Abstract
Purpose
The impact of immunosuppression on intensive care unit (ICU)-acquired colonization and infection related to multidrug-resistant (MDR) bacteria (ICU-MDR-col and ICU-MDR-inf, respectively) is unknown.
Methods
We carried out an observational prospective cohort study in 8 ICUs in France (all with single-bed rooms and similar organizational characteristics). All consecutive patients with an ICU stay > 48 h were included, regardless of immune status, and followed for 28 days. Patients underwent systematic screening for colonization with MDR bacteria upon admission and every week subsequently. Immunosuppression was defined as active cancer or hematologic malignancy, neutropenia, solid-organ transplant, use of steroids or immunosuppressive drugs, human immunodeficiency virus infection and genetic. The primary endpoint was the incidence rate of a composite outcome including ICU-MDR-col and/or ICU-MDR-inf.
Results
750 patients (65.9% males, median age 65 years) were included, among whom 264 (35.2%) were immunocompromised. Reasons for ICU admission, severity scores and exposure to invasive devices and antibiotics during ICU stay were comparable between groups. After adjustment for center and pre-specified baseline confounders, immunocompromised patients had a lower incidence rate of ICU-MDR-col and/or ICU-MDR-inf (adjusted incidence ratio 0.68, 95% CI 0.52–0.91). When considered separately, the difference was significant for ICU-MDR-col, but not for ICU-MDR-inf. The distribution of MDR bacteria was comparable between groups, with a majority of Enterobacteriacae resistant to third-generation cephalosporins (~ 74%).
Conclusion
Immunocompromised patients had a significantly lower incidence rate of a composite outcome including ICU-MDR-col and/or ICU-MDR-inf. This finding points to the role of contact precautions and isolation measures, and could have important implications on antibiotic stewardship in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This observational prospective multicenter cohort study shows that immunocompromised patients have a lower incidence rate of intensive care unit-acquired colonization and/or infection with multidrug-resistant bacteria, both prior to and after adjustment for pre-specified confounders. |
Introduction
Antimicrobial resistance (AMR) is an emerging global threat to human health, food safety and economic development [1]. Intensive care units (ICUs) are a ‘hot spot’ for the emergence and diffusion of AMR [2]. Critically ill patients often harbor risk factors for colonization and infection with multidrug-resistant (MDR) bacteria and are frequently exposed to antibiotics, leading to sustained selection pressure [3]. Furthermore, there is an increased risk for cross-transmission of MDR bacteria in ICUs due to close contacts between patients and healthcare workers [4]. ICU-acquired colonization with MDR bacteria (ICU-MDR-col) has been associated with a prolonged ICU stay [5], and ICU-acquired infection with MDR bacteria (ICU-MDR-inf) has been linked to a longer duration of invasive mechanical ventilation (IMV) [6] and to higher mortality [7].
In the last 2 decades, the mortality of critically ill immunocompromised patients has markedly declined [8], leading intensivists to generally be more confident in their likelihood to benefit from intensive care. Consequently, their proportion among the typical ICU case mix has increased [9]. Immunocompromised patients are particularly at risk of developing healthcare-associated infections (HAIs), a significant proportion of which are caused by MDR bacteria [10]. Thus, choosing an appropriate empiric antibiotic regimen for a critically ill immunocompromised patient is a frequent yet challenging clinical problem, and one that could be alleviated by a detailed understanding of the epidemiology of AMR in this population [11].
Numerous studies have investigated the prevalence of MDR organisms among bacteria causing infections in immunocompromised patients [12]. However, few have focused on immunocompromised patients in the ICU, or systematically compared these estimates with those obtained in a comparable cohort of immunocompetent patients. Several studies have documented that immunosuppression was associated with a higher incidence of colonization and/or infection with MDR pathogens (including vancomycin-resistant enterococci (VRE) [13], carbapenem-resistant Acinetobacter baumannii (CRAB) [14] and other Gram-negative bacteria [15]) in ICU patients [16,17,18]. However, in a single-center case–control study, immunosuppression was only associated with ICU-MDR-col and ICU-MDR-inf in univariate analysis, but not in multivariate analysis after adjustment for antibiotic exposure prior to and during ICU stay [19].
We conducted the CIMDREA study, an observational prospective multicenter cohort study in 8 French ICUs, with the primary objective to investigate the association of immunosuppression with the incidence rate of a composite outcome including ICU-MDR-col and/or ICU-MDR-inf within 28 days of ICU stay. Secondary objectives were to assess the impact of immunosuppression on the 28-day cumulative incidence of ICU-MDR-col and ICU-MDR-inf (combined and separately), to investigate the microbiology of ICU-MDR-col and ICU-MDR-inf in immunocompromised and immunocompetent patients, and to determine whether immunosuppression modifies the impact of ICU-MDR-col and ICU-MDR-inf on ICU length-of-stay, IMV duration and 28-day mortality.
