Abstract
Purpose
To systematically identify predictors of gastrointestinal (GI) bleeding in adult intensive care unit (ICU) patients.
Methods
We conducted a systematic review and meta-analysis of cohort studies including trial cohorts. We searched MEDLINE, EMBASE, and trial registries up to March 2019. Eligible studies assessed potential predictors of clinically important GI bleeding (CIB; primary outcome) or overt GI bleeding (secondary outcome), had > 20 events, and presented adjusted effect estimates. Two reviewers assessed study eligibility, extracted data, and assessed risk of bias and certainty of evidence using GRADE. We meta-analysed adjusted effect estimates if data from ≥ 2 studies were available.
Results
We included 8 studies (116,497 patients). 4 studies (including 74,456 patients) assessed potential predictors of CIB, and we meta-analysed 12 potential predictors from these. Acute kidney injury (relative effect [RE] 2.38, 95% confidence interval [CI] 1.07–5.28, moderate certainty) and male gender (RE 1.24, 95% CI 1.03–1.50, low certainty) were associated with increased incidence of CIB. After excluding high risk of bias studies, coagulopathy (RE 4.76, 95% CI 2.62–8.63, moderate certainty), shock (RE 2.60, 95% CI 1.25–5.42, low certainty), and chronic liver disease (RE 7.64, 95% CI 3.32–17.58, moderate certainty) were associated with increased incidence of CIB. The effect of mechanical ventilation on CIB was unclear (RE 1.93, 0.57–6.50, very low certainty).
Conclusions
We identified predictors of CIB and overt GI bleeding in adult ICU patients. These findings may be used to identify ICU patients at higher risk of GI bleeding who are most likely to benefit from stress ulcer prophylaxis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We identified and summarised predictors of clinically important and overt GI bleeding in adult ICU patients. The findings will help clinicians and guideline developers to identify high-risk patients who are likely to benefit the most from stress ulcer prophylaxis. |
Introduction
Gastrointestinal (GI) stress ulceration is a well-recognized condition that affects critically ill patients in the intensive care unit (ICU) and has been associated with increased morbidity and mortality [1, 2]. It has been thought for decades that mechanical ventilation for more than 48 h and coagulopathy are the strongest, and possibly the only, predictors of clinically important GI bleeding (CIB) [2, 3], which is usually defined as overt GI bleeding (visual evidence of GI bleeding) with affection of haemodynamics, haemoglobin levels, or transfusion requirements. In a recent large cohort study, coagulopathy was associated with higher incidence of CIB, while mechanical ventilation was not [4]. In that study, other predictors of CIB were identified, i.e., number of comorbidities, need for renal replacement therapy, and chronic liver disease [4]. Furthermore, while crude mortality was increased in patients with CIB, this association was not significant after adjustment for potential confounders related to severity of illness [4].
Providing stress ulcer prophylaxis (SUP) in the ICU is a topic of ongoing debate. Although SUP is commonly prescribed in the ICU [4], there have been concerns about potential harms including pneumonia, Clostridioides difficile infection and myocardial ischemia [5, 6]. The recent Stress Ulcer Prophylaxis in the Intensive Care Unit (SUP-ICU) trial showed that proton pump inhibitors (PPIs) reduce both overt GI bleeding and CIB, with no effect on mortality and infectious complications [7]. A recent meta-analysis confirmed these findings, although the effects on CIB and serious adverse events were less certain [8]. The remaining uncertainty about the safety of using SUP warrants careful selection of patients who may benefit the most from prophylaxis (i.e., those at highest risk of bleeding).
In this systematic review and meta-analysis of cohort studies and randomised clinical trials (RCTs), we aimed to identify and summarise predictors of CIB and overt GI bleeding in adult ICU patients. We hypothesised that we could identify clinically relevant predictors of CIB and overt GI bleeding.
Methods
This systematic review and meta-analysis was conducted in accordance with recent recommendations [9] and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [10] [completed checklist included in the Electronic Supplementary Material (ESM)]. The review was conducted according to a pre-specified internal protocol, which was not published or prospectively registered.
