Abstract
Purpose
The autologous collagen-induced chondrogenesis technique is described, and the results of a 6-year follow-up clinical study using this technique are presented.
Methods
30 patients with International Cartilage Repair Society (ICRS) Grade III/IVa symptomatic chondral defects of the knee treated with enhanced microdrilling using atelocollagen were prospectively examined in this clinical series. The median age of the patients was 39.0 years (range 19–61 years). Patients were followed up to 72 months. Clinical evaluation was performed using functional knee scores and radiologically. Both quantitative and qualitative assessments were performed.
Results
Statistically significant and clinically relevant improvement was observed in 2 years and was sustained for the 6 years of the study observation. At 6 years, the mean Lysholm score was 79.7 (SD 6.8) compared to 52.6 (SD 10.7) pre-operatively (p < 0.05). The symptomatic Knee Injury and Osteoarthritis Outcome Score (KOOS) improved from 68.3 (SD 11.4) to 90.2 (SD 4.3) (p < 0.05). The subjective International Knee Documentation Committee (IKDC) also showed improvement from 39.1 (SD 4.1) to 81.6 (SD 7.8) (p < 0.05). The calculated T2* relaxation times were 26.0 (SD 4.2) seconds and 30.3 (SD 6.2) seconds for the repair tissue and native cartilage, respectively. The average magnetic resonance observation of cartilage repair tissue (MOCART) score was 78.5 (SD 9.6) for all lesions.
Conclusion
The enhanced microdrilling using atelocollagen is an enhancement of the traditional microfracture method using an off-the-shelf product. When used to treat moderate to severe chondral lesions, this enhancement produces hyaline-like cartilage with a corresponding improvement in symptoms.
Level of evidence
IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The last 2 decades have seen growing amounts of research dedicated to the topic of articular cartilage defects, which continue to be of high prevalence and a significant source of disability worldwide. Full-thickness chondral lesions show a limited ability to regenerate and are a cause of symptoms such as pain, locking, and swelling. Untreated, the natural history of these defects may lead to progressive and irreversible architectural changes of osteoarthritis, thus considerably increasing the socioeconomic burden on the healthcare system. Despite the development of numerous successful cartilage regenerative procedures, the microfracture approach remains the most popular technique among orthopaedic surgeons globally. Although it has been demonstrated extensively in the literature that this technique produces inferior fibrocartilage with shorter duration of clinical improvement, it is the technical simplicity, cost-effectiveness, and all-arthroscopic approach of this procedure that make it appealing to clinicians [1, 2]
The challenge remains that of identifying a practical, minimally invasive technique that is sensitive to the health economic constraints in the provision of modern orthopaedic services, and most importantly, in the ability to regenerate normal hyaline cartilage. There is also a need to identify an off-the-shelf solution for the surgeon who identifies a potentially treatable lesion during other surgical procedures.
The development of an enhanced microdrilling technique using atelocollagen, autologous collagen-induced chondrogenesis, progressing from in vitro analysis through to animal model pre-clinical trials has been previously described [3,4,5,6]. Atelocollagen, a purified type I collagen rendered non-immunogenic by the removal of telopeptides has been used as a scaffold [7]. Collagen is known to conduct chondrogenic cells and is naturally remodelled as opposed to synthetic scaffolds, which degrade hydrolytically [8, 9]. The scaffold is rendered adherent using fibrin glue.
Further, the absence of a cellular component to the repair scaffold would allow for reduced complexity, cost, and infrastructure requirements. The purpose of the study was to validate a pragmatic off-the-shelf regenerative technique to manage the challenge of moderate to large chondral lesions. The hypothesis of the study was that the microfracture technique could be augmented by the atelocollagen scaffold through arthroscopic application with carbon dioxide (CO2) insufflation of the joint to improve the quality of repair tissue.
Materials and methods
After obtaining National Research Ethics Committee approval (NREC 13/LO/1780), 30 patients were recruited with International Cartilage Repair Society (ICRS) Grade III and IVa chondral lesions identified by magnetic resonance imaging (MRI) to undergo enhanced microdrilling using atelocollagen. Inclusion criteria comprised knees with a mechanical axis that did not deviate more than 5° from normal, no active inflammatory process, and intact cruciate and collateral ligaments. No age or body mass index (BMI) restriction was imposed. Pre-operative symptom scoring was assessed using the Knee Injury and Osteoarthritis Outcome Score (KOOS), International Knee Documentation Committee score (IKDC), and Lysholm scoring systems. A pre-operative MRI was obtained for all patients and the cartilage defect on MRI findings was correlated with physical examination by direct compression or rubbing the cartilage defect area. Notably, all of the patients showed considerable discomfort with this clinical examination.