Methods
Design and setting
CIMDREA was an observational prospective multicenter cohort study conducted in the ICUs of Lille, Croix-Rousse (Lyon), Lyon-Sud and Amiens University-affiliated hospitals, and Roubaix, Marne-La-Vallée, Lens and Béthune hospitals. All participating centers had single-bed rooms and shared similar characteristics (see Supplementary Table 1 for details on the organizational setup of each center).
Patients and definition of immunosuppression
All adult patients hospitalized for > 48 h in the participating ICUs were eligible, regardless of their immune status. Patients were included consecutively if they fulfilled the following criteria: MDR bacteria screening by rectal and nasal swabbing in the 48 h following ICU admission; at least one MDR bacteria screening by rectal or nasal swabbing after the 48th hour in the ICU and before ICU discharge; non-opposition to participate.
Immunosuppression was defined as: solid cancer (active or in remission for less than 5 years), active hematologic malignancy, neutropenia (< 0.7G/L for ≥ 7 days), solid-organ transplant, long-term (≥ 28 days), use of steroids (≥ 10 mg of prednisone per day or equivalent) or other immunosuppressant drugs, human immunodeficiency virus (HIV) infection, and genetic immunodeficiency [20]. Immune status was recorded on the last day of the follow-up period, to accurately classify patients in whom a diagnosis associated with immunosuppression was established during ICU stay.
Microbiology
In the participating ICUs, patients underwent rectal and nasal swabbing upon admission (at the latest on the 48th hour following admission) and every week until ICU discharge (or until day 28, whichever came first). Colonization with MDR bacteria was detected by streaking swabs onto selective culture media, followed by species identification.
Routine microbiology data, i.e., the results of bacterial cultures ordered by attending physicians as part of patient care, were also collected. Antibiotic susceptibility was defined according to breaking points recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [21]. MDR bacteria were defined as follows: third-generation cephalosporins (3GC)-resistant Enterobacteriaceae, including through expression of an extended spectrum beta-lactamase (ESBL); carbapenem-resistant Enterobacteriaceae; methicillin-resistant Staphylococcus aureus (MRSA); vancomycin-resistant Enterococcus faecalis and Enterococcus faecium (VRE); Pseudomonas aeruginosa resistant to imipenem and ceftazidime; and carbapenem-resistant Acinetobacter baumannii (CRAB) [22].
Clinical variables and outcomes
The primary endpoint was the incidence rate of a composite outcome including ICU-MDR-col and/or ICU-MDR-inf within 28 days of ICU stay. Secondary endpoints included: the incidence rates of ICU-MDR-col and ICU-MDR-inf, considered separately, and the 28-day cumulative incidence of ICU-MDR-col and ICU-MDR-inf (combined and separately). ICU-MDR-col was defined as the colonization by MDR bacteria isolated on a rectal or a nasal swab collected ≥ 48 h following admission, or on any other sample if it was not considered to be related to an infection (see below). In patients colonized with MDR bacteria at ICU admission, only ICU-acquired colonization related to other MDR bacterial species was taken into account. ICU-MDR-inf was defined as an infection related to MDR bacteria occurring > 48 h following ICU admission. As opposed to colonization (asymptomatic carrier state), infections were defined by clinical, biological and imaging characteristics compatible with the definitions published by international societies on healthcare- and ventilator-associated pneumonia (HAP, VAP) [23], blood-stream and catheter-related infections [24], urinary tract infections [25] and other community- and healthcare-associated infections [26,27,28,29]. No specific treatment protocols targeting immunocompromised patients were implemented during the study, all treatments (including antibiotic regimens) were decided by attending physicians, following relevant guidelines [30,31,32] (Supplementary Table 1).
Clinical variables potentially associated with MDR-ICU-col and MDR-ICU-inf were recorded: demographics, comorbidities, recent hospitalization and antibiotic exposure (< 3 months before ICU admission), organ failures at admission, exposure to invasive devices and antibiotics during ICU stay. When recording antibiotic exposure, we did not consider antimycobacterial drugs, fidaxomicine, erythromycin (as a prokinetic), low-dose trimethoprim-sulfamethoxazole (for prophylaxis against Pneumocystis pneumonia), antifungals and antivirals. When recording steroids, we did not consider hydrocortisone (often prescribed as substitutive hormonotherapy or in the context of refractory shock).
Patients were followed and data were collected on an electronic Case Report Form (eCRF) until ICU discharge or until day 28, whichever came first.
Statistical analysis
Patient characteristics were described according to immune status without statistical comparisons. Categorical variables are reported as number and percentage, whereas quantitative variables are expressed as median with interquartile range (IQR; 25th–75th percentile).