Data sources and searches
We searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (ICTRP) on March 12–14th 2019 using relevant search terms and filters to identify studies of prognosis. The search was conducted by a medical librarian and the electronic search strategy is presented in the ESM. No restriction on year of publication or language was used. For included studies that were secondary publications of another study (e.g., secondary cohort studies based on RCTs), we also obtained the original publications. For two trial registrations without available data, we attempted to contact authors once and excluded both entries, as no response was obtained.
Study selection and eligibility criteria
We included retrospective and prospective cohort studies including RCT cohorts assessing potential predictors of GI bleeding in adult ICU patients. Adult patients were defined by the included studies; if no definition was provided, we included studies if at least 80% of the included population were at least 18 years of age. ICUs were defined according to the included studies. To decrease confounding, uncertainty, and chance findings, studies with 20 or fewer GI bleeding events and studies that did not report multivariable adjusted estimates for at least one of the outcomes of interest were excluded.
For studies in languages not spoken by any of the authors, we used Google Translate (Google LLC, CA, USA) to assess eligibility [11]. Studies reported as abstracts only and records without any data presented were excluded.
Two reviewers (AG and LZ) independently and in duplicate screened titles and abstracts, followed by full-text screening of potentially eligible studies using a standardised screening form and the Covidence platform (https://www.covidence.org; Veritas Health Innovation, Melbourne, Australia). Discrepancies were solved through consensus or by involvement of a third reviewer (MHM or WA).
Data extraction, risk of bias assessments, and certainty of evidence
We extracted the following data from eligible studies: study type, countries, period of enrolment, setting, number of centres, enrolment criteria, age, gender, severity of illness, use of SUP (medication used, route of administration and dose), definitions of overt/any GI bleeding and CIB, duration of follow-up, number of patients and events, potential predictors [including effect estimates and confidence intervals (CIs) and/or P values] and analytic strategy including the covariates adjusted for. We did not contact study authors for any additional data, as this was not deemed necessary for any of the included studies.
We assessed risk of bias in the included studies using the Quality In Prognosis Studies (QUIPS) tool [12]. The domains patient selection, study attrition, measurement of prognostic factors, outcome measurement, study confounding, and statistical analysis and reporting were rated as low, moderate, or high risk of bias. We classified studies with 5 or 6 low risk of bias domains as overall low risk of bias; studies with 2 or more high risk of bias domains as overall high risk of bias; and all other studies as overall moderate risk of bias [13].
The certainty of evidence was assessed as high, moderate, low, or very low using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach adapted to studies of prognosis, where observational studies start as high certainty of evidence [14]. This assessment was based on risk of bias, consistency, precision, directness, and other concerns including publication bias (which we planned to assess by visual inspection of funnel plots if at least 10 studies were included [15]).
Two reviewers (AG and LZ) independently and in duplicate extracted data, assessed risk of bias, and assessed certainty of evidence using GRADE. Any discrepancies were resolved by consensus or through involvement of a third reviewer (MHM or WA).
Outcomes and predictors
The primary outcome was CIB during the ICU stay, based on definitions in the included studies. The secondary outcome was overt GI bleeding during the ICU stay, based on definitions in the included studies, including GI bleeding events without further specifications. For studies that only assessed potential predictors of CIB, we also included data for this outcome in the analyses of overt GI bleeding. We were primarily interested in upper GI bleeding, but as clinical differentiation of upper and lower GI bleeding is sometimes difficult without diagnostic endoscopy, we included any GI bleeding unless specifically stated that it was lower GI in origin.
We considered all reported potential predictors of GI bleeding, except all forms of pharmacologic SUP [including PPIs, histamine-2-receptor antagonists (H2RAs), sucralfate, and antacids], as this was beyond the scope of this review, and the effect of SUP has already been assessed in recent systematic reviews and meta-analyses of RCTs [8, 16].
Data synthesis and statistical analyses
Potential predictors reported in two or more included studies are presented in the main text; potential predictors assessed in only one study are presented in the ESM. When meta-analysis was not possible (due to different definitions, categorisations, or other dissimilarities), we qualitatively summarised potential predictors across studies.
