Abstract
Purpose
To assess the effect of low dose corticosteroids on outcomes in adults with septic shock.
Methods
We systematically reviewed randomised clinical trials (RCTs) comparing low-dose corticosteroids to placebo in adults with septic shock. Trial selection, data abstraction and risk of bias assessment were performed in duplicate. The primary outcome was short-term mortality. Secondary and tertiary outcomes included longer-term mortality, adverse events, quality of life, and duration of shock, mechanical ventilation and ICU stay.
Results
There were 22 RCTs, including 7297 participants, providing data on short-term mortality. In two low risk of bias trials, the relative risk (RR) of short-term mortality with corticosteroid versus placebo was 0.98 [95% confidence interval (CI) 0.89–1.08, p = 0.71]. Sensitivity analysis including all trials was similar (RR 0.96; 95% CI 0.91–1.02, p = 0.21) as was analysis of longer-term mortality (RR 0.96; 95% CI 0.90–1.02, p = 0.18). In low risk of bias trials, the risk of experiencing any adverse event was higher with corticosteroids; however, there was substantial heterogeneity (RR 1.66; 95% CI 1.03–2.70, p = 0.04, I2 = 78%). No trials reported quality of life outcomes. Duration of shock [mean difference (MD) −1.52 days; 95% CI −1.71 to −1.32, p < 0.0001], duration of mechanical ventilation (MD −1.38 days; 95% CI −1.96 to −0.80, p < 0.0001), and ICU stay (MD −0.75 days; 95% CI −1.34 to −0.17, p = 0.01) were shorter with corticosteroids versus placebo.
Conclusions
In adults with septic shock treated with low dose corticosteroids, short- and longer-term mortality are unaffected, adverse events increase, but duration of shock, mechanical ventilation and ICU stay are reduced.
PROSPERO registration no. CRD42017084037.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In adults with septic shock, treatment with corticosteroids does not affect short- or longer-term mortality, adverse events are increased but duration of shock, mechanical ventilation and ICU admission are reduced. |
Background
Corticosteroids, acting to both modulate the immune response to infection [1] and to enhance the cardiovascular response to exogenous catecholamines [2], have been administered to patients with sepsis since the 1950s [3]. Early randomised clinical trials (RCTs), using high-dose corticosteroids in patients with septic shock, demonstrated no beneficial treatment effect, with a suggestion that treatment may even increase mortality [4]. As a result, treatment of septic shock with high-dose corticosteroids declined. Interest in the use of lower-dose corticosteroids, often referred to as “stress-dose” steroids, was revived in the late 1990s when two RCTs reported significant improvements in haemodynamic parameters [5, 6] and suggested improved mortality [6].
Subsequent RCTs have reported divergent results [7, 8], and, to date, systematic reviews [9, 10] have not resolved whether use of corticosteroids in patients with septic shock improves outcomes. The ongoing debate has been fuelled by the recent publication of two large RCTs [11, 12] without clear conclusions. Therefore, to provide an updated summary of the evidence, we conducted a systematic review and meta-analysis with trial sequential analysis to assess the effect of low-dose corticosteroids compared to placebo or usual care on patient-centred outcomes, including mortality, adverse events and quality-of-life in adult patients with septic shock.
Methods
The systematic review was conducted according to a pre-specified protocol registered at the international prospective register of systematic reviews (PROSPERO registration CRD42017084037). The full details of the protocol are available in the Electronic Supplementary Material (ESM).
Search and eligibility criteria
We searched for RCTs of adult patients with septic shock, where a corticosteroid in a dose of less than 500 mg per day of hydrocortisone (or equivalent) was compared to placebo, no corticosteroid or any other control intervention, and at least one of the outcomes outlined below were reported. Studies in which the population was not limited to patients with septic shock, but in which data from an identifiable sub-group of patients with septic shock were included, when authors of the studies could provide data on the group of patients with septic shock, were eligible for inclusion. We excluded studies in which both experimental groups received corticosteroids. We applied no language restriction and we included all reports including studies only reported in abstract form.