We estimated and compared the incidence rate (expressed as number of events per 1000 patients × ICU days) of ICU-MDR-col and ICU-MDR-inf (combined and separately) according to immune status using a negative binomial regression model, using ICU length-of-stay (censored at 28 days) as offset variable (after applying a log-transformation). We estimated the 28-day cumulative incidence of ICU-MDR-col and ICU-MDR-inf (combined and separately) using the Kalbfleisch and Prentice method [33], considering ICU discharge (alive or dead) as competing event. The associations with immune status were assessed using cause-specific Cox’s proportional hazard models. Associations of immune status with the incidence rates of ICU-MDR-col and ICU-MDR-inf were further investigated after adjustment for pre-specified baseline confounders [34] [age, gender, Simplified Acute Physiology Score (SAPS) II, recent ICU hospitalization, recent MDR colonization or infection, and recent antibiotic treatment (recent indicating in the 3 months prior to ICU admission)]. Additional adjustments on time-dependent covariates (use of IMV and antibiotic treatment during ICU stay) were performed in the Cox’s regression models.
We investigated the association between occurrence of ICU-MDR-col/ICU-MDR-inf and 28-day prognostic outcomes (28-day mortality, ICU length-of-stay and IMV duration) using Cox’s regression models, treating ICU-MDR-col/ICU-MDR-inf as time-varying covariates, before and after adjustment for pre-specified confounders (age, gender, SAPS-II, diabetes mellitus, heart disease, lung disease, cerebrovascular disease, chronic kidney disease, and liver cirrhosis). Subgroup analyses according to immune status were done by including into the Cox’s regression models immune status and the interaction term between ICU-MDR-col/ICU-MDR-inf and immune status.
To avoid case deletion in multivariate analyses due to the presence of missing data in covariates, multivariable regression models were performed after handling missing data using a multiple imputation procedure (detailed in the Supplementary Material) [35].
Full details on the statistical analysis (including sample size calculation) are provided in the Supplementary Material.
Ethics
The study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. The Ethics Committee and Institutional Review Board (Comité de Protection des Personnes Ouest I) approved the study protocol (registration number 2019T3-07_RIPH3 HPS) on March 26th, 2019 as minimal-risk research using data collected for routine clinical practice. Patients or their next of kins received information about the study and were given the possibility to refuse using their personal data. In accordance with the French law, the database was registered to the Commission Nationale l’Informatique et des Libertés. The study was registered on ClinicalTrials.gov (NCT04043793).
Results
Patient characteristics
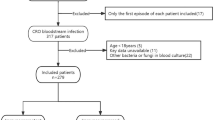
From May 5th, 2019 to January 31st, 2020, 750 patients were included, among whom 486 (64.8%) were immunocompetent and 264 (35.2%) were immunocompromised. During the study period, 2309 patients were screened but excluded, mainly because their ICU length-of-stay was ≤ 48 h (n = 853), or because rectal or nasal swabs were not performed upon admission (n = 609) or subsequently (n = 581) (Supplementary Fig. 1). The main causes of immunosuppression were cancer (n = 148, 56.1%), hematological malignancy (n = 68, 25.8%), and use of steroids and/or other immunosuppressive therapies (n = 66, 25%). These causes were distributed similarly across centers. Thirty-four (4.5%) patients had more than one cause of immunosuppression (Supplementary Table 2).
Patients were mostly male (65.9%), with a median age of 65 years (Table 1). Some comorbidities were less prevalent in immunocompromised patients, including diabetes (22 vs. 30.7%), heart disease (26.5% vs. 34.6%), lung disease (21.2% vs. 27.2%), and alcohol use (18.6% vs. 24.6%). Immunocompromised patients were less likely to live in a nursing home (2.7% vs. 6.8%), but more likely to have been hospitalized for > 48 h (71% vs. 36.4%) and to have received antibiotics (61% vs. 40.1%) in the 3 months prior to ICU admission. Reasons for ICU admission, Sequential Organ Failure Assessment (SOFA) score and SAPS-II scores, exposure to invasive devices, and antibiotics during ICU stay were comparable between groups. Transfusions of blood products were more frequent among immunocompromised patients (47.1% vs. 27.7%) (Table 2).
ICU-acquired colonization and infection with MDR bacteria
A total of 196 ICU-MDR-col and/or ICU-MDR-inf occurred among 154 immunocompetent patients (incidence rate 27.3 [95% IC 23.5–31.6] per 1000 patients × ICU days), in comparison to 75 events among 63 immunocompromised patients (20.8, 95% CI 16.4–26.4) (Table 3). Among patients with ICU-MDR-col and/or ICU-MDR-inf, the first event occurred with a median delay of 7 days (IQR 4–11) following ICU admission (6 (IQR 4–12) and 7 (IQR 4–11) days in immunocompromised and immunocompetent patients, respectively), and colonization was the first event in the majority of cases in both groups (Fig. 1).