Surgical technique
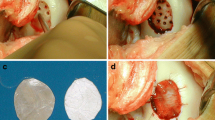
The surgical procedure began as a routine knee arthroscopy. The lesion was mapped according to ICRS guidelines. The lesion was then debrided to stable vertical margins with every attempt to ensure that the resulting defect was contained (i.e. there was a rim of native cartilage surrounding the treatment area). The surface was lightly abraded with a burr to reduce the subchondral sclerosis without altering the contour. Microdrilling was carried out. The subchondral bone of the medial femoral condyle was drilled with a 3.5-mm-diameter drill up to a depth of 10 mm with a 3-mm interval. The subchondral bone of the patella was drilled with a 45° angled drill (PowerPick drill, Arthrex, UK). The drilling was performed under water. The joint was then washed out and insufflated with CO2. The surface was dried with cotton buds to promote adhesion. The gel was prepared and applied using a duel syringe (ratio—fibrin 1:thrombin 0.2:atelocollagen 0.8) used in the application of fibrin glue, via a 20-gauge spinal needle (Fig. 1a, b). Once the gel was set and found to be stable to cycling, the instruments were removed and the portals were closed. A local anaesthetic was applied to the portals.
Clinical case. a Grade IVa chondral defect of the medial femoral condyle. b Atelocollagen is applied to the defect site under CO2 insufflation after lesion preparation and microdrilling. c Second-look arthroscopy at 2-year follow-up showing complete cartilage growth. d Trochlear defect with T2* values of 26 ms for the repair area and 26 ms for the surrounding healthy cartilage. This suggests a similar biochemical composition of the repair area and the native cartilage, which indicates hyaline-like repair tissue
Post-operative protocol
Patients were provided continuous passive motion (CPM) rehabilitation for 4 h post-operatively and were discharged on the same day. CPM was continued at home for 4–6 weeks. Patients were instructed by the physiotherapist on the initial knee exercises and the use of appropriate crutches along with partial weight bearing for 6 weeks. The cartilage repair for patellar and trochlear lesions was further protected with a knee brace locked at 0–20° movement, which was gradually increased to 90° over 6 weeks.
Follow-up was assessed by symptom scoring (KOOS, IKDC and Lysholm scoring systems) at 6 weeks, 6 months, 1 year, and then yearly thereafter with the last follow-up occurring at 6 years. Radiological evaluation included morphological MRI and quantitative assessment using T2* mapping and delayed gadolinium-enhanced MRI of cartilage (d-GEMRIC) scans. This was performed to assess the status of cartilage repair at 6 months, 1 year, and 3 years, while the subsequent yearly assessments were carried out using the magnetic resonance observation of cartilage repair tissue (MOCART) scoring system (Fig. 1c, d).
Statistical analysis
G*power software version 3.1.3 was used to estimate sample size, which was obtained based on repeated measure and within-factor analysis. This study compared symptom scores obtained preoperatively to 6 years post-operatively. The large effect size of 0.40 was verified from a previous study indicating that there was a statistically significant difference in 7 variables among 12 variables in each group [10]. The sample size of eight patients was calculated based on a significance level of 0.05, power of 0.80, six repeated measures, the default value of correlation among repeated measures, and nonsphericity correlation.
Statistical analysis was performed using SAS software version 9.2 (SAS Institute Inc., Cary, NC). All variables were summarized with standard descriptive statistics such as mean, standard deviation (SD), median, and range.
The Mann–Whitney test was used to compare improvement between the groups. Statistical significance was defined as p < 0.05. Continuous data analysis was performed through descriptive statistics. The Wilcoxon signed rank test was used for the comparison of scores between pre-operative and post-operative status.
Results
The median age of the patients was 39.0 years (range 19–61 years) and they were followed up to 72 months. The mean lesion size was 4.6 cm2 (SD 2.0). At 6 years, the mean Lysholm score was 79.7 (SD 6.8), compared to 52.6 (SD 10.7) pre-operatively (p < 0.05). Symptomatic KOOS improved to 90.2 (SD 4.3) from 68.3 (SD 11.4) (p < 0.05). Subjective IKDC also showed improvement from 39.1 (SD 4.1) to 81.6 (SD 7.8) (p< 0.05) (Fig. 2). The mean T2* relaxation times calculated were 26.0 (SD 4.2) and 30.3 (SD 6.2) for the repair tissue and native cartilage, respectively. The mean MOCART score was 78.5 (SD 9.6) for all lesions.