We performed a search of electronic databases, Medline (via the PUBMED interface), EMBASE and The Cochrane Central Registry of Controlled Trials (via the Ovid interface). All searches were conducted from inception through to March 3, 2018. We used search terms for septic shock, sepsis and septicaemia combined with terms for corticosteroids and sensitive filters specific to each database to identify randomised clinical trials [13,14,15]. We also performed an electronic search of conference abstracts and clinical trial registries. The full details of the electronic search strategy are available in the ESM. We also conducted a manual search of reference lists of relevant primary studies and previous review articles, and contacted experts in the field.
Study selection
Two investigators independently screened articles for inclusion based on study title and abstract. The full text of articles deemed relevant during preliminary screening were retrieved and reviewed for inclusion by two reviewers. Disagreement during the review process was resolved by discussion with a third reviewer and by consensus.
Data extraction
Two investigators independently extracted information from each included trial. We extracted all available data as outlined in the protocol, including characteristics of the included studies, details of the population enrolled, details of the intervention including type of corticosteroid, dose and regimen, mode of discontinuation and whether the comparison group received placebo or usual care. Data specified in the protocol that were not available in trial reports were requested from the corresponding authors of included studies.
Risk of bias assessment
Two investigators, with no affiliation with any of the included trials, independently assessed risk of bias of the included trials. Disagreements were resolved by discussion with a third reviewer and by consensus. Clarifications regarding additional details of the methods of included studies required to assess risk of bias were sought from corresponding authors where these were not clear in the available reports. We used the Cochrane risk of bias tool [16], along with specific criteria developed for the purpose of this review, to ensure consistency across trials (details supplied in ESM). We adjudicated risk of bias across all predefined outcome measures, and overall risk of bias was adjudicated low only if all domains were assessed as low risk of bias.
Outcomes
The primary outcome was short-term mortality (death within 90 days of randomisation) in trials adjudicated as low risk of bias in all domains of the Cochrane risk of bias tool [16]. Secondary outcomes were longer-term mortality (death occurring within and beyond 90 days of randomisation), patient-reported health-related quality of life at final follow-up and the proportion of patients experiencing at least one adverse event. Tertiary outcomes were time to resolution of shock, duration of mechanical ventilation, duration of ICU and hospital length of stay, and the incidence of specific adverse events; secondary infection, gastrointestinal bleeding, delirium, hyperglycaemia and hypernatraemia, each recorded as defined in the included trials.
Subgroup analyses
We planned to assess nine subgroups for short-term mortality based upon: adjudication of risk of bias, dose of corticosteroid, bolus or infusion dosing, time allowed from eligibility to randomisation, ICU population (medical, surgical or mixed), pulmonary versus non-pulmonary source of sepsis, type of corticosteroid, duration of intervention and mode of treatment cessation.
Data synthesis
We evaluated statistical heterogeneity by inspecting forest plots and quantitatively by using diversity (D2) [17] and inconsistency (I2) [18] statistics. Furthermore, clinical heterogeneity was evaluated by performing subgroup analyses for the primary outcome. For subgroup analyses, we used χ2 tests to investigate heterogeneity (test-of-interaction or test for subgroup differences), and p < 0.1 was considered statistically significant. We assessed reporting bias for outcomes in which 10 or more studies provided data, by funnel plot inspection and the Harbord test [19] for dichotomous outcomes and the regression asymmetry test [20] and adjusted rank correlation [21] for continuous outcomes.
For dichotomous outcomes, we calculated relative risks (RRs) with 95% confidence intervals (CIs). For continuous outcomes, we calculated mean differences (MDs) with 95% CIs.
The primary analysis was conducted using a fixed effect model [22] and included only trials adjudicated as overall low risk of bias. We conducted a sensitivity analysis by pooling data with a random effects model, and by pooling data from all trials regardless of adjudication of risk of bias. We assessed the potential effect of missing outcome data by performing a best and worst case analysis [23], assuming in the best–worst case scenario that all patients lost to outcome assessment (follow-up) in the intervention group had a beneficial outcome, whereas all patients lost to outcome assessment in the control group had a detrimental outcome, and in the worst–best case scenario that all patients lost to outcome assessment in the intervention group had a detrimental outcome, whereas all patients lost to outcome assessment in the control group had a beneficial outcome.
Secondary and tertiary outcomes were pooled using a fixed effect model using pooled RRs for dichotomous outcomes and mean differences for continuous outcomes that were available as means with standard deviations. For continuous measures reported in other metrics, not amenable to statistical pooling, we reported the results from each individual trial.