In univariate analysis, the incidence rate of the primary endpoint was lower in immunocompromised than in immunocompetent patients, with a difference of borderline significance (unadjusted incidence rate ratio (IRR) 0.76, 95% CI 0.57–1.01 (Table 4). This difference reached the significance level after adjustment for center and pre-specified baseline confounders, with an adjusted IRR (aIRR) 0.68, 95% CI 0.52–0.91. A similar difference was found when ICU-MDR-col was analyzed separately (aIRR 0.63, 95% CI 0.45–0.86). However, the incidence rate of ICU-MDR-inf was not significantly different between the two groups (aIRR 0.66, 95% CI 0.38–1.11).
Similar associations were found when analyzing the 28-day cumulative incidence of ICU-MDR-col and ICU-MDR-inf, both before and after adjustment for pre-specified baseline confounders and time-varying covariates, with an adjusted cause-specific hazard ratio (cHR) 0.63 (95% CI 0.45–0.86) for ICU-MDR-col and/or ICU-MDR-inf, cHR 0.57 (95% CI 0.4–0.79) for ICU-MDR-col, and cHR 0.55 (95% CI 0.3–0.97) for ICU-MDR-inf. The incidence of ICU-MDR-col and ICU-MDR-inf in subgroups defined according to the type of immunosuppression is presented in Supplementary Table 3.
Microbiology
Among MDR bacteria responsible for ICU-MDR-col and ICU-MDR-inf, 3GC-resistant Enterobacteriacae were the most frequently isolated organisms (~ 74%), followed by carbapenem-resistant Enterobacteriacae, MRSA and MDR P. aeruginosa (Table 3). The distribution of MDR bacteria was comparable between groups. Among patients with ICU-acquired infections related to MDR bacteria, the distribution of affected organs was similar between groups, with a majority of VAP, followed by HAP and primary blood-stream infections.
ICU-acquired MDR colonization and infection and prognostic outcomes
In the overall cohort, there was no statistically significant association between the occurrence of ICU-MDR-col and ICU-MDR-inf (combined and separately) and overall 28-day survival, ICU length-of-stay, and IMV duration (among the 499 patients receiving IMV), both in univariate analysis and after adjustment for center and pre-specified baseline confounders. Similar results were also found when considering the sub-populations of immunocompromised and immunocompetent patients separately (Supplementary Table 4).
Discussion
In this observational prospective multicenter cohort study, we found that immunocompromised patients had a significantly lower incidence rate of a composite outcome including ICU-MDR-col and/or ICU-MDR-inf. This was found both in univariate analysis and after adjustment for predefined baseline confounders, and when considering the cumulative incidence for the primary outcome. When considered separately, the difference was significant for ICU-MDR-col, but not for ICU-MDR-inf.
These findings contradict our original hypothesis, based on the dominant results from most recent investigations, i.e., that immunocompromised patients would be at higher risk to be colonized and/or infected with MDR bacteria during ICU stay. For example, in a case–control study in 16 ICUs in the United States of America (n = 298), immunosuppression was independently associated with HAIs caused by extremely drug-resistant Gram-negative bacilli (OR 1.55, 95% CI 1.01–2.39) [15]. In a prospective study in ten Japanese centers focusing on patients with HAP (n = 526), immunodepression was independently associated with MDR pathogens (aOR 2.31, 95% CI 1.05–5.11) [16]. And in a multicenter study on ICU patients with HAP and VAP (n = 2297), the incidence of infection with MDR bacteria was higher in immunocompromised vs. immunocompetent patients (OR 1.75, 95% CI 1.13–2.71) [17]. Conversely, we found in a single-center case–control study that immunocompromised patients had a higher incidence of ICU-MDR-inf and ICU-MDR-col in univariate analysis, but not after adjustment for antibiotic exposure prior to and during ICU stay [19]. This suggested that the increased risk of ICU-MDR-col and ICU-MDR-inf in immunocompromised patients could be explained by a higher prevalence of risk factors for MDR bacteria (e.g., antibiotic exposure), more so than by their immune deficiency itself. Of note, the incidence of ICU-MDR-col and ICU-MDR-inf was higher in our cohort than in other studies with similar methodology [36], but our results are generally in line with a previous investigation conducted in our center [19], where the global cumulative incidence of ICU-MDR-col and ICU-MDR-inf was 24.5% and 19.5%, respectively.