We performed meta-analyses by extracting and pooling adjusted relative effect (RE) estimates and their standard errors using DerSimonian-Laird random effects models and the inverse variance method [9, 17]. Standard errors were calculated from 95% CIs or from effect estimates and P values [18]. Where P < 0.001 was reported, we assumed P to be exactly 0.001 to allow estimation [18]. If only P values reported as larger inequalities were available, results were not meta-analysed.
Statistical heterogeneity was addressed through consistency of point estimates and the extent of overlap of CIs. Heterogeneity was not assessed with I2 statistics (although these are presented in the forest plots), as they are uniformly high and not useful in prognostic studies with large sample sizes and relatively precise estimates [14].
We conducted pre-specified subgroup analyses according to the effect measures used (hazard ratios [HRs] and odds ratios [ORs]), to assess if any systematic differences caused by the use of different effect measures were present [9]. Furthermore, we conducted pre-specified subgroup analyses according to overall risk of bias where this differed for studies included in the same meta-analysis, to assess the influence of risk of bias on the results [14]. Results from both the analyses including all studies and from studies adjudicated as overall moderate or low risk of bias (i.e., excluding high risk of bias studies) are presented in the evidence profiles.
All analyses were conducted using R version 3.5.3 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria) with the meta package v. 4.9-5, which was also used for producing forest plots, and the ggplot2 package v. 3.1.1. Two-sided P values < 0.05 and 95% CIs not including 1.00 were considered statistically significant.
Results
Study selection and characteristics
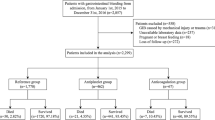
We screened 5352 abstracts and 143 full-text papers and included 8 studies including a total of 116,497 patients [2, 4, 5, 19,20,21,22,23] (Fig. 1). Four studies including 74,456 patients assessed potential predictors of CIB [2, 4, 19, 22]; 2 studies were prospective cohort studies [2, 4], 2 were secondary studies of RCTs [19, 20, 24, 25], and 4 were retrospective cohort studies [5, 21,22,23]. All studies were conducted in multiple centres. Inclusion criteria in most studies were broad; 1 study included neurocritically ill patients only [23], and 2 studies included patients mechanically ventilated for at least 24 [5] or 48 h [19]. More than 70% of the patients received SUP in all but 1 study, where only 30% received SUP [2]; in 3 studies, all patients received SUP [5, 19, 22], and enrolment in 2 of these studies was restricted to patients who received SUP for at least 2 [5] or 3 days [22].
Risk of bias was low in 1 study [19], moderate in 3 studies [2, 4, 20], and high in 4 studies [5, 21,22,23]. There was substantial variation in the analytic strategies used including the variables adjusted for, and in the definitions of some of the included predictors (ESM). Additional study characteristics are presented in Table 1 and Tables S1-S8 in the ESM.
The incidences of CIB and overt GI bleeding in the included studies ranged from 0.6 to 2.8% and 1.3 to 12.8%, respectively.
Predictors assessed
We included a median of 8 potential predictors per study (range 2–21) in our meta-analyses and reported summary estimates for 12 potential predictors of CIB and 21 for overt GI bleeding (Tables 2, 3). All individual forest plots (including results from all subgroup analyses) and additional details are presented in the ESM.
Predictors of CIB
We performed meta-analyses of adjusted estimates for 12 potential predictors of CIB (Table 2 and Fig. 2). Acute kidney injury was associated with a statistically significant increase in the risk of CIB (3 studies [2, 4, 22], 484 events/73,379 patients, RE 2.38, 95% CI 1.07–5.28, moderate certainty). Male gender was also associated with a small increase in the risk of CIB (2 studies [4, 22], 451 events/71,127 patients, RE 1.24, 95% CI 1.03–1.50, low certainty). The effect of mechanical ventilation on CIB risk was unclear (3 studies [2, 4, 22], 484 events/73,379 patients, RE 1.93, 95% CI 0.57–6.50, very low certainty).
Overview of potential predictors of clinically important GI bleeding. This figure presents all relative effects (points) with 95% confidence intervals (horizontal lines) for all included potential predictors of clinically important gastrointestinal (GI) bleeding. Arrows indicate values outside the plot (95% confidence interval smaller than 0.1 or larger than 10.0). Point estimates and 95% confidence intervals (in square brackets) are presented on the right side of the plot. Additional details can be found in Table 2 and the electronic supplementary material (ESM), where a similar figure for overt GI bleeding also can be found
When high risk of bias studies were excluded, coagulopathy (2 studies [2, 4], 60 events/3286 patients, RE 4.76, 95% CI 2.62–8.63, moderate certainty), shock (2 studies [2, 4], 60 events/3286 patients, RE 2.60, 95% CI 1.25–5.42, low certainty), and chronic liver disease (1 study [4], 27 events/1034 patients, RE 7.64, 95% CI 3.32–17.58, moderate certainty) were associated with a statistically significant increase in risk of CIB.
The 95% CIs for all remaining potential predictors included both increased and decreased risk of CIB; the certainty of evidence was very low, low, or moderate for most potential predictors, primarily due to inconsistency or imprecision (Table 2).
Results from subgroup analyses according to the effect measures used are presented in the ESM; these estimates of subgroup effects are highly uncertain and difficult to interpret due to the low number of studies and the overlap between differences in effect measures and according to risk of bias adjudications.
Predictors of overt GI bleeding
We performed meta-analyses of adjusted estimates for 21 potential predictors of overt GI bleeding (Table 3 and Figure S1 in the ESM); 8 predictors were associated with a statistically significant increase in the risk of overt GI bleeding: coagulopathy (4 studies [2, 4, 5, 22], 2069 events/108,691 patients, RE 2.13, 95% CI 1.31–3.45, moderate certainty), shock (4 studies [2, 4, 5, 22], 2069 events/108,691 patients, RE 1.34, 95% CI 1.03–1.74, low certainty), sepsis (3 studies [2, 5, 22], 2020 events/107,657 patients, RE 1.16, 95% CI 1.02–1.32, moderate certainty), acute hepatic failure (4 studies [2, 5, 20, 22], 2096 events/108,531 patients, RE 1.76, 95% CI 1.13–2.74, moderate certainty), chronic liver disease (3 studies [4, 5, 22], 2036 events/106,439 patients, RE 2.16, 95% CI 1.25–3.71, moderate certainty), acute kidney injury (4 studies [2, 4, 5, 22], 2069 events/108,691 patients, RE 1.90, 95% CI 1.20–3.02, moderate certainty), male gender (4 studies [4, 5, 21, 22], 2094 events/110,878 patients, RE 1.18, 95% CI 1.07–1.31, low certainty), and acute myocardial infarction (2 studies [5, 22], 1987 events/105,405 patients, RE 1.65, 95% CI 1.41–1.93, low certainty). The effect of mechanical ventilation was unclear (5 studies [2, 4, 21,22,23], 764 events/79,234 patients, RE 1.11, 95% CI 0.64–1.91, very low certainty).
When high risk of bias studies were excluded, coagulopathy (2 studies [2, 4], 82 events/3286 patients, RE 4.14, 95% CI 2.69–6.90, moderate certainty), shock (2 studies [2, 4], 82 events/3286 patients, RE 2.56, 95% CI 1.44–4.54, low certainty), and chronic liver disease (1 study [4], 49 events/1034 patients, RE 4.51, 95% CI 2.30–8.85, moderate certainty) remained statistically significantly associated with overt GI bleeding.
The 95% CIs for all remaining potential predictors included both increased and decreased risk of overt GI bleeding; the certainty of evidence was very low, low or moderate for most potential predictors, primarily due to risk of bias, inconsistency, or imprecision (Table 3).
The interpretation of the results from subgroup analyses according to effect measures was uncertain for similar reasons as for CIB (ESM).
Predictors not included in the meta-analyses
Potential predictors not meta-analysed are presented in Table S9 in the ESM. Age was associated with increased risk of overt GI bleeding in 1 study [5], but not in any of the other studies [4, 21, 22]. Increased creatinine was associated with increased risk of CIB and overt GI bleeding in 2 studies [19, 21], and thrombocytopenia was associated with decreased CIB in 1 study [5], while no effect on overt GI bleeding was seen in another study [22].
Discussion
This is, to our knowledge, the first systematic review and meta-analysis to provide an overview of predictors of CIB and overt GI bleeding in adult ICU patients and will help clinicians, guideline developers, and investigators to identify adult ICU patients who may benefit the most from SUP.