We assessed statistical significance for the primary outcome at p < 0.05. Given the multiple outcomes reported we assessed the statistical significance of results of the secondary outcomes at p < 0.025 and tertiary outcomes at p < 0.01.
Trial sequential analysis
We conducted trial sequential analysis (TSA) in order to assess the risk of random errors [24]. We used a random effects model for all overall low risk of bias trials included in the primary analyses [24]. We used a family-wise error rate, the probability of making one or more false positive assertions when performing multiple hypothesis tests, of 5% [23] with a statistical significance level of 5% for the primary outcome; 2.5% for the three secondary outcomes, and 1% for the tertiary outcomes. We used a beta of 20% and a diversity (D2) [17] as suggested by the included trials [23]. For dichotomous outcomes, a pre-specified relative risk of 15% was used. We present TSA-adjusted CIs for estimates where these were calculated.
Analyses were conducted using Review Manager v.5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark), Trial Sequential Analysis v.0.9.5.10 beta (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark, available from www.ctu.dk/tsa) and R v.3.4.3 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria) with the meta package v.4.9-0.
Grading the quality of evidence
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach [25] to assess the overall quality of evidence for each outcome measure and present the results in the ‘summary of findings’ table. The quality of evidence and our confidence in the effect-estimates were evaluated on the basis of study design, study quality, precision, consistency, directness and the risk of reporting bias. Consequently, the overall quality of evidence is rated “high”, “moderate”, “low” or “very low” for each outcome.
Results
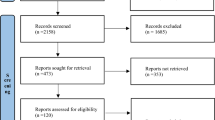
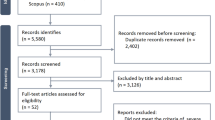
The search for studies was completed on March 3, 2018. Figure 1 shows the results of the search and the reasons for exclusion of studies. A total of 15,588 records were retrieved with 23 studies [5,6,7,8, 11, 12, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42], including a total of 7688 trial participants, included in the systematic review. One study did not report any outcomes of interest and was not included in the quantitative synthesis [31]. Additional data and clarifications were obtained from authors of 15 studies [5,6,7,8, 12, 26, 27, 29, 32, 33, 37, 38, 41,42,43]. Data were obtained from two studies [41, 42], in which the primary population was not specifically septic shock but in which a group of patients with septic shock was identifiable at baseline. The characteristics of the included studies are shown in Table 1 and ESM Table 1. There were two studies adjudicated as low risk of bias in all domains [12, 33], with all other studies rated as unclear or high risk of bias in at least one domain of potential bias. The summary of the risk of bias assessments is shown in Fig. 2 and ESM Fig. 1, with the full details of the risk of bias assessments presented in ESM Table 2.
Primary outcome
There was no definitive evidence of reporting bias evident on inspection of the funnel plot (ESM Fig. 2) or via the Harbord test (p = 0.39). There were 22 studies including a total of 7297 participants with data available regarding short-term (≤ 90 days) mortality [5,6,7,8, 11, 12, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. The pooled estimate of the RR for short-term mortality for corticosteroids compared to placebo in the trials adjudicated as low risk of bias was 0.98 (95% CI 0.89–1.08, p = 0.71, I2 = 0%). The TSA for the primary outcome (shown in ESM Figs. 3, 3a), showed a required information size was exceeded, indicating sufficient events had been accrued in the current trials to exclude a 15% RR (from a baseline event rate of 28.9%), and the TSA adjusted 95% CI remained the same (0.89–1.08). The estimate of the RR for short-term mortality in trials not adjudicated as low risk of bias was similar (RR 0.95, 95% CI 0.88–1.02, p = 0.15, I2 = 39%) to that from the trials estimated as low risk of bias (test for subgroup differences p = 0.58), as shown in Fig. 3. The results of the sensitivity analyses and subgroup analyses were largely consistent with the primary analysis, with subgroups defined by mode of cessation of the intervention and by type of corticosteroid used showing potentially differential treatment effects, as shown in Table 2. Data were not available to assess the effect of pulmonary versus non-pulmonary source of sepsis.
Secondary outcomes
For longer-term mortality, there were a total of 5667 trial participants from three trials that reported mortality at 12 months [5,6,7] and two trials that reported mortality at 180 days [11, 44]. The pooled RR for longer-term mortality for corticosteroids compared to placebo (ESM Fig. 4) was 0.96 (95% CI 0.90–1.02, p = 0.18, I2 = 0%). The proportion of trial participants reporting any adverse event was available in 10 trials [5,6,7, 11, 12, 27, 29, 32, 33, 37]. The fixed effect estimated RR of reporting an adverse event for trial participants randomised to receive corticosteroids compared to control in trials adjudicated as low risk of bias was 1.66 (1.03–2.70, p = 0.04, I2 = 78%). The TSA analysis (ESM Fig. 5a), included too few events to calculate TSA-adjusted CI. When the pooled RR of reporting an adverse event was estimated from all trials (ESM Fig. 5), the estimated RR was 0.98 (95% CI 0.90–1.08, p = 0.73, I2 = 54%, test for subgroup difference p = 0.02). The results of the sensitivity analyses for the secondary outcomes are shown in ESM Table 3. No trial included in this review reported health-related quality of life outcomes.
Tertiary outcomes
Tertiary outcomes were assessed on all trials regardless of adjudication of risk of bias. The time to resolution of shock was shorter in trial participants assigned to receive corticosteroids, with data available from 7 trials in a metric suitable for pooling [12, 29, 34, 36,37,38, 41]; the results of this pooled analysis are shown in Table 3 and ESM Fig. 6. There were an additional 9 trials [5,6,7,8, 11, 28, 32, 33, 39] that reported time to resolution of shock using metrics that were not amenable to statistical pooling; all but one [33] showed a shorter duration of shock in the group assigned corticosteroids (ESM Table 4).
Based on 5 trials [5, 12, 29, 37, 41] with data available in a format that allowed pooling, duration of mechanical ventilation was shorter in patients assigned to steroid treatment (Table 3; ESM Fig. 7). An additional 4 trials [11, 27, 32, 33] reported data in metrics that were not amenable to statistical pooling (ESM Table 4); in these the duration of ventilation was similar in the two groups.
In 13 trials [6,7,8, 12, 27, 28, 33, 34, 36,37,38, 42] that reported duration of ICU admission in a manner that allowed pooling of data, duration was shorter in patients assigned steroids (Table 3; ESM Fig. 8). There was no evidence of reporting bias on inspection of the funnel plot (ESM Fig. 9) or the regression asymmetry test (p = 0.24) or the adjusted rank correlation test (p = 0.33). An additional 3 trials [11, 32, 35] reported data in metrics that did not allow pooling, two reported a shorter duration of ICU admission in the corticosteroid group (ESM Table 4).
In 11 trials that reported data that could be pooled [6,7,8, 12, 26,27,28, 33, 36,37,38, 42] there was no significant difference in duration of hospital admission (Table 3; ESM Fig. 10). There was no evidence of reporting bias on inspection of the funnel plot (ESM Fig. 11) or via the regression asymmetry test (p = 0.19) or the adjusted rank correlation test (p = 0.93). There were two additional trials that reported duration of hospital admission in metrics not amenable to statistical pooling [11, 31], neither showed a significant difference between the two groups.
The effect of corticosteroids on the incidence of individual adverse events, as defined in the included studies, is reported in Table 3 and ESM Figs. 12, 14–17. Corticosteroid treatment was associated with increased reporting of hypernatraemia and hyperglycaemia but not secondary infection (Table 3). There was no evidence of reporting bias for the incidence of secondary infection on inspection of the funnel plot (ESM Fig. 13) or from the Harbord test, 0.99.
Summary of findings and recommendations
The quality of evidence for all outcomes (summary of findings) is presented in Table 4.
Discussion
The results of this systematic review provide an evidence summary to inform clinicians regarding decisions to use corticosteroids in adult patients with septic shock. We found that assignment to treatment with corticosteroids had no effect on either short-term or longer-term mortality. To date, health-related quality of life has not been reported by any trial. Adverse events were increased in patients assigned to corticosteroids. The time to resolution of shock was shorter, as was duration of mechanical ventilation, and ICU admission. The use of corticosteroids was not associated with increased incidence of secondary infection.