Among the main factors leading an ICU patient to become colonized by MDR bacteria are the selection of endogenous-resistant strains through antibiotic pressure, and cross-transmission from other patients through contacts with healthcare workers or surfaces. As antibiotic exposure was similar between immunocompromised and immunocompetent patients, our results point to the potential effect of contact precautions (CP) and isolation measures—which are more frequently applied in immunocompromised patients—to prevent cross-transmission of MDR bacteria. Contact precautions (the use of gowns and gloves) and isolation measures (use of single-bed rooms and application of positive air flows) have been a matter of intense debate in the critical care literature, as observational and interventional studies have documented conflicting results on their efficacy, depending on epidemiological settings and the type of MDR bacteria considered [37,38,39]. Nonetheless, they have been endorsed by international recommendations in patients with proven colonization or infection by MDR bacteria [40, 41]. Importantly, the actual adherence of healthcare workers to CPs—which strongly influences their efficacy—is difficult to monitor accurately, and this has prevented us from fully characterizing their impact of the incidence of ICU-MDR-col and ICU-MDR-inf in our cohort. The extent to which they might actually contribute to our findings remains to be specifically investigated.
Our study also sheds light on the impact of immunosuppression on the dynamics of colonization and infection with MDR bacteria in ICU patients. As documented in previous studies [42, 43], colonization with a given MDR strain precedes infection in the majority of cases in our cohort. This was true both in immunocompromised and immunocompetent patients, suggesting that colonization is more influenced by external factors (such as cross-transmission) than by immune status. Finally, it has been documented that resistance and virulence could be negatively correlated in bacteria, as the emergence of resistance is often associated with changes in important biological functions in bacteria [44]. It has thus been hypothesized that an impaired immune system, more permissible to less-virulent strains, could favor the emergence of AMR [45]. Our results argue against this hypothesis, as immunosuppression was not associated with a higher incidence of ICU-MDR-inf.
Extending beyond a merely descriptive epidemiological investigation, our study may also have practical therapeutic implications. Because of the previously documented positive association between immune deficiency and MDR bacteria in critically ill patients, most guidelines suggest that immunocompromised patients should receive broad-spectrum antibiotics in case of suspected healthcare- or ICU-associated infections [30, 31]. If confirmed in subsequent studies, our findings would not support the idea that immunocompromised patients are at higher risk of being colonized and infected with MDR bacteria, which could have a broad impact on antibiotic stewardship in this population.
There was no significant association between ICU-MDR-col/ICU-MDR-inf and 28-day mortality, ICU length-of-stay, and IMV duration. In previous studies, ICU-MDR-col has been associated with a higher ICU length-of-stay [5], and ICU-MDR-inf has been linked to longer IMV duration [6] and higher mortality [7, 15, 46]. However, these findings are not universal, and it can be difficult to disentangle the direct effect of MDR bacteria from the several confounders that can potentially contribute to the associations found in these observational studies [47, 48]. Exploring the prognostic impact of ICU-MDR-col and ICU-MDR-inf was a secondary objective in our study, and possibly, its sample size was not sufficient to explore this association with enough statistical power. Importantly, immune status did not change the observed lack of association between colonization/infection and outcome, arguing against a specific prognostic impact of MDR bacteria among immunocompromised patients.
Our study has several limitations. We chose a composite outcome as primary endpoint (because colonization precedes infection in the majority of cases), and calculated the study sample size accordingly. However, the lack of a statistically significant association between immunosuppression and ICU-MDR-inf could potentially be explained by an insufficient power, due to the low incidence of this outcome. In the same line, the limited sample size has precluded any firm conclusion regarding potential differences in the incidence of ICU-MDR-col/ICU-MDR-inf in subgroups of immunocompromised patients, as well as across bacterial species. A more detailed recording of CP and isolation measures at the individual level would have enabled to study their impact on ICU-MRD-col/ICU-MDR-inf more precisely. Similarly, a more precise analysis of antibiotic treatments (molecules, doses) received prior to and during ICU stay could lead to a better understanding of the role of selection pressure on the emergence and spread of AMR. Of note, while antibiotic exposure has mainly been associated with an increased risk of ICU-MDR-col/inf, it can also be hypothesized that broad-spectrum antibiotics could lead to a higher rate of false negatives (reduced sensitivity) when attempting to detect colonization with MDR bacteria. Follow-up was limited to ICU stay, and assessment of colonization through rectal and nasal swabs was not maintained after ICU discharge. The definition of immunosuppression used in this study was in line with previous work on ICU patients [20], but may not fully take into account significant residual heterogeneity in the immune function of these patients. Furthermore, we acknowledge that some patients with baseline comorbidities known to negatively affect immune defenses (e.g., diabetes, chronic obstructive pulmonary disease, and cirrhosis), or those who develop acquired immune defects as a consequence of critical illness [49] might be overlooked (and thus inaccurately classified) by this definition. Reasons for treatment with steroids, as well as types and doses (prior to and during ICU stay) were not recorded. Finally, microbiology labs in some centers did not routinely report on the mechanism of resistance to 3GC in Enterobacteriaceae. Thus, it was sometimes impossible to accurately distinguish between expression of ESBL and other resistance mechanisms (including high-level cephalosporinase), yet their potential for cross-transmission differs markedly, with ESBL, often encoded on mobile genetic elements (e.g., plasmids), being at higher risk for horizontal gene transfer.
