Abstract
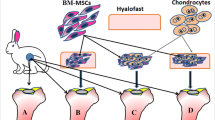
The purpose of this study was to evaluate the efficiency of using mesenchymal stem cells (MSC) in a hyaluronan scaffold for repair of an osteochondral defect in rabbit knee. Bone marrow was harvested from the posterior iliac crest in 11 New Zealand White rabbits. MSC were isolated and cultured in autologous serum for 28 days and transferred to a hyaluronan scaffold 24 h prior to implantation. A 4 mm diameter and 1.5 mm deep defect was created in the medial femoral condyle of both knees and the scaffold with MSC was implanted in one knee while an empty scaffold was implanted in the contra-lateral knee. After 24 weeks the rabbits were killed and histological sections were subjected to semiquantitative and quantitative evaluation by observers blinded regarding treatment modality. High degree of filling was obtained, but there was no statistically significant difference between the two treatments. However, there was a tendency for a better quality of repair in the MSC treated knees. No hypertrophy was observed by either method. MSC in a hyaluronan scaffold may be a promising treatment approach, but further studies are needed to determine the best combination of scaffold and cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Focal cartilage and osteochondral injuries are common [1] and injured articular cartilage has limited capacity for complete spontaneous healing. With the aim of increasing the healing potential, autologous chondrocyte implantation (ACI) was introduced, and the first clinical results using this approach in treatment of human knees were published in 1994 [5]. In this so-called first generation chondrocyte implantation procedure, the defect is covered by a periosteum flap sutured to the rim of the defect and the chondrocytes as a cell suspension are implanted under the flap. The second generation chondrocyte implantation procedure involves the use of autologous chondrocytes implanted in scaffolds [4, 29]. With both first and second generation approaches the chondrocytes are harvested from the joint and then expanded in vitro. In this process the cells dedifferentiate and lose their ability to produce collagen type II, the major collagen component of normal hyaline cartilage. Before implantation the cells are transferred to the scaffold which provides the cells with a three dimensional framework for growth. In vitro studies have shown that growth in such an environment allows the cells to redifferentiate and resume synthesis of collagen type II [13]. Different kinds of scaffolds have been developed, of which one is based on hyaluronic acid [6, 30], a component of normal cartilage. A commercially available scaffold based on hyaluronan is HYAFF-11® (Fidia Advanced Biopolymers, Abano Terme, Italy). Promising clinical results have been published using chondrocytes added to this polymer [23].
Mesenchymal stem cells (MSC) are multipotent progenitor cells that may differentiate into several cell lineages including chondrocytes. MSC have theoretical advantages compared to chondrocytes when it comes to potential for healing. Such cells have the ability to proliferate without losing their ability to differentiate into mature chondrocytes producing collagen II and aggrecan, or osteoblasts producing osteoid. MSC thus may induce repair of both bone and cartilage in an osteochondral defect. One concern using ACI is a re-arthroscopy rate of 20–25% during the first 1–2 years [16, 20] where in most cases hypertrophy of the repair tissue is found. This is believed to be a hypertrophy of the periosteum flap, although hypertrophy has also been reported with the use of a collagen membrane [4]. This may be associated with pain and catching symptoms and is trimmed down when detected at arthroscopy. Another concern is donor site morbidity, which is the term used when pain and functional impairment is believed to be a result of the cartilage biopsy procedure itself. Seven of ten patients treated with ACI in the ankle, with cartilage harvest from an asymptomatic knee, suffered sustained knee pain (reflected by a 15 point reduction in Lysholm score) after 12 months [33]. With the use of MSC, donor site morbidity from the joint for cartilage harvest will be avoided. MSC may be isolated in humans from sources with little donor site morbidity, such as bone marrow, adipose or synovial tissue [35].
The purpose of this study was to test the hypothesis that expanded bone marrow derived MSC in a hyaluronan scaffold will improve healing compared to the same scaffold without cells in an established model of an osteochondral injury in the rabbit knee.
Methods
Animal care
Twelve New Zealand White rabbits were initially included in the study. One died during anaesthesia at the bone marrow harvesting procedure. The remaining 11 rabbits completed the study. Animal environment, diet, anaesthesia, analgesia and the procedure of sacrifice were the same as in previous studies [2, 3]. At the time of bone marrow harvesting the rabbits weighed 3,087 (SD 140) g. At the time of implantation of cells and biomaterials, at 24 weeks of age, the mean weight was 3,333 (SD 191) g, and when killed at 24 weeks post implantation the rabbits weighed 3,903 (SD 394) g. The animals were allowed to move freely in their cages immediately after surgery, and most animals were immediately able to bear weight on both extremities. The experiment was performed according to the guidelines for animal research at the University of Oslo and approved by the Norwegian Government Committee for Experimental Animal Care.
Obtaining bone marrow and serum
A 2 cm skin incision was made over the posterior iliac crest under sterile conditions. A small piece of the cortical bone from the posterior iliac crest was removed and 1 ml bone marrow was aspirated. The skin was closed using resorbable suture. Thirty millilitres of blood was drawn from the ear-vein for the preparation of autologous serum (AS) and repeated after 2 weeks to get a sufficient volume of serum. The blood was drained into 10 ml Vacutainer tubes without anti-coagulants (BD, Plymouth, UK) and allowed to clot for 4 h at 4–8°C. Subsequently, the blood was centrifuged at 1,800×g at 4°C for 15 min. Serum was collected and filtered through a 0.2 μm membrane (Sarstedt, Nümbrecht, Germany). Aliquots of sterile AS were stored at −20°C.
Isolation and expansion of mesenchymal stem cells
The bone marrow was diluted immediately in 10 ml DMEM/F12 medium (Gibco, Paisley, UK) containing 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml amphotericin B (Sigma, St Louis, MO) and stored for 1–2 h at 4–8ºC. Diluted bone marrow was then supplemented with 2.4 ml AS (20%), seeded in 25 cm2 flask (Nunc, Roskilde, Denmark) and cultured at 37ºC with 5% humidified CO2 for 24 h. At that time non-adherent cells were discarded, adherent cells were washed with PBS (Gibco) to isolate the MSC and subsequently cultured in DMEM/F12 medium with antibiotics, amphotericin B, and 20% AS. The culture medium was replaced every 3–4 days. At approximately 50% confluence the cells were detached from plastic adherence using trypsin–EDTA (Gibco) and replated at about 1,000 cells/cm2 in 75 cm3 flask (Nunc). After the first passage, amphotericin B was removed and 10% instead of 20% AS was used for further cell cultures.
Biomaterial and preparation of implants
The hyaluronan biomaterial (HYAFF-11®, Fidia) was sterilized by the provider using γ-irradiation. Circular pieces of the scaffold biomaterial were cut using a 5 mm biopsy punch (Stiefel Laboratories Ltd., Ireland). The preparation of scaffolds with MSC and empty scaffolds were carried out according to Grigolo et al. [12]. Twenty-four hours prior to seeding of cells in the scaffolds, the wells in 96-well culture plates were pre-coated with a 1% w/v sterile PolyHEMA/ethanol solution (Sigma) to avoid adherent growth. Forty-eight hours prior to implantation cells were trypsinised and counted. After 27 days of culture (three passages) and 48 h prior to implantation 2 × 106 MSC were resuspended in 35 μl of cell culture medium and seeded into one piece of biomaterial giving a cell density of 10 × 106 cm−2. Medium was changed three times before implantation. Pieces of HYAFF-11® for the control defects were immersed in cell culture medium immediately prior to implantation because a scaffold without cells disintegrates rapidly in medium.