Discussion
This study demonstrates sustained good clinical results from an enhancement of a standard microfracture technique that is reinforced with MRI evidence suggestive of successful chondral regeneration. The many diverse approaches used to regenerate lost articular cartilage can be broadly divided into techniques that may stimulate native tissue, deliver a scaffold able to conduct chondrogenic tissue, deliver a cellular component that will transform into cartilage, and a combination of the above [6, 11,12,13]. Osteochondral transplantation using allografts or autografts aims to provide hyaline or hyaline-like repair for articular defects and has shown good long-term results in some papers [14, 15], but these techniques are restricted by limited donor site availability, incongruent grafts, and alteration of biomechanical properties of the joint [12, 16].
Many techniques are prohibitively expensive for the general public health services [16, 17], while others require extensive arthrotomy with the associated morbidity [16]. The overhead of the application of cultured cells, whether chondrocytes or mesenchymal stem cells, adds yet another obstacle to progressing away from a microfracture technique known to produce inferior fibrocartilaginous regenerate deficient in bound hydrophilic components [1, 18]. Thus, despite the numerous modern regenerative techniques available, the standard microfracture (or microdrilling) technique remains the mainstay of surgical therapy [19].
Currently, the majority of chondral lesions have been identified incidentally when performing arthroscopy for other conditions [20, 21]. In such cases, either the patient is given a microfracture or is brought back at a later date for definitive chondro-regenerative therapy in a second surgical procedure. The high costs of cartilage repair procedures, repeated surgeries, and waiting periods are all factors the surgeon should consider. Nonetheless, another barrier to adoption of more complex techniques is the associated learning curve, along with the motivation for their implementation. Thus, such techniques often fail to achieve widespread mainstream adoption. While most surgeons would not treat asymptomatic lesions, the natural history of moderate chondral and osteochondral lesions found incidentally is a steady progression toward ones that are symptomatic and associated with structural subchondral changes. An incidental discovery on MRI scan or at arthroscopy thus offers the opportunity of preventing this progression, yet there are clearly ethical issues in treating such lesions with procedures which themselves may have significant morbidity.
The enhanced microdrilling technique using atelocollagen followed in our series is an augmentation of the standard microfracture procedure with no appreciable increase in morbidity [3, 5]. It is an all-arthroscopic procedure unique in two aspects. First, the use of CO2 for joint insufflation is a major advantage because of its proven excellent safety profile. It allows for gravity-independent application of the gel mixture and contributes to the technical simplicity. Second, the technique involves the use of atelocollagen, which is a nonantigenic and highly purified type I collagen. The advantage of the atelocollagen–fibrin mixture is that it can be used irrespective of the depth or size of the defect. Furthermore, the clotting properties and mouldable nature of the mixture ensures a good and stable coverage of the defect.
The authors preferred microdrilling to the microfracture technique. The disadvantage of bone compaction associated with microfracture and the resulting reduced bone marrow, which can potentially affect cartilage repair, is avoided by microdrilling [22]. Furthermore, even the potential for heat necrosis reported to be associated with microdrilling has been disproved by some studies [23]. It is possible that these changes in technique, however minor they may appear, may have a considerable impact on the long-term success of cartilage repair.
The significantly improved functional scores (IKDC, Lysholm and KOOS) noted at the 2-year mark were maintained at the 6-year follow-up. The results are comparable to similar studies with a medium term follow-up. Improved outcomes in younger patients and in patients with normal BMI were observed, but the small number of patients enrolled makes stratification by age or BMI difficult to interpret. However, very few studies on regenerative neochondrogenesis show results beyond the 5-year follow-up.
To assess the true nature of the regenerate, a non-invasive system of evaluation including MRI was used, which compared signals from the regenerate against the normal native articular cartilage [24]. Quantitative T2* mapping at follow-up demonstrated identical values in the repair tissue and native cartilage. Because this modality has been shown to have low sensitivity for glycosaminoglycan content, an additional d-GEMRIC scan was performed. This scan performed at the 1-year follow-up confirmed the hyaline-like properties of the enhanced microdrilling using atelocollagen repair tissue. An average MOCART score of 78 was similar to other successful cartilage repair techniques like the matrix-induced autologous chondrocyte implantation (MACI) [25]. Patello-femoral chondral defects had similar MOCART scores as those for condylar lesions and there was no significant difference in outcome between the two groups.