Conclusion
In this observational prospective multicenter cohort study, immunocompromised patients had a lower incidence rate of a composite outcome including ICU-acquired colonization and/or infection with MDR bacteria, both prior to and after adjustment for pre-specified confounders. If confirmed, this could have important implications with regards to the choice of empiric antibiotic regimens in critically ill immunocompromised patients. Future studies should attempt to investigate the precise impact of contact precautions and isolation measures on the transmission dynamics of MDR bacteria in this population.
Availability of data and materials
Not applicable.
Abbreviations
- 3GC:
-
Third-generation cephalosporins
- AMR:
-
Antimicrobial resistance
- CARB:
-
Carbapenem-resistant Acinetobacter baumannii
- COPD:
-
Chronic obstructive pulmonary disease
- ECLS:
-
Extracorporeal Life Support
- ECMO:
-
Extracorporeal Membrane Oxygenation
- ESBL:
-
Extended-spectrum beta-lactamase
- EUCAST:
-
European Committee on Antimicrobial Susceptibility Testing
- HAI:
-
Healthcare-associated infection
- HAP:
-
Healthcare-associated pneumonia
- HIV:
-
Human immunodeficiency virus
- ICU:
-
Intensive care unit
- ICU-MDR-col:
-
ICU-acquired colonization with multidrug-resistant bacteria
- ICU-MDR-inf:
-
ICU-acquired infection with multidrug-resistant bacteria
- IQR:
-
Interquartile range
- IMV:
-
Invasive mechanical ventilation
- MDR:
-
Multidrug-resistant
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- SAPS-II:
-
Simplified Acute Physiology Score II
- SOFA:
-
Sequential Organ Failure Assessment
- SOT:
-
Solid organ transplant
- VAP:
-
Ventilator-associated pneumonia
- VRE:
-
Vancomycin-resistant Enterococcus sp.
References
Murray CJ, Ikuta KS, Sharara F et al (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399:629–655. https://doi.org/10.1016/S0140-6736(21)02724-0
De Waele JJ, Boelens J, Leroux-Roels I (2020) Multidrug-resistant bacteria in ICU: fact or myth. Curr Opin Anaesthesiol 33:156–161. https://doi.org/10.1097/ACO.0000000000000830
Vincent J-L, Rello J, Marshall J et al (2009) International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–2329. https://doi.org/10.1001/jama.2009.1754
Masse J, Elkalioubie A, Blazejewski C et al (2017) Colonization pressure as a risk factor of ICU-acquired multidrug resistant bacteria: a prospective observational study. Eur J Clin Microbiol Infect Dis 36:797–805. https://doi.org/10.1007/s10096-016-2863-x
Barbier F, Pommier C, Essaied W et al (2016) Colonization and infection with extended-spectrum β-lactamase-producing Enterobacteriaceae in ICU patients: what impact on outcomes and carbapenem exposure? J Antimicrob Chemother 71:1088–1097. https://doi.org/10.1093/jac/dkv423
Bickenbach J, Schöneis D, Marx G et al (2018) Impact of multidrug-resistant bacteria on outcome in patients with prolonged weaning. BMC Pulm Med. https://doi.org/10.1186/s12890-018-0708-3
Barbier F, Lisboa T, Nseir S (2016) Understanding why resistant bacteria are associated with higher mortality in ICU patients. Intensive Care Med 42:2066–2069. https://doi.org/10.1007/s00134-015-4138-x
Mokart D, Pastores SM, Darmon M (2014) Has survival increased in cancer patients admitted to the ICU? Yes. Intensive Care Med 40:1570–1572. https://doi.org/10.1007/s00134-014-3433-2
van Vliet M, Verburg IWM, van den Boogaard M et al (2014) Trends in admission prevalence, illness severity and survival of haematological patients treated in Dutch intensive care units. Intensive Care Med 40:1275–1284. https://doi.org/10.1007/s00134-014-3373-x
Detsis M, Karanika S, Mylonakis E (2017) ICU acquisition rate, risk factors, and clinical significance of digestive tract colonization with extended-spectrum beta-lactamase-producing enterobacteriaceae: a systematic review and meta-analysis. Crit Care Med 45:705–714. https://doi.org/10.1097/CCM.0000000000002253
Strich JR, Heil EL, Masur H (2020) Considerations for empiric antimicrobial therapy in sepsis and septic shock in an era of antimicrobial resistance. J Infect Dis 222:S119–S131. https://doi.org/10.1093/infdis/jiaa221
Dumford D, Skalweit MJ (2020) Antibiotic-resistant infections and treatment challenges in the immunocompromised host: an update. Infect Dis Clin N Am 34:821–847. https://doi.org/10.1016/j.idc.2020.08.005