Creation and repair of the osteochondral defects
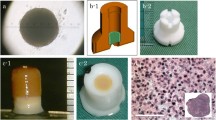
Four weeks after bone marrow harvest the 11 rabbits (now 24 weeks old) underwent surgery in both knees under sterile conditions. Through a medial parapatellar incision, a 4 mm diameter and 1.5 mm deep defect was created on the load bearing area of the medial femoral condyle so that the posterior border was approximately 1 mm from the anterior horn of the medial meniscus with the knee maximally flexed (Fig. 1). A motorised drill with a 4 mm diameter bur with a 0.5 mm central tip was used. A sleeve around the bur secured the depth of 1.5 mm. The hyaluronan scaffold containing MSC was implanted in one knee (left and right side were alternated) and scaffold without cells was implanted in the opposite knee (Fig. 2). Thus, we treated 22 defects; 11 defects with scaffolds and cells and 11 defects with scaffold alone. After implantation, 50 passive flexion/extension repetitions of the knees were made. Subsequently we checked that the implants were still in place. The joint capsule and skin were closed in layers using resorbable suture. In three knees a postoperative partial rupture of the skin suture occurred with the joint capsule still closed. These were resutured within the first postoperative days and no further complications were seen. In two rabbits a luxation of the patella in one of the knees was treated by closed reduction after 6 and 9 days, and no further reluxations were seen.
Sacrifice of animals and evaluation of the cartilage repair
Twenty-four weeks after implantation, the rabbits (now 48 weeks old) were killed. The femoral condyles were dissected free, cut and immersed in phosphate-buffered 4% formaldehyde solution for 1 week, and then decalcified using a 20% formic acid solution for 3 weeks. The specimens were then divided into two, through the centre of the defect in random directions [14]. The specimens were embedded in paraffin wax and stained with hematoxylin-eosin. Two or three sections, 4–5 μm thick were made from each of the two parts, parallel to and as close as possible to, the edge facing the centre of the defect. Thus, the sections were all close to the centre of the defect. The sections from each of the halves judged to be technically best were subjected to morphometry using point counting [14] and, consequently, each defect was represented by two sections. Each section selected for analysis was photographed at 40× magnification using a digital camera (Color View III, Olympus Soft Imaging Solutions®, Münster, Germany). Using a computer program (Analysis Pro®, Olympus Soft Imaging Solutions®, Münster, Germany), a straight line was drawn between the tidemarks on either side of the defect. Ideally, this line should have been curved parallel to the original surface of the cartilage. However, due to large variation in the curvature in different planes of the condyle, such a line was not possible to reconstruct reproducibly. A rectangle of 1 mm height below the line, and 0.6 mm height above the line was drawn. As all sections were from near the centre of the defect the width of the rectangles were close to 4 mm. Using the computer program, a grid with 200 μm between test lines was superimposed to the picture (Fig. 3). This resulted in a total of approximately 160 test points (60 above and 100 below the tidemark line). Test points overlaying cartilage, bone or no tissue, respectively, were recorded. The analyses were performed by two independent observers (AÅ and SL) blinded to the surgical procedure. The two observers counted each specimen twice and this was repeated after 3–4 weeks. The relative filling was expressed as the number of test points overlaying cartilage and bone, respectively relative to the total number of test points, and was calculated for the area of the rectangle above and below the tidemark line, and for the total area of the rectangle. The relative filling for each defect was expressed as the average calculated from all countings for each defect. Bone filling below our drawn tidemark line was used as an estimate of the regeneration of the original bony part of the osteochondral defect, while cartilage filling above the tidemark line was used as an estimate of the regeneration of the original cartilaginous part. To characterize the quality of the repair, selected parameters (modified from the O’Driscoll score [24], Table 1) were given a score of 0 (lowest score), 1 or 2 (highest score): The scores were reported for each parameter separately, without combining them to a total score. The evaluation was performed by two observers (FPR and SL) together and repeated at two occasions separated by 3 weeks. Again, the observers were blinded to the type of surgery performed. If there was a discrepancy in the scoring for one parameter in a specimen, this parameter was re-evaluated by the same observers and a final consensus was made.