A significant advantage of this technique is its cost-effectiveness compared to the currently practised cell-based strategies. The enhanced microdrilling using atelocollagen procedure performed in the UK would cost around £5,168, which is less than one-third of the cost of the standard two-stage MACI technique (£16,226) [16] and continues to remain the near gold standard. In addition to having demonstrated good functional and clinical outcomes at the midterm follow-up, other advantages of this technique include its minimally invasive nature and off-the-shelf availability. In particular, a Human Tissue Authority license is not required for the use of atelocollagen.
The many recent innovations in management of chondral lesions highlight the importance of the disease and the difficulties in identifying a management algorithm that is acceptable to the patient, clinician, and health service provider. This rules out the classical autologous chondrocyte implantation technique and its subsequent cell culture-dependent variations by virtue of these techniques needing an expensive intermediate stage. The goal of reversing the degenerative process for every patient is incompletely satisfied by most regenerative procedures; for this reason, a pragmatic approach is more desirable. The economic impracticality of most advanced chondro-regenerative procedures is highlighted by the fact that microfracture still remains the most common method of treating such lesions. To reach mainstream, a technique must have low barriers to adoption.
There are some limitations that should be mentioned. The study design was a prospective case series of a relatively small sample size. While the results are positive and statistically significant for multiple observed parameters, the small cohort size makes it impossible to draw firm conclusions regarding the efficacy of this treatment.
Nevertheless, the overall results are very encouraging. Performing a randomized control trial (RCT) would be ideal but challenging at the same time. A study by Engen et al. published an RCT on cartilage repair and concluded that the results of published RCTs may not actually represent the entire cartilage population [26]. Another limitation could be the lack of restrictions on age or BMI as inclusion criteria. Older age and higher BMI may have a negative influence on the overall scores, although there was no statistically significant difference observed in our series.
Conclusion
This arthroscopic method enhances a widely performed chondro-stimulation procedure using off-the-shelf products with minimal costs compared to cell-based methods. Extending the scope of this procedure to target patients with moderate to large symptomatic lesions who would conventionally be offered implant arthroplasty benefits the patient with reduced morbidity and the health service with reduced cost. Further study with a larger cohort is required.
References
Case JM, Scopp JM (2016) Treatment of articular cartilage defects of the knee with microfracture and enhanced microfracture techniques. Sports Med Arthrosc Rev 24(2):63–68
Redondo ML, Beer AJ, Yanke AB (2018) Cartilage restoration: microfracture and osteochondral autograft transplantation. J Knee Surg 31(3):231–238
Jeong IH, Shetty AA, Kim SJ, Jang JD, Kim YJ, Chung YG, Choi NY, Liu CH (2013) Autologous collagen-induced chondrogenesis using fibrin and atelocollagen mixture. Cells Tissues Organs 198(4):278–288
Shetty AA, Kim SJ, Shetty V, Jang JD, Huh SW, Lee DH (2016) Autologous collagen induced chondrogenesis (ACIC: Shetty-Kim technique)—a matrix based acellular single stage arthroscopic cartilage repair technique. J Clin Orthop Trauma 7(3):164–169
Shetty AA, Kim SJ, Bilagi P, Stelzeneder D (2013) Autologous collagen-induced chondrogenesis: single-stage arthroscopic cartilage repair technique. Orthopedics 36(5):e648–652
Stelzeneder D, Shetty AA, Kim SJ, Trattnig S, Domayer SE, Shetty V, Bilagi P (2013) Repair tissue quality after arthroscopic autologous collagen-induced chondrogenesis (ACIC) assessed via T2* mapping. Skeletal Radiol 42(12):1657–1664
Lee HS, Oh KJ, Moon YW, In Y, Lee HJ, Kwon SY (2019) Intra-articular injection of type I atelocollagen to alleviate knee pain: a double-blind, randomized controlled trial. Cartilage. https://doi.org/10.1177/1947603519865304
Irawan V, Sung TC, Higuchi A, Ikoma T (2018) Collagen scaffolds in cartilage tissue engineering and relevant approaches for future development. Tissue Eng Regen Med 15(6):673–697