Bhorade SM, Christenson J, Pohlman AS et al (1999) The incidence of and clinical variables associated with vancomycin-resistant enterococcal colonization in mechanically ventilated patients. Chest 115:1085–1091. https://doi.org/10.1378/chest.115.4.1085
Biderman P, Bugaevsky Y, Ben-Zvi H et al (2015) Multidrug-resistant Acinetobacter baumannii infections in lung transplant patients in the cardiothoracic intensive care unit. Clin Transplant 29:756–762. https://doi.org/10.1111/ctr.12575
Patel SJ, Oliveira AP, Zhou JJ et al (2014) Risk factors and outcomes of infections caused by extremely drug-resistant gram-negative bacilli in patients hospitalized in intensive care units. Am J Infect Control 42:626–631. https://doi.org/10.1016/j.ajic.2014.01.027
Shindo Y, Ito R, Kobayashi D et al (2013) Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med 188:985–995. https://doi.org/10.1164/rccm.201301-0079OC
Moreau A-S, Martin-Loeches I, Povoa P et al (2018) Impact of immunosuppression on incidence, aetiology and outcome of ventilator-associated lower respiratory tract infections. Eur Respir J. https://doi.org/10.1183/13993003.01656-2017
Shorr AF, Zilberberg MD, Micek ST, Kollef MH (2008) Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care-associated pneumonia. Arch Intern Med 168:2205–2210. https://doi.org/10.1001/archinte.168.20.2205
Nseir S, Di Pompeo C, Diarra M et al (2007) Relationship between immunosuppression and intensive care unit-acquired multidrug-resistant bacteria: a case-control study. Crit Care Med 35:1318–1323. https://doi.org/10.1097/01.CCM.0000261885.50604.20
Lemiale V, Mokart D, Resche-Rigon M et al (2015) Effect of noninvasive ventilation vs oxygen therapy on mortality among immunocompromised patients with acute respiratory failure: a randomized clinical trial. JAMA 314:1711–1719. https://doi.org/10.1001/jama.2015.12402
EUCAST (2020) The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 10.0, 2020
Magiorakos A-P, Srinivasan A, Carey RB et al (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x
Kalil AC, Metersky ML, Klompas M et al (2016) Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. https://doi.org/10.1093/cid/ciw353
Mermel LA, Allon M, Bouza E et al (2009) Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious Diseases Society of America. Clin Infect Dis 49:1–45. https://doi.org/10.1086/599376
Hooton TM, Bradley SF, Cardenas DD et al (2010) Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 50:625–663. https://doi.org/10.1086/650482
Solomkin JS, Mazuski JE, Bradley JS et al (2010) Diagnosis and management of complicated intra-abdominal infection in adults and children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 50:133–164. https://doi.org/10.1086/649554
Osmon DR, Berbari EF, Berendt AR et al (2013) Diagnosis and management of prosthetic joint infection: clinical practice Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. https://doi.org/10.1093/cid/cis803
Berbari EF, Kanj SS, Kowalski TJ et al (2015) 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 61:e26–e46. https://doi.org/10.1093/cid/civ482
Habib G, Lancellotti P, Antunes MJ et al (2015) 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36:3075–3128. https://doi.org/10.1093/eurheartj/ehv319
Torres A, Niederman MS, Chastre J et al (2017) International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J. https://doi.org/10.1183/13993003.00582-2017
Guillamet CV, Kollef MH (2015) Update on ventilator-associated pneumonia. Curr Opin Crit Care 21:430–438. https://doi.org/10.1097/MCC.0000000000000231
Freifeld AG, Bow EJ, Sepkowitz KA et al (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis 52:e56-93. https://doi.org/10.1093/cid/cir073
Prentice RL, Kalbfleisch JD, Peterson AV et al (1978) The analysis of failure times in the presence of competing risks. Biometrics 34:541–554
Lederer DJ, Bell SC, Branson RD et al (2019) Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc 16:22–28. https://doi.org/10.1513/AnnalsATS.201808-564PS
White IR, Royston P, Wood AM (2011) Multiple imputation using chained equations: issues and guidance for practice. Stat Med 30:377–399. https://doi.org/10.1002/sim.4067