The first large study on predictors of CIB in adult ICU patients, published 25 years ago, identified 2 independent predictors: mechanical ventilation (for more than 48 h) and coagulopathy [2], which have been highlighted as important predictors since [3]. In this systematic review, we found no clear association between mechanical ventilation and CIB or overt GI bleeding. This could be explained by the use of lung-protective mechanical ventilation (lower pressures and tidal volumes) in the setting of contemporary practice of critical care compared to decades ago [26, 27]. It could also be speculated that the results are unclear due to different definitions of the predictor (primarily related to the duration of mechanical ventilation) or differences in populations, as almost half the patients included in the aforementioned study were cardiac surgical patients [2].
We found that coagulopathy was a predictor of CIB in moderate–low risk of bias studies and for overt GI bleeding regardless of risk of bias. Coagulopathy was defined using biochemical variables in two studies (platelets < 50,000/mm3 or international normalised ratio > 1.5 in both studies [2, 4], or partial thromboplastin time > 2 times the refence value in one study [2]) and using International Classification of Diseases (ICD)-9 codes in two studies [5, 22]. Enteral nutrition has also been mentioned as a possible protective factor [3]. Although enteral nutrition was associated with a decreased risk of CIB in 1 study not at high risk of bias [19], the pooled estimate (RE 0.63, 95% CI 0.17–2.37) suggests that this effect is unclear.
Acute kidney injury was associated with increased risk of CIB and overt GI bleeding in studies irrespective of risk of bias. Acute kidney injury was defined using biochemical variables and oliguria in 1 study [2] (creatinine clearance < 40 ml/min, urine output < 500 ml/day, or creatinine > 2.8 mg/dl [248 µmol/l]), as the need for renal replacement therapy on the first day of admission in 1 study [4], and according to ICD-9 codes in two studies [5, 22]. Additional identified predictors of overt GI bleeding included shock, sepsis, acute hepatic failure, chronic liver disease, and acute myocardial infarction. The identified predictors (except male gender) are all related to severity of illness and involved in mechanisms proposed to lead to stress-related GI bleeding in the critically ill, by being physiological stressors or by decreasing splanchnic perfusion or haemostatic competence [3].
Finally, our results indicate that male gender could be a predictor of both outcomes, but this could be a chance finding, and the point estimate from the 1 study not at high risk of bias [4] indicated a decreased although uncertain risk in males.
It is important to highlight that some analyses for overt GI bleeding included almost four times as many events as the corresponding analyses for CIB, leading to more precise estimates and thus more statistically significant predictors than for CIB.
The results of this systematic review and meta-analysis could prove useful for several reasons. First, the identification of patients at risk of CIB and overt GI bleeding may help clinicians consider, identify and prevent GI bleeding, which may improve outcomes. Second, as current knowledge on the potential adverse effects of SUP is uncertain [7, 8, 28], it may help clinicians target this intervention to patients who are most likely to benefit overall. The clinical applicability of these results, however, is somewhat hampered by most of the available evidence being from patients who received SUP, and it should be stressed that predictors of CIB and overt GI bleeding could be different in patients not receiving SUP.
Future research on this topic is needed, including studies conducted in populations not exposed to SUP or alternatively after adjustment for the use of SUP. All included studies assessed individual potential predictors; however, the cumulative risk of being exposed to multiple predictors or specific combinations of predictors may also be important [29]. Assessing the influence of simultaneous predictors and severity of illness on the risk of CIB and overt GI bleeding could be useful, as could dedicated clinical prediction models [30].
Strengths and limitations of this review
This systematic review comes with several strengths. First, we performed a comprehensive and systematic literature search with no language or temporal restrictions. Second, our review was conducted according to recent recommendations for systematic reviews of prognostic factors [9], and study selection, data extraction, risk of bias assessments, and certainty of evidence assessments were performed independently and in duplicate using the QUIPS tool and the GRADE approach [12, 14]. Third, we included all potential predictors assessed in at least 2 studies, and when meta-analysis was not appropriate, we presented the results qualitatively. Fourth, we excluded studies with 20 or fewer events. While this led to exclusion of 53 studies (of which a substantial proportion likely fulfilled multiple exclusion criteria), it also increased the confidence in the estimates presented, as studies with few events provide uncertain estimates and are at high risk of chance findings [31].