This systematic review and meta-analysis has a number of methodological strengths. The research question was focussed to include a specific clinically relevant population and a specific intervention. The study was conducted in accordance with current best research practice and followed a pre-published protocol. A trial sequential analysis was used to assess the risk of random errors (spurious findings), with results supporting the contention that a 15% relative increase or decrease in short-term mortality can be confidently excluded. The risk of bias assessment was conducted in a robust fashion, by using reviewers not involved in any of the included studies. There are also a number of limitations. As with all meta-analyses, the strength of conclusions that can be drawn are dependent on the strength of the included trials. What is less often recognised is the problems that may arise from differing definitions of outcomes used by studies. The two largest trials reported incidences of hyperglycaemia in the control groups as 3/1829 (0.16%) [12] and 520/626 (83.1%) [11], respectively. Pooling data that is clearly as disparate as these leads to reduced confidence in the results of the analysis, as can be seen in the GRADE summary of findings table. Pooling time-to-event outcomes, such as time to resolution of shock and duration of mechanical ventilation is also difficult in a trial-level meta-analysis. These outcomes are prone to bias due to competing risk [45], and the data included in this systematic review and meta-analysis highlights the particular difficulty of pooling trial-level data for time to event outcomes in critically ill patients. We anticipated reporting data on health-related quality of life, but found these data were not reported by the included trials.
The results of this systematic review and meta-analysis differ from the previous review published in the Cochrane Database of Systematic Reviews, which found a reduction in mortality when corticosteroids were used in patients with sepsis [46]. In contrast, our study was restricted to trials in which the study population was septic shock, excluding trials in which corticosteroids were used for other indications such as pneumonia [47], trials in which both experimental groups received corticosteroids [9, 48], and trials which used larger doses of corticosteroids [49]. The inclusion of the two recently published, largest trials [11, 12] aids the interpretation of the results of these trials to allow clinicians, researchers and those directing health policy to make decisions regarding the use of corticosteroids in this population.
The results of this systematic review and meta-analysis indicate that, while there is no discernible mortality benefit, a significant reduction in duration of ventilation, if confirmed with more specific analyses, may represent a patient-centred outcome. Subsequent confirmation of the benefits related to a reduction in duration of ICU admission in specific cost-effectiveness and health economic analyses might provide justification for recommending the use of corticosteroids in future clinical practice guidelines, if these analyses confirm that these benefits outweigh the potential effects related to increased risk of adverse events. It was notable that the risk of experiencing any adverse event was higher in trial participants assigned to corticosteroids. The data would suggest that this effect was greatest on biochemical events such as hyperglycaemia and hypernatraemia, but the clinical significance of these events is not clear. The subgroup of trials which used hydrocortisone and fludrocortisone did suggest the possibility of a mortality benefit but, given this was based on trials not adjudicated as low risk of bias and was of marginal significance, we cannot draw strong inferences from this result.
Further research is needed to clarify some issues. The publication of the longer-term outcomes from all trials included in this review may add some clarity regarding the effect of corticosteroids on long-term mortality. Pooling all the trial data in an individual patient data meta-analysis would allow more accurate assessment of the effect of corticosteroids on time-to-event outcomes as well as subgroups pf patients based on clinical characteristics, such as time to commencement of the intervention or possibly response to a corticotropin stimulation test. It may also allow for a more nuanced assessment of the role of corticosteroids in those with more severe shock, defined by dose of vasopressor, while accounting for confounders in this relationship such as volume of fluid administered and sedative regimen. More information regarding the effect of corticosteroids on longer-term quality of life is required.
In conclusion, there is high-quality evidence that, in adult patients with septic shock, corticosteroids compared to placebo or control therapy had no significant effect on short-term or longer-term mortality. Among patients treated with corticosteroids, there was an increased incidence of adverse events and an association with shorter duration of shock, mechanical ventilation and ICU admission, but these latter conclusions are based on lower-quality evidence.