Statistics
SPSS statistical package version 14 (Chicago, IL, USA, 2005) was used for statistical analysis. Pre-experimental analysis using a power of 0.80 and a significance level of 0.05 and a standard deviation for the differences of less than 24% indicated a need of nine animals. Due to the risk of losing animals during the experiment, a decision to use 12 rabbits was made. Based on previous experimental studies [3] a filling difference of more than 25% was considered as a proper level to disregard the null hypothesis of no difference in filling between the two treatments. Each animal served as its own control, and thus a paired Student’s t test could be used to compare degree of filling of cartilage and bone. The mean differences and standard deviation of the differences were used to assess the agreement between the two observers and between measurements at the two time points for each observer. Wilcoxon rank test for non parametric paired samples was used for comparison of the semiquantitative evaluation of the repair.
Results
Macroscopically, no signs of hypertrophy of the repair tissue, degenerative change or inflammation were observed in any of the knees. Quantitative histological evaluation showed a high degree of filling, but no significant difference in the degree of filling was observed between the two treatment modalities. This was the findings both for cartilage and bone filling in the entire defect, and the parts of the defect below and above tidemark (Table 2). The differences in measured filling between each observer and between measured filling at two separate time points were small reflecting a satisfactory inter and intra observer variability (Table 3). Semiquantitative data on the quality of the repair are given in Table 4; there was a significantly higher score for the degree of chondrocyte cluster formation (i.e. more clustering, p = 0.03) in the defects treated with MSC-loaded scaffolds compared to empty scaffolds. There were no significant difference in score for the parameters hyaline like cartilage, integration to surrounding cartilage, amount of necrosis and surface integrity. No traces of the biomaterial were seen. Selected histology samples are shown in Fig. 4.
Discussion
We did not find any difference in the degree of filling when using scaffolds with MSC compared to scaffolds without cells as judged by morphometry. In the semi-quantitative analysis there was an increased cluster formation in the MSC treated defects, but no statistical difference in the other parameters. Cluster formation is a sign of repair in early osteoarthritis [8], and in cartilage repair, cluster formation may be interpreted as a positive phenomenon as cell proliferation is central to new tissue formation. Consequently, cluster formation should be considered a positive sign and indicating some additive effect of the MSCs in our study. In addition there was a trend towards higher scores for all parameters in the cell treated defects, and if combined to a total score there is a significant higher sum for the cell treated defects. However, the relevance of a sum score may be questioned as the importance of each parameter in relation to the others is unknown. Our approach is also in line with the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS) in their proposed scoring system for biopsies from cartilage repair tissue in humans [22].
Thus, in this study there was a trend towards a positive effect of added MSC in the repair of an osteochondral defect in a rabbit knee, but this effect appears to be limited with the current set up.
The reproducibility of semi-quantitative histological scoring systems has been poor and, thus, the validity is questionable [17]. We therefore, chose to let our conclusions rest mainly on the results of a quantitative measurement of filling [14] that turned out to be reproducible with very good agreement between the observers and between the two time-points.
Two previous studies have used hyaluronan scaffold with and without MSC in rabbits [19, 25]. Radice et al. [25] did not find any difference between the hyaluronan scaffold with or without MSC after observation periods of 8 and 16 weeks. Kayakabe et al. [19] observed a better filling compared to empty defects (with no scaffold) only when fibroblast growth factor-2 (FGF-2) was added to the MSC-loaded hyaluronan scaffold. The current study supports previous findings with relatively small differences when comparing hyaluronan scaffold with and without MSC in a slightly different model. Both Radice et al. [25] and Kayabe et al. [19] studied trochlear defects, while we studied medial femoral condyle defects. Cartilage stiffness and thickness varies between different localizations in the joint [26], and the course and outcome of a repair may vary accordingly. We chose to study defects on the medial femoral condyle as the majority of cartilage lesions in humans are located there [1].