Our review also has limitations. First, the populations and potential predictors assessed were heterogenous, which may affect the interpretation and generalisability of the results. Second, as we only included adjusted estimates, the substantial variation in the used analytical strategies and adjustments may have affected the results. This is a common limitation in meta-analyses of prognostic factors and is hard to avoid; despite this obstacle, it is recommended to primarily focus on adjusted estimates [9]. Third, 3 of the studies presenting potential predictors for our secondary outcome (overt GI bleeding) may not have fulfilled the outcome and follow-up definitions completely; 1 study included a small proportion of lower GI bleeding [21], and in 2 studies, some events could have happened shortly after ICU discharge [5, 23] (details in ESM). Fourth, the evidence base was sparse with few events included in some of the analyses, which affects the certainty of evidence due to imprecision. Fifth, we meta-analysed different relative effect measures (ORs and HRs) together, which may have affected the summarised estimates [9]. As recommended [9], we presented separate summary estimates according to the effect measure used, but where differences were present, it was not possible to determine if this was due to a true heterogeneity or differences in effect measures used. Sixth, we did not estimate absolute risk differences for each potential predictor. This was not done due to several complicating factors, including the different effect estimates used, the varying event rates in the included studies, and the lack of baseline risks estimates from a population without any of the predictors, as most ICU patients will have one or more of the potential predictors assessed. Consequently, clinicians and guideline developers will have to estimate absolute risk differences based on these results and estimated baseline risks in the populations of interest. Seventh, there is a risk of chance findings due to the low event rate and the large number of analyses conducted. Finally, this review was undertaken in a short-time frame to inform the development of a guideline on the use of SUP; consequently, we did not register the review in PROSPERO or publish the protocol prior to conduct.
Conclusions
In this systematic review and meta-analysis of potential predictors of GI bleeding in adult ICU patients, we assessed 12 and 21 potential predictors of CIB and overt GI bleeding, respectively. Acute kidney injury, coagulopathy, shock, and chronic liver disease were consistently associated with increased risk of GI bleeding. These findings may help clinicians, guideline developers, and investigators to identify high-risk patients most likely to benefit from prophylactic acid suppression.
References
Cook DJ, Griffith LE, Walter SD et al (2001) The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care 5:368–375
Cook DJ, Fuller HD, Guyatt GH et al (1994) Risk factors for gastrointestinal bleeding in critically Ill patients. N Engl J Med 330:377–381
Cook D, Guyatt G (2018) Prophylaxis against upper gastrointestinal bleeding in hospitalized patients. N Engl J Med 378:2506–2516
Krag M, Perner A, Wetterslev J et al (2015) Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med 41:833–845
MacLaren R, Reynolds PM, Allen RR (2014) Histamine-2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med 174:564–574
Charlot M, Ahlehoff O, Norgaard ML et al (2010) Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: a nationwide cohort study. Ann Intern Med 153:378–386
Krag M, Marker S, Perner A et al (2018) Pantoprazole in patients at risk for gastrointestinal bleeding in the ICU. N Engl J Med 379:2199–2208
Barbateskovic M, Marker S, Granholm A et al (2019) Stress ulcer prophylaxis with proton pump inhibitors or histamin-2 receptor antagonists in adult intensive care patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 45:143–158
Riley RD, Moons KGM, Snell KIE et al (2019) A guide to systematic review and meta-analysis of prognostic factor studies. BMJ 364:k4597
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Walpole SC (2019) Including papers in languages other than English in systematic reviews: important, feasible, yet often omitted. J Clin Epidemiol. https://doi.org/10.1016/j.jclinepi.2019.03.004
Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C (2013) Assessing bias in studies of prognostic factors. Ann Intern Med 158:280–286