References
Annane D (2005) Glucocorticoids in the treatment of severe sepsis and septic shock. Curr Opin Crit Care 11:449–453
Chrousos GP (1995) The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. New Engl J Med 332:1351–1362
Wagner HN Jr, Bennett IL Jr, Lasagna L, Cluff LE, Rosenthal MB, Mirick GS (1956) The Effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp 98:197–215
Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, Fisher CJ Jr (1995) Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med 23:1430–1439
Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, Hemmer B, Hummel T, Lenhart A, Heyduck M, Stoll C, Peter K (1999) Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med 27:723–732
Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A (1998) Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med 26:645–650
Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaut P, Bellissant E (2002) Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. J Am Med Assoc 288:862–871
Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J (2008) Hydrocortisone therapy for patients with septic shock. New Engl J Med 358:111–124
Annane D, Cariou A, Maxime V, Azoulay E, D’Honneur G, Timsit JF, Cohen Y, Wolf M, Fartoukh M, Adrie C, Santre C, Bollaert PE, Mathonet A, Amathieu R, Tabah A, Clec’h C, Mayaud J, Lejeune J, Chevret S (2010) Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. J Am Med Assoc 303:341–348
Moran JL, Graham PL, Rockliff S, Bersten AD (2010) Updating the evidence for the role of corticosteroids in severe sepsis and septic shock: a bayesian meta-analytic perspective. Crit Care 14:R134
Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, Cariou A, Forceville X, Schwebel C, Martin C, Timsit JF, Misset B, Ali Benali M, Colin G, Souweine B, Asehnoune K, Mercier E, Chimot L, Charpentier C, Francois B, Boulain T, Petitpas F, Constantin JM, Dhonneur G, Baudin F, Combes A, Bohe J, Loriferne JF, Amathieu R, Cook F, Slama M, Leroy O, Capellier G, Dargent A, Hissem T, Maxime V, Bellissant E (2018) Hydrocortisone plus fludrocortisone for adults with septic shock. New Engl J Med 378:809–818
Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, Billot L, Correa M, Glass P, Harward M, Joyce C, Li Q, McArthur C, Perner A, Rhodes A, Thompson K, Webb S, Myburgh J (2018) Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med 378(9):797–808
Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Werre SR (2005) Optimal search strategies for retrieving scientifically strong studies of treatment from medline: analytical survey. BMJ 330:1179
Wilczynski NL, McKibbon KA, Walter SD, Garg AX, Haynes RB (2013) Medline clinical queries are robust when searching in recent publishing years. J Am Med Inform Assoc 20:363–368
Wong SS, Wilczynski NL, Haynes RB (2006) Developing optimal search strategies for detecting clinically sound treatment studies in embase. J Med Libr Assoc 94:41–47
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC (2011) The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Wetterslev J, Thorlund K, Brok J, Gluud C (2009) Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol 9:86
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Harbord RM, Egger M, Sterne JA (2006) A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25:3443–3457
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Villar J, Mackey ME, Carroli G, Donner A (2001) Meta-analyses in systematic reviews of randomized controlled trials in perinatal medicine: comparison of fixed and random effects models. Stat Med 20:3635–3647
Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C (2014) Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol 14:120
Wetterslev J, Jakobsen JC, Gluud C (2017) Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol 17:39
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S (2004) Grading quality of evidence and strength of recommendations. BMJ 328:1490
Aboab J, Polito A, Orlikowski D, Sharshar T, Castel M, Annane D (2008) Hydrocortisone effects on cardiovascular variability in septic shock: a spectral analysis approach. Crit Care Med 36:1481–1486
Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, Al Knawy B, Hajeer AH, Tamimi W, Cherfan A (2010) Low-dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. Can Med Assoc J 182:1971–1977
Chawla K, Kupfer Y, Tessler S (1999) Hydrocortisone reverses refractory septic shock. Crit Care Med 27:A33
Cicarelli DD, Vieira JE, Bensenor FE (2007) Early dexamethasone treatment for septic shock patients: a prospective randomized clinical trial. Sao Paulo Med J 125:237–241
Cooperative Study Group (1963) The effectiveness of hydrocortisone in the management of severe infections: a double-blind study. JAMA 183:462–465