Our findings are also in accordance with a study by Solchaga et al. [30] who compared different scaffolds without cells in a similar rabbit model: 3 mm diameter and 1.5 mm deep defects on the medial femoral condyle were created and the scaffolds studied were HYAFF®-11, auto cross-linked polysaccharide polymer (ACP) and two different polyester-based scaffolds: Poly(DL-lactic-co-glycolic acid) (PLGA) and poly(l-lactic acid) (PLLA). The filling of cartilage and bone above and below tidemark was measured in this study, and the degree of filling at 20 weeks seems to be in the same order of magnitude as in the current study, although a slightly different calculation method was used. They also found that rapidly resolving scaffolds (ACP and PLGA) resulted in a bone regeneration beyond the tidemark line, while the more slowly resolving scaffolds (HYAFF®-11 and PLLA) resulted in an incomplete bone regeneration, but with an increased filling from 12 to 20 weeks. On the other hand HYAFF®-11 resulted in more cartilage formation than the other implants. In the present study, we observed that approximately two-thirds of the bony part of the defect was filled with bone at 24 weeks. However, according to the development from 12 to 20 weeks in the study of Solchaga et al., there may be a delayed bone filling due to a slowly resolving scaffold. Thus, improved bone filling and reestablishment of the original tidemark might have been obtained with a longer observation period.
In an osteochondral lesion, cells from the bone marrow adjacent to the lesion may contribute to the repair and partly outweigh the effect of the added cells. Interestingly, Gao et al. [11] found better healing of a 3 mm osteochondral defect in the rabbit with hyaluronan scaffold loaded with mesenchymal progenitor cells compared to empty scaffolds in an experiment where the bony part of the defects were first filled up with calcium phosphate. Hyaluronan scaffold loaded with autologous chondrocytes showed better healing than empty scaffold and empty defects in the rabbit when 6 × 5 mm pure chondral defects were created [12]. Thus, the effect of adding MSC or chondrocytes may be more important when the access to cells from the bone marrow is limited.
A considerable component of spontaneous healing of the defects could explain the small differences in the current study. A high degree of spontaneous healing is known to occur with defect diameter up to 3 mm in a rabbit model with less spontaneous healing in larger defects [28]. To limit the impact of this confounding factor 4 mm defects were chosen, as larger defects could possibly exceed the width of the rabbit femoral condyle.
Other biomaterials have been loaded with or without MSC in studies of rabbit knees. Some authors report better filling or higher score with cells in a scaffold than without, both for MSC [15, 32] and for chondrocytes [9, 34]. Hybrid scaffolds combining hyaluronan with other components have shown promising results [7, 10]. An injectable synthetic extracellular matrix composed of chemically modified hyaluronic acid and collagen loaded with MSC induced complete filling and superior integration in osteochondral defects in rabbits after 12 weeks [21]. According to the authors this matrix may be implanted arthroscopically in patients.
Growth factors may promote chondrocyte differentiation of MSC [18]. We did not supplement growth factors in our study, and this may partly explain the limited effect of the added MSC. However, in contrast to similar studies the cells were cultured in AS known to contain several growth factors [31]. Previous experiments from our group have also shown that AS induces a more rapid proliferation and a more stable gene expression of MSC compared to foetal bovine serum [27].
The current animal model was chosen because the rabbit knee is widely used in experimental cartilage repair studies, and the model has been used previously by our group in experimental cartilage surgery [2]. The thickness of the cartilage of the medial femoral condyle in these animals varies from 0.3 to 0.4 mm [26] while the HYAFF-11® is approximately 1 mm thick. To ensure the containment of the implant in the defect we chose a depth of 1.5 mm of our osteochondral defect. Radice et al. [25] have shown that HYAFF-11® implanted in a trochlea defect 3 mm diameter and 0.5 mm deep was still in place after 1 week.
In the present study MSC were seeded to the scaffold 48 h prior to implantation. In the commercial use of Hyaff-11 with chondrocytes the cells are cultured 14 days in the scaffold before implantation [23]. In experimental studies with MSC in scaffolds the time from seeding to implantation, when stated, is less than 48 h [15, 19, 21, 32]. A reason to choose a relatively short interval from seeding to implant is that differentiation of MSC into chondrocytes is probably facilitated by local factors in the joint and in the cartilage.
Different time intervals have been used to study cartilage repair. In a clinical setting it is the long term results that are of greatest interest. To obtain a sufficiently long observation time, and to keep the number of animals needed to a reasonable level with maintained statistical power, we opted for 24 weeks as the only time point.
