Stahli A (2008) Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthritis Cartilage 16:1067–1073
Irrgang JJ, Ho H, Harner CD, Fu FH (1998) Use of the international knee documentation committee guidelines to assess outcome following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 6:107–114
Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O (2007) A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am 89:2105–2112
Kramer J, Bohrnsen F, Lindner U, Behrens P, Schlenke P, Rohwedel J (2006) In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci 63:616–626
Kreuz PC, Muller S, Ossendorf C, Kaps C, Erggelet C (2009) Treatment of focal degenerative cartilage defects with polymer-based autologous chondrocyte grafts: four-year clinical results. Arthritis Res Ther 11:R33
Kreuz PC, Steinwachs M, Erggelet C, Lahm A, Krause S, Ossendorf C, Meier D, Ghanem N, Uhl M (2007) Importance of sports in cartilage regeneration after autologous chondrocyte implantation: a prospective study with a 3-year follow-up. Am J Sports Med 35:1261–1268
Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, Sudkamp N (2006) Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage 14:1119–1125
Lee CR, Breinan HA, Nehrer S, Spector M (2000) Articular cartilage chondrocytes in type I and type II collagen-GAG matrices exhibit contractile behavior in vitro. Tissue Eng 6:555–565
Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S (2006) Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol 57:16–23
Minas T (2001) Autologous chondrocyte implantation for focal chondral defects of the knee. Clin Orthop Relat Res 391:349–361
Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T (2009) Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med 37:902–908
Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR (2009) Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med 37:2053–2063
Nehrer S, Breinan HA, Ramappa A, Shortkroff S, Young G, Minas T, Sledge CB, Yannas IV, Spector M (1997) Canine chondrocytes seeded in type I and type II collagen implants investigated in vitro. J Biomed Mater Res 38:95–104
Niemeyer P, Pestka JM, Kreuz PC, Erggelet C, Schmal H, Suedkamp NP, Steinwachs M (2008) Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med 36:2091–2099
Ossendorf C, Kaps C, Kreuz PC, Burmester GR, Sittinger M, Erggelet C (2007) Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res Ther 9:R41
Outerbridge RE (1961) The etiology of chondromalacia patellae. J Bone Joint Surg Br 43-B:752–757
Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A (2000) Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res 374:212–234
Rosenberger RE, Gomoll AH, Bryant T, Minas T (2008) Repair of large chondral defects of the knee with autologous chondrocyte implantation in patients 45 years or older. Am J Sports Med 36:2336–2344
Rosenstein AD, Veazey B, Shephard D, Xu KT (2008) Gender differences in the distal femur dimensions and variation patterns in relation to TKA component sizing. Orthopedics 31:652
Russlies M, Behrens P, Ehlers EM, Brohl C, Vindigni C, Spector M, Kurz B (2005) Periosteum stimulates subchondral bone densification in autologous chondrocyte transplantation in a sheep model. Cell Tissue Res 319:133–142
Steadman JR, Rodkey WG, Briggs KK (2002) Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg 15:170–176
Steinwachs M (2009) New technique for cell-seeded collagen matrix-supported autologous chondrocyte transplantation. Arthroscopy 25:208–211
Trattnig S, Ba-Ssalamah A, Pinker K, Plank C, Vecsei V, Marlovits S (2005) Matrix-based autologous chondrocyte implantation for cartilage repair: noninvasive monitoring by high-resolution magnetic resonance imaging. Magn Reson Imaging 23:779–787
Welsch GH, Trattnig S, Scheffler K, Szomonanyi P, Quirbach S, Marlovits S, Domayer S, Bieri O, Mamisch TC (2008) Magnetization transfer contrast and T2 mapping in the evaluation of cartilage repair tissue with 3T MRI. J Magn Reson Imaging 28:979–986
Wright RW (2009) Knee injury outcomes measures. J Am Acad Orthop Surg 17:31–39
Acknowledgments
Written consent for publication was obtained from the patient or their relative. The authors would like to thank Katja Martin for reviewing the early manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gille, J., Schuseil, E., Wimmer, J. et al. Mid-term results of Autologous Matrix-Induced Chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc 18, 1456–1464 (2010). https://doi.org/10.1007/s00167-010-1042-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-010-1042-3