Foroutan F, Guyatt GH, O’Brien K et al (2016) Prognosis after surgical replacement with a bioprosthetic aortic valve in patients with severe symptomatic aortic stenosis: systematic review of observational studies. BMJ 354:i5065
Iorio A, Spencer FA, Falavigna M et al (2015) Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 350:h870
Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I (2006) The case of the misleading funnel plot. BMJ 333:597–600
Alhazzani W, Alshamsi F, Belley-Cote E et al (2018) Efficacy and safety of stress ulcer prophylaxis in critically ill patients: a network meta-analysis of randomized trials. Intensive Care Med 44:1–11
Higgins JPT, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. http://handbook.cochrane.org
Altman DG, Bland JM (2011) How to obtain the confidence interval from a P value. BMJ 343:d2090
Cook D, Heyland D, Griffith L, Cook R, Marshall J, Pagliarello J (1999) Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. Crit Care Med 27:2812–2817
Ellison RT, Perez-Perez G, Welsh CH et al (1996) Risk factors for upper gastrointestinal bleeding in intensive care unit patients: role of helicobacter pylori. Federal hyperimmune immunoglobulin therapy study group. Crit Care Med 24:1974–1981
Kumar S, Ramos C, Garcia-Carrasquillo RJ, Green PH, Lebwohl B (2017) Incidence and risk factors for gastrointestinal bleeding among patients admitted to medical intensive care units. Frontline Gastroenterol 8(1):67–173
Lilly CM, Aljawadi M, Badawi O et al (2018) Comparative effectiveness of proton pump inhibitors vs histamine type 2 receptor blockers for preventing clinically important gastrointestinal bleeding during intensive care: a population-based study. Chest 154:557–566
Wei J, Jiang R, Li L et al (2019) Stress-related upper gastrointestinal bleeding in adult neurocritical care patients: a Chinese multicenter, retrospective study. Curr Med Res Opin 35:181–187
Cook D, Guyatt G, Marshall J et al (1998) A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med 338:791–797
Donta ST, Peduzzi P, Cross AS et al (1996) Immunoprophylaxis against klebsiella and pseudomonas aeruginosa infections. The Federal Hyperimmune Immunoglobulin Trial Study Group. J Infect Dis 174:537–543
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA et al (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Petrucci N, De Feo C (2013) Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003844.pub4
Marker S, Perner A, Wetterslev J et al (2019) Pantoprazole prophylaxis in ICU patients with high severity of disease: a post hoc analysis of the placebo-controlled SUP-ICU trial. Intensive Care Med 45:609–618
Iwashyna TJ, Burke JF, Sussman JB, Prescott HC, Hayward RA, Angus DC (2015) Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med 192:1045–1051
Labarère J, Bertrand R, Fine MJ (2014) How to derive and validate clinical prediction models for use in intensive care medicine. Intensive Care Med 40:513–527
Vetter TR, Mascha EJ (2017) Bias, confounding, and interaction: lions and tigers, and bears, oh my! Anesth Analg 125:1042–1048
Acknowledgements
The authors thank Ms. Sarah Culgin, research coordinator at the GUIDE Group and the Research Institute of St. Joseph’s Healthcare Hamilton, who coordinated practical aspects of this review; Ms. Karin Dearness, director of the Library Services at St. Joseph’s Healthcare Hamilton, who developed the electronic search strategy and conducted the searches; and Mr. Farid Foroutan, Department of Health Research Methods, Evidence, and Impact at McMaster University, who provided templates and materials used for the review.
Funding
The Guidelines in Intensive Care, Development and Evaluation (GUIDE) Group.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
The Department of Intensive Care at Rigshospitalet—Copenhagen University Hospital receives support for other research projects from Ferring Pharmaceuticals, Denmark, and the Novo Nordisk Foundation, Denmark. RM received support for other research projects from CSL Behring, USA. AG, AP, SM, MK, and MHM are involved in the SUP-ICU research programme, and AP, MK, and MHM were directly involved in one of the studies included [4]. RM was the lead author of one of the included studies [5]. Two of the included studies [2, 19] were led from McMaster University, Hamilton, Canada, where LZ, JCD, ZY, and WA are employed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Granholm, A., Zeng, L., Dionne, J.C. et al. Predictors of gastrointestinal bleeding in adult ICU patients: a systematic review and meta-analysis. Intensive Care Med 45, 1347–1359 (2019). https://doi.org/10.1007/s00134-019-05751-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05751-6