Zahar J-R, Blot S, Nordmann P et al (2019) Screening for intestinal carriage of extended-spectrum beta-lactamase-producing enterobacteriaceae in critically ill patients: expected benefits and evidence-based controversies. Clin Infect Dis 68:2125–2130. https://doi.org/10.1093/cid/ciy864
Lucet JC, Harris AD, Guidet B (2020) Less contact isolation is more in the ICU: not sure. Intensive Care Med 46:1735–1738. https://doi.org/10.1007/s00134-019-05809-5
Birgand G, Schouten J, Ruppé E (2020) Less contact isolation is more in the ICU: con. Intensive Care Med 46:1732–1734. https://doi.org/10.1007/s00134-019-05887-5
Poulakou G, Nseir S, Daikos GL (2020) Less contact isolation is more in the ICU: pro. Intensive Care Med 46:1727–1731. https://doi.org/10.1007/s00134-020-06173-5
Yokoe DS, Anderson DJ, Berenholtz SM et al (2014) A compendium of strategies to prevent healthcare-associated infections in acute care hospitals: 2014 updates. Infect Control Hosp Epidemiol 35:967–977. https://doi.org/10.1086/677216
Tacconelli E, Cataldo MA, Dancer SJ et al (2014) ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect 20(Suppl 1):1–55. https://doi.org/10.1111/1469-0691.12427
Kallel H, Houcke S, Resiere D et al (2022) Prior carriage predicts intensive care unit infections caused by extended-spectrum beta-lactamase-producing enterobacteriaceae. Am J Trop Med Hyg 106:525–531. https://doi.org/10.4269/ajtmh.20-1436
Papadomichelakis E, Kontopidou F, Antoniadou A et al (2008) Screening for resistant gram-negative microorganisms to guide empiric therapy of subsequent infection. Intensive Care Med 34:2169–2175. https://doi.org/10.1007/s00134-008-1247-9
Melnyk AH, Wong A, Kassen R (2015) The fitness costs of antibiotic resistance mutations. Evol Appl 8:273–283. https://doi.org/10.1111/eva.12196
Margolis E, Rosch JW (2018) Fitness landscape of the immune compromised favors the emergence of antibiotic resistance. ACS Infect Dis 4:1275–1277. https://doi.org/10.1021/acsinfecdis.8b00158
Martin-Loeches I, Torres A, Rinaudo M et al (2015) Resistance patterns and outcomes in intensive care unit (ICU)-acquired pneumonia. Validation of European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) classification of multidrug resistant organisms. J Infect 70:213–222. https://doi.org/10.1016/j.jinf.2014.10.004
Zahar J-R, Clec’h C, Tafflet M et al (2005) Is methicillin resistance associated with a worse prognosis in Staphylococcus aureus ventilator-associated pneumonia? Clin Infect Dis 41:1224–1231. https://doi.org/10.1086/496923
Committee GS, Bertolini G, Nattino G et al (2018) Mortality attributable to different Klebsiella susceptibility patterns and to the coverage of empirical antibiotic therapy: a cohort study on patients admitted to the ICU with infection. Intensive Care Med 44:1709–1719. https://doi.org/10.1007/s00134-018-5360-0
Venet F, Monneret G (2018) Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol 14:121–137. https://doi.org/10.1038/nrneph.2017.165
Acknowledgements
We thank Hugo Coronado, Apolline Briatte, Nafas Abdallah Paune, Hajar Chouiki, Lisa Villequey, Stéphanie Beaussart, Fabienne Jarosz-Thévenin, Anne Dewatine, and Sabine Janowski for their help in data curation and study supervision. We thank Damien Ming and Paul Vasikasin for their critical insights during manuscript drafting.
Funding
There was no specific funding for this study.
Author information
Authors and Affiliations
Contributions
Study conception and design: LK and SN. Statistical analysis: CP and JL. Data curation: LK, MV, SJ, JP, MC, EN, JCR, FW, PG, YZ, NVGDB, and CV. Manuscript drafting: LK, MV, JL, and SN. Critical revision: all authors.
Corresponding author
Ethics declarations
Conflicts of interests
LK has received speaking fees and a research scholarship from BioMérieux, and has been employed by Transgene. SN has received speaking fees from MSD, Pfizer, Gilead, BioMérieux, Fischer and Paykel, and BioRad. JCR received a grant from Hamilton Medical for an experimental study. Other authors have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kreitmann, L., Vasseur, M., Jermoumi, S. et al. Relationship between immunosuppression and intensive care unit-acquired colonization and infection related to multidrug-resistant bacteria: a prospective multicenter cohort study. Intensive Care Med 49, 154–165 (2023). https://doi.org/10.1007/s00134-022-06954-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-022-06954-0