Deng QM, Shang D, Wan XY (2011) Effects of low-dose glucocorticoid on renal function and prognosis in patients with sepsis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 23:183–184
Gordon AC, Mason AJ, Perkins GD, Stotz M, Terblanche M, Ashby D, Brett SJ (2014) The interaction of vasopressin and corticosteroids in septic shock: a pilot randomized controlled trial. Crit Care Med 42:1325–1333
Gordon AC, Mason AJ, Thirunavukkarasu N, Perkins GD, Cecconi M, Cepkova M, Pogson DG, Aya HD, Anjum A, Frazier GJ, Santhakumaran S, Ashby D, Brett SJ (2016) Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: the vanish randomized clinical trial. JAMA 316:509–518
Hu B, Li JG, Liang H, Zhou Q, Yu Z, Li L, Luo Y, Liu C, Gan Q (2009) The effect of low-dose hydrocortisone on requirement of norepinephrine and lactate clearance in patients with refractory septic shock. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 21:529–531
Kurugundla N, Irugulapati L, Kilari D, Amchentsev A, Devakonda A, George L, Raoof S (2008) Effect of steroids in septic shock patients without relative adrenal insufficiency. International conference A, American Thoracic Society 116
Lv QQ, Gu XH, Chen QH, Yu JQ, Zheng RQ (2017) Early initiation of low-dose hydrocortisone treatment for septic shock in adults: a randomized clinical trial. Am J Emerg Med 35(12):1810–1814
Meduri GU, Golden E, Umberger R (2009) Prospective double-blind randomized clinical trial on the effects of low-dose hydrocortisone infusion in patients with severe sepsis [abstract]. Chest 136:45S
Mirea L, Ungureanu R, Pavelescu D, Grintescu IC, Dumitrache C, Grintescu I, Mirea D (2014) Continuous administration of corticosteroids in septic shock can reduce risk of hypernatremia. Crit Care 18:S86
Oppert M, Schindler R, Husung C, Offermann K, Graf KJ, Boenisch O, Barckow D, Frei U, Eckardt KU (2005) Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med 33:2457–2464
Tandan S, Guleria R, Gupta N (2005) Low dose steroids and adrenocortical insufficiency in septic shock: a double-blind randomised controlled trial from India. Am J Respir Crit Care Med 171:A43
Tongyoo S, Permpikul C, Mongkolpun W, Vattanavanit V, Udompanturak S, Kocak M, Meduri GU (2016) Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial. Crit Care 20:329
Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, Gabarrus A, Sellares J, Restrepo MI, Anzueto A, Niederman MS, Agusti C (2015) Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 313:677–686
Rinaldi L, Ferrari E, Marietta M, Donno L, Trevisan D, Codeluppi M, Busani S, Girardis M (2013) Effectiveness of sepsis bundle application in cirrhotic patients with septic shock: a single-center experience. J Crit Care 28:152–157
Venkatesh B, Finfer S, Myburgh J, Cohen J, Billot L (2018) Long-term outcomes of the ADRENAL trial. N Engl J Med 378(18):1744–1745
Resche-Rigon M, Azoulay E, Chevret S (2006) Evaluating mortality in intensive care units: contribution of competing risks analyses. Crit Care 10:R5
Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y (2015) Corticosteroids for treating sepsis. Cochrane Database Syst Rev: Cd002243
Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, Della Porta R, Giorgio C, Blasi F, Umberger R, Meduri GU (2005) Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med 171:242–248
Keh D, Boehnke T, Weber-Cartens S, Schulz C, Ahlers O, Bercker S, Volk HD, Doecke WD, Falke KJ, Gerlach H (2003) Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med 167:512–520
Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA (1987) A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. New Engl J Med 317:653–658
Acknowledgements
We would like to thank Agnes Wroblewski from the Douglas Piper Medical Library at RNSH for her assistance in obtaining elusive manuscripts included in this review. We are indebted to Dr Arne Andreasen, and Dr Soo Wan Kim for providing translations. We are deeply appreciative of Professors Annane, Arabi, Bollaert, Briegel, Gordon, Meduri, Sprung, and Torres, and Drs Cicarelli, Mirea, Rinaldi, Tongyoo, Gabarus-Barri for supplying additional details and clarifications regarding their studies.
Funding
This study received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Professors Venkatesh, Finfer, Myburgh, Perner and Associate Professor Cohen were all members of the management committee of the ADRENAL study. The other authors have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rygård, S.L., Butler, E., Granholm, A. et al. Low-dose corticosteroids for adult patients with septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 44, 1003–1016 (2018). https://doi.org/10.1007/s00134-018-5197-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-018-5197-6