Park YB, Ha CW, Rhim JH, Lee HJ (2018) Stem cell therapy for articular cartilage repair: review of the entity of cell populations used and the result of the clinical application of each entity. Am J Sports Med 46(10):2540–2552
Kusano T, Jakob RP, Gautier E, Magnussen RA, Hoogewoud H, Jacobi M (2012) Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg Sports Traumatol Arthrosc 20(10):2109–2115
Feng H (2018) Is stem cell therapy the future of orthopedics? Knee Surg Relat Res 30(3):177–178
Huh SW, Shetty AA, Kim JM, Cho ML, Kim SA, Yang S, Kim YJ, Javaregowda PK, Choi NY, Kang J, Kim SJ (2016) Autologous bone marrow mesenchymal cell induced chondrogenesis for the treatment of osteoarthritis of knee. J Tissue Eng Regen Med 13(2):200–209
Welton KL, Logterman S, Bartley JH, Vidal AF, McCarty EC (2018) Knee cartilage repair and restoration: common problems and solutions. Clin Sports Med 37(2):307–330
Haien Z, Jiachang W, Qiang L, Yufeng M, Zhenwei J (2018) Osteochondral autologous transplantation compared to microfracture for treating osteochondral defect: an updated meta-analysis of randomized controlled trials. J Knee Surg 31(4):341–347
Pareek A, Reardon PJ, Maak TG, Levy BA, Stuart MJ, Krych AJ (2016) Long-term outcomes after osteochondral autograft transfer: a systematic review at mean follow-up of 10.2 years. Arthroscopy 32(6):1174–1184
Mistry H, Connock M, Pink J, Shyangdan D, Clar C, Royle P, Court R, Biant LC, Metcalfe A, Waugh N (2017) Autologous chondrocyte implantation in the knee : systematic review and economic evaluation. Health Technol Assess 21(6):1–294. https://doi.org/10.3310/hta21060
de Windt TS, Sorel JC, Vonk LA, Kip MMA, Ijzerman MJ, Saris DBF (2017) Early health economic modelling of single-stage cartilage repair guiding implementation of technologies in regenerative medicine. J Tissue Eng Regen Med 11(10):2950–2959
Albright JC, Daoud AK (2017) Microfracture and microfracture plus. Clin Sports Med 36(3):501–507
Erggelet C, Vavken P (2016) Microfracture for the treatment of cartilage defects in the knee joint—a golden standard? J Clin Orthop Trauma 7(3):145–152
Fodor P, Solyom A, Ivanescu A, Fodor R, Bataga T (2018) Prevalence of chondral lesions in knee arthroscopy. J Interdiscip Med 3(1):21–24
Makovicka JL, Patel KA, Hassebrock JD, Hartigan DE, Wong M, Chhabra A (2019) Arthroscopic evaluation of knee cartilage using optical reflection spectroscopy. Arthrosc Tech 8(4):e399. https://doi.org/10.1016/j.eats.2018.11.019
Eldracher M, Orth P, Cucchiarini M, Pape D, Madry H (2014) Small subchondral drill holes improve marrow stimulation of articular cartilage defects. Am J Sports Med 42(11):2741–2750
Tahmasbi V, Ghoreishi M, Zolfaghari M (2018) Sensitivity analysis of temperature and force in robotic bone drilling process using Sobol statistical method. Biotechnol Biotechnol Equip 32(1):130–141
Chen Z, Yan C, Yan S, Liu Q, Hou M, Xu Y, Guo R (2018) Non-invasive monitoring of in vivo hydrogel degradation and cartilage regeneration by multiparametric MR imaging. Theranostics 8(4):1146–1158
Aldrian S, Zak L, Wondrasch B, Albrecht C, Stelzeneder B, Binder H, Kovar F, Trattnig S, Marlovits S (2014) Clinical and radiological long-term outcomes after matrix-induced autologous chondrocyte transplantation. Am J Sports Med 42(11):2680–2688
Engen CN, Engebretsen L, Årøen A (2010) Knee cartilage defect patients enrolled in randomized controlled trials are not representative of patients in orthopedic practice. Cartilage 1(4):312–319
Acknowledgements
SK was involved in reviewing the literature and drafting the manuscript as the main author. NK, NS, DS, and SA were involved in documentation and data preparation. SL, YS, and YC were involved in the interpretation of the results and giving intellectual contributions. AS gave an original concept of this paper and was responsible for the final proofreading of the manuscript. All authors read and approved the final manuscript.
Funding
This study had no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee (Health Research Authority, NHS) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, S.J., Shetty, A.A., Kurian, N.M. et al. Articular cartilage repair using autologous collagen-induced chondrogenesis (ACIC): a pragmatic and cost-effective enhancement of a traditional technique. Knee Surg Sports Traumatol Arthrosc 28, 2598–2603 (2020). https://doi.org/10.1007/s00167-020-05884-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-020-05884-y