MSC in a hyaluronan scaffold may be a promising treatment approach, but further studies are needed to establish the most suitable scaffold for MSC, to optimise the handling of the cells before implantation and to increase hyaline cartilage synthesis following implantation of the cells. If the MSC under such optimised conditions turn out to be superior to chondrocyte implantation in experimental cartilage repair the procedure should be introduced to clinical practice following well controlled randomised clinical trials.
References
Aroen A, Loken S, Heir S, Alvik E, Ekeland A, Granlund OG, Engebretsen L (2004) Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med 32:211–215
Aroen A, Heir S, Loken S, Reinholt FP, Engebretsen L (2005) Articular cartilage defects in a rabbit model, retention rate of periosteal flap cover. Acta Orthop 76:220–224
Aroen A, Heir S, Loken S, Engebretsen L, Reinholt FP (2006) Healing of articular cartilage defects. An experimental study of vascular and minimal vascular microenvironment. J Orthop Res 24:1069–1077
Bartlett W, Skinner JA, Gooding CR, Carrington RWJ, Flanagan AM, Briggs TWR, Bentley G (2005) Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective randomised study. J Bone Joint Surg Br 87-B:640–645
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Campoccia D, Doherty P, Radice M, Brun P, Abatangelo G, Williams DF (1998) Semisynthetic resorbable materials from hyaluronan esterification. Biomaterials 19:2101–2127
Fan H, Hu Y, Zhang C, Li X, LV R, Qin L, Zhu R (2006) Cartilage regeneration using mesenchymal stem cells and a PLGA-gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials 27:4573–4580
Frenkel SR, Di Cesare PE (1999) Degradation and repair of articular cartilage. Front Biosci 4:D671–D685
Frenkel SR, Toolan B, Menche D, Pitman MI, Pachence JM (1997) Chondrocyte transplantation using a collagen bilayer matrix for cartilage repair. J Bone Joint Surg Br 79:831–836
Frenkel SR, Bradica G, Brekke JH, Goldman SM, Ieska K, Issack P, Bong MR, Tian H, Gokhale J, Coutts RD, Kronengold RT (2005) Regeneration of articular cartilage–evaluation of osteochondral defect repair in the rabbit using multiphasic implants. Osteoarthr Cartil 13:798–807
Gao J, Dennis JE, Solchaga LA, Goldberg VM, Caplan AI (2002) Repair of osteochondral defect with tissue-engineered two-phase composite material of injectable calcium phosphate and hyaluronan sponge. Tissue Eng 8:827–837
Grigolo B, Roseti L, Fiorini M, Fini M, Giavaresi G, Aldini NN, Giardino R, Facchini A (2001) Transplantation of chondrocytes seeded on a hyaluronan derivative (Hyaff–11) into cartilage defects in rabbits. Biomaterials 22:2417–2424
Grigolo B, Lisignoli G, Piacentini A, Fiorini M, Gobbi P, Mazzotti G, Duca M, Pavesio A, Facchini A (2002) Evidence for redifferentiation of human chondrocytes grown on a hyaluronan-based biomaterial (HYAFF-11): molecular, immunohistochemical and ultrastructural analysis. Biomaterials 23:1187–1195
Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A (1988) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96:379–394
Guo X, Wang C, Zhang Y, Xia R, Hu M, Duan C, Zhao Q, Dong L, Lu J, Qing SY (2004) Repair of large articular cartilage defects with implants of autologous mesenchymal stem cells seeded into beta-tricalcium phosphate in a sheep model. Tissue Eng 10:1818–1829
Henderson I, Gui J, Lavigne P (2006) Autologous chondrocyte implantation: natural history of postimplantation periosteal hypertrophy and effects of repair-site debridement on outcome. Arthroscopy 22:1318–1324
Hyllested JL, Veje K, Ostergaard K (2002) Histochemical studies of the extracellular matrix of human articular cartilage—a review. Osteoarthr Cartil 10:333–343
Im GI, Jung NH, Tae SK (2006) Chondrogenic differentiation of mesenchymal stem cells isolated from patients in late adulthood: the optimal conditions of growth factors. Tissue Eng 12:527–536
Kayakabe M, Tsutsumi S, Watanabe H, Kato Y, Takagishi K (2006) Transplantation of autologous rabbit BM-derived mesenchymal stromal cells embedded in hyaluronic acid gel sponge into osteochondral defects of the knee. Cytotherapy 8:343–353
Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O (2004) Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am 86:455–464
Liu Y, Shu XZ, Prestwich GD (2006) Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng 12:3405–3416
Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker K, Roberts S, Stauffer E (2003) Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am 85-A(suppl 2):45–57
Marcacci M, Berruto M, Brocchetta D, Delcogliano A, Ghinelli D, Gobbi A, Kon E, Pederzini L, Rosa D, Sacchetti GL, Stefani G, Zanasi S (2005) Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res 96–105
O’Driscoll SW, Keeley FW, Salter RB (1988) Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J Bone Joint Surg Am 70:595–606
Radice M, Brun P, Cortivo R, Scapinelli R, Battaliard C, Abatangelo G (2000) Hyaluronan-based biopolymers as delivery vehicles for bone-marrow-derived mesenchymal progenitors. J Biomed Mater Res 50:101–109
Rasanen T, Messner K (1996) Regional variations of indentation stiffness and thickness of normal rabbit knee articular cartilage. J Biomed Mater Res 31:519–524
Shahdadfar A, Fronsdal K, Haug T, Reinholt FP, Brinchmann JE (2005) In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells 23:1357–1366
Shapiro F, Koide S, Glimcher MJ (1993) Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am 75:532–553
Solchaga LA, Dennis JE, Goldberg VM, Caplan AI (1999) Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. J Orthop Res 17:205–213
Solchaga LA, Temenoff JS, Gao J, Mikos AG, Caplan AI, Goldberg VM (2005) Repair of osteochondral defects with hyaluronan- and polyester-based scaffolds. Osteoarthr Cartil 13:297–309
Tallheden T, van der Lee J, Brantsing C, Mansson JE, Sjogren-Jansson E, Lindahl A (2005) Human serum for culture of articular chondrocytes. Cell Transplant 14:469–479
Uematsu K, Hattori K, Ishimoto Y, Yamauchi J, Habata T, Takakura Y, Ohgushi H, Fukuchi T, Sato M (2005) Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials 26:4273–4279
Whittaker JP, Smith G, Makwana N, Roberts S, Harrison PE, Laing P, Richardson JB (2005) Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br 87:179–183
Willers C, Chen J, Wood D, Xu J, Zheng MH (2005) Autologous chondrocyte implantation with collagen bioscaffold for the treatment of osteochondral defects in rabbits. Tissue Eng 11:1065–1076
Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I (2007) Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 327:449–462
Acknowledgments
The authors thank Ingar Holme, Ph.D., for statistical advice and Bioengineer Aileen Murdoch Larsen for technical assistance. We also thank Fidia Advanced Biopolymers, Abano Terme, Italy for supplying the biomaterial. The study was supported by grants from the EXTRA funds from the Norwegian Foundation for Health and Rehabilitation, Norwegian Center for Stem Cell Research by the Research Council of Norway, Gidske og Peter Jacob Sørensens Foundation for the Promotion of Science and Oslo Sports Trauma Center (OSTRC). The centre is financed by the South-Eastern Norway Regional Health Authority, the Royal Norwegian Ministry of Education and Research, the Norwegian Olympic Committee & Confederation of Sport & Norsk Tipping. No affiliations or conflicting interests declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Løken, S., Jakobsen, R.B., Årøen, A. et al. Bone marrow mesenchymal stem cells in a hyaluronan scaffold for treatment of an osteochondral defect in a rabbit model. Knee Surg Sports Traumatol Arthr 16, 896–903 (2008). https://doi.org/10.1007/s00167-008-0566-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-008-0566-2