Abstract
Objective
It was to systematically evaluate the effect of corticosteroids on 28d all-cause mortality (ACM), in-hospital death rate, and ICU death rate in critically ill sepsis patients.
Methods
PubMed, Embase, and Medline databases were used to screen the published literatures on the therapeutic effect of corticosteroids in the treatment of critically ill sepsis patients. After evaluating the quality of the included literatures, RevMan 5.3 software was used for meta-analysis. 4524 literatures regarding the application of corticosteroids to treat critically ill sepsis patients were preliminarily searched. After screening was carried out, 9 literatures were finally included. 2,850 patients were treated with corticosteroids and 2867 patients were treated with placebo.
Results
The meta-analysis of the effect of corticosteroids versus placebo on 28dACM showed [OR = 0.87, 95% CI 0.78–0.98, Z = 2.22, P = 0.03], P < 0.05; the meta-analysis of the outcome of corticosteroids versus placebo on ICU death rate showed [OR = 0.77, 95% CI 0.63–0.94, Z = 2.60, P = 0.009], P < 0.05; and the meta-analysis of the effect of corticosteroids versus placebo on in-hospital death rate showed [OR = 0.80, 95% CI 0.66–0.96, Z = 2.34, P = 0.002], P < 0.05.
Conclusion
In summary, corticosteroids can reduce the death rate of critically ill sepsis patients to a certain extent and have good clinical application value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is caused by infection of the human body with pathogenic microorganisms such as bacteria that cause an inflammatory response (Esposito et al. 2017). In addition to the manifestations of systemic inflammatory response syndrome and primary infectious lesions, critically ill patients often have manifestations of organ hypoperfusion. Sepsis generally includes previous sepsis and sepsis (Hecker et al. 2019). There are many ways that can cause sepsis, such as the common clinical lung, peritoneum, bile duct, urinary system, meninges, and other parts of the inflammation (Font et al. 2020). Pathogenic microorganisms causing sepsis include common bacteria, fungi, viruses, and some parasites, but positive blood culture results find that not every patient with sepsis has pathogenic microorganisms causing infection. According to statistics, the proportion of positive blood culture results is only about 45%. Myocardial injury is also one of the most common complications of sepsis (Thompson et al. 2019). When a patient develops sepsis for a long period of time, it may cause damage to the heart muscle and affect the function of the heart. Clinical studies have found that the main clinical manifestations of these patients are that the heart cannot perform well contraction, the diastolic ability of the heart will appear a certain degree of decline, pumping function will also appear a certain degree of decline after illness, the failure of these functions will make the heart cannot get adequate blood moisturization, thus indirectly leading to systemic ischemia. Sepsis is characterized by a poor prognosis in patients with myocardial injury. Although the current medical level has made great progress in anti-infective treatment and organ function support surgery, it also has certain results. According to statistics, the death rate of sepsis can still reach 30% or even 70% (Osborn 2017; Greer et al. 2019). Such a high death rate has also successfully made sepsis the second cause of death in intensive care unit (ICU) after heart disease (Taeb et al. 2017).
During the early systemic inflammatory response syndrome of sepsis, proinflammatory mediators are released uncontrollably, resulting in waterfall chain reaction. Proinflammatory mediators: tumor necrosis factor-α (TNF-a), interleukin-1 (IL-1), IL-6, IL-8, platelet activating factor (PAF), prostaglandins and leukotrienes secrete and activate granulocytes, resulting in endothelial cell injury (Gaballa et al. 2021; Liyanage et al. 2017); platelets adhere to damaged endothelial cells and release many oxygen free radicals and lipid metabolites, resulting in further damage or even failure of important tissues and organs (Filippone et al. 2019). In the anti-inflammatory treatment of septic patients, corticosteroids, as a compound composed of fat-soluble small molecules, easily bind to receptors in the cytoplasm, affect gene transcription, change the level of transmitter-associated proteins, and then inhibit the corresponding inflammatory response, reduce systemic inflammatory response syndrome and tissue damage, improve the body’s tolerance to bacterial endotoxin, and restore microcirculatory hemodynamics (Annane et al. 2015). Corticosteroids have been shown not only to alleviate severe inflammatory responses and organ dysfunction during systemic inflammatory response syndrome, but also to downregulate uncontrolled pro-inflammatory responses, inhibit excessive consumption of immune factors and cells, and maintain the body’s innate immune capacity (Heming et al. 2018). Corticosteroids have also been used in clinical practice for a long time because of their strong anti-inflammatory ability and their low price (Fujii et al. 2020). For more than 4 decades, corticosteroids have become one of the therapies of considerable interest in the treatment of patients with sepsis and septic shock, because they can downregulate uncontrolled pro-inflammatory responses while maintaining innate immune function, but their benefit–risk ratio in the treatment of sepsis remains controversial (Taeb et al. 2017). Literature on the effects of corticosteroids in critically ill sepsis patients was collected and analyzed. Meta-analysis systematically evaluated the application of corticosteroids on 28dACM, inpatient deaths, and ICU deaths in critically ill sepsis patients, in order to provide some reference for the selection of efficient treatment for critically ill sepsis patients in clinical treatment.
Materials and methods
Documents retrieval
Independent searching Pubmed, Embase, Medline, Cochrane Online Library, Web of science, and other databases by two persons for clinical research experiments, and the range of time was from database establishment to now. The subjects included in the literature had no age restriction and no language restriction. The search keywords were “sepsis”, “corticosteroids”, “methylprednisolone”, “Hydrocortisone”, “Fludrocortisone” and “placebo”, and the keywords were combined in “or” and “and”.
Inclusion and exclusion criteria of literatures
Inclusion criteria: (1) more than one critically ill sepsis patient; (2) corticosteroids were selected as the therapeutic drug; (3) prospective randomized study, randomized controlled study, and cohort study were selected as the study type; (4) the number of patients included, grouping, number of patients in different groups, age, and death rate indicators were recorded in detail and completely; (5) the number of samples included in the study was at least 15; (6) data available for analysis were provided, such as RR or OR and 95% CI.
Exclusion criteria: (1) articles of non-clinical trials; (2) review articles, case studies, repeated publications, and basic studies; (3) missing outcome measures or key data; (4) studies unrelated to the topic of this study; (5) no study controls were set in the literature, or studies without comparable samples between groups; (6) the literature with the highest quality was selected between or among articles published with the same group of data. (7) studies with small sample size; (8) study type was not fully specified, incorrect randomized controlled studies; (9) incomplete articles, and full text not available from authors; (10) no valid outcome data could be extracted from literature; (11) specialty-related exclusion requirements.
Extraction of information
Two investigators independently extracted the basic data of the included articles as well as the outcome measure data. The basic data included the first author, date of publication, patient disease diagnosis, interventions, and concomitant medications. Outcome measures included “inpatientdeaths, 28dACM, ICU deaths”. Cross-checking of extracted outcome data was performed by the evaluator and disagreement was decided for agreement; if disagreement remained after discussion, a third investigator joined the discussion.
Methodological quality evaluation
Methodological quality evaluation of the included articles was carried out independently by two investigators using the Cochrane Collaboration’s tool for assessing risk of bias (RoB) provided by the Cochrane Collaboration. The evaluation items include six dimensions, which are: (1) whether the literature describes the random allocation method; (2) whether the literature is sufficient to have allocation concealment and whether the reasons are sufficient; (3) whether the literature implements the blinding method, and the implementation objects of the blinding method are the study subjects, medical staff or outcome indicator evaluators, respectively; (4) whether the literature describes the withdrawal or loss to follow-up of personnel; (5) whether the literature performs the selective reporting of outcome indicators; and (6) whether there are other factors that can affect the quality of the experiment (Salluh and Póvoa 2017).
Statistical analysis
Statistical analysis was performed using Review Manager (Version 5.3) software. Binary variables were presented using OR or RR, along with 95% CI, for the combined model analysis. Continuous variable was shown by mean difference (MD) or standard MD (SMD) with 95% CI for the combined model analysis. Evaluation of overall heterogeneity was performed using the χ2 test. Heterogeneity analysis of indicators was performed using the I2 test, and when I2 ≥ 50% and P ≤ 0.05, statistical analysis was performed using the random effect model, and sensitivity analysis was performed to determine the source of heterogeneity; when I2 < 50% and P > 0.05, it should perform statistical analysis applying the fixed effect model. According to the criteria provided by Cochrane Collaboration, the bias evaluation tool (funnel plot) provided by Cochrane Collaboration was used to evaluate the publication bias of the literature (Salluh et al. 2010).
Results
Screening procedure of literature
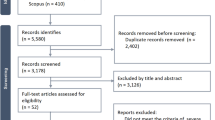
According to the search subject terms and search strategy, 4524 literatures involving in the outcome of corticosteroids treating critically ill sepsis patients were preliminarily retrieved from Pubmed, Embase, Medline, Cochrane Online Library, and Web of science databases with the search keywords “sepsis”, “corticosteroids”, “methylprednisolone”, “Hydrocortisone”, “Fludrocortisone” and “placebo”. 2366 literatures that did not meet the requirements such as repeated publication, case studies, basic studies, and missing outcome measures were excluded. The abstracts of the articles were read and 1685 articles that did not meet the requirements were excluded based on the inclusion and exclusion criteria. Preliminary eligible articles were downloaded and 353 non-eligible articles and 111 articles with missing outcome data were excluded. Finally, nine articles were included for meta-analysis (Torres et al. 2015; Annane et al. 2002, 2018; Venkatesh et al. 2018; Arabi et al. 2010; Gordon et al. 2014; Yildiz et al. 2002; Lv et al. 2017; Menon et al. 2017). The literature search process is illustrated in Fig. 1.
Basic data of included literature
Finally, 9 literatures were included, including 2850 subjects in experimental group (corticosteroids) and 2867 subjects in controls (placebo). The specific information of the first author, publication year, and study subject of the literatures is shown in Table 1.
Quality evaluation of included literature
9 included articles were evaluated for quality using Cochrane Reviewer’ Handbook and assessment charts were drawn for overall evaluation of literature quality, and the evaluation results are shown in Fig. 2. All included articles were found to have “allocation concealment (selection bias)”, “binding of participants and personnel (performance bias)” and “selective reporting (reporting bias)” entries evaluated as “Low risk”. Only individual articles have “random sequence generation (selection bias)”, “incomplete outcome data (attrition bias)”, and “binding of outcome assessment (detection bias)” with “high risk” or “unclear risk”. Therefore, the Cochrane Reviewer’ Handbook was used to evaluate the quality of literatures with grade B or above. The quality of the included articles was subsequently evaluated using the Jadad scale, and the assessment found that the Jadad scale scores of the included articles were all greater than 3, so sensitivity analysis was not required (Fig. 3).
Comparison of 28d all-cause mortality (28dACM) between the two groups
Among the included literatures, 7 literatures involved corticosteroids and placebo on 28dACM. The heterogeneity analysis showed I2 = 0%, P = 0.42. Therefore, the fixed effect model was applied for analyzing. Meta-comprehensive model suggested that OR = 0.87, 95% CI 0.78–0.98, Z = 2.22, P = 0.03. The results showed that the 28dACM rate of sepsis patients treated with corticosteroids was lower (P < 0.05). The relevant forest map is shown in Fig. 4. Funnel plot distribution was essentially symmetrical and most of the data was on either side of the central axis, suggesting that there was no significant publication bias, as illustrated in Fig. 5.
ICU mortality in the two groups
Among the included literatures, a total of five literatures involved the effect of corticosteroids and placebo on ICU mortality. The heterogeneity analysis showed I2 = 0%, P = 0.96, so the fixed effect model was used for analysis. The results of meta-analysis showed that OR = 0.77, 95% CI 0.63–0.94, Z = 2.60, P = 0.009. The results showed that ICU mortality in sepsis patients treated with corticosteroids was lower than that in patients treated with placebo, and the difference had statistical significance (P < 0.05). The relevant forest map is illustrated in Fig. 6. Funnel plot distribution was basically symmetrical and most of the data were on either side of the central axis. It reveals that there was no obvious publication bias (Fig. 7).
In-hospital mortality in both groups
Among the included literatures, six literatures involved the effect of corticosteroids and placebo on inpatient mortality. The results of heterogeneity analysis showed I2 = 0%, P = 0.76. Therefore, the fixed effect model was adopted for analyzing. Meta-comprehensive model indicated that OR = 0.80, 95% CI 0.66–0.96, Z = 2.34, P = 0.002. The results showed that the inpatient mortality rate of sepsis patients treated with corticosteroids was lower than that of patients applying placebo, and the distinction was statistically meaningful (P < 0.05). The relevant forest plot is shown in Fig. 8. Funnel plot distribution was basically symmetrical and most of the data were on either side of the central axis. It suggests no apparent publication bias, as shown in Fig. 9.
Discussion
Sepsis is a life-threatening organ dysfunction caused by a dysregulated body response induced by infection (Wan et al. 2021). Systematic review of published national or local population estimates in 2017 showed that sepsis infects 30 million people and kills 6 million people annually (MOCORSEP Study Group 2021). The study showed that ICU, inpatient, and 1-year mortality were 15.5%, 28.3%, and 40.9%, respectively, while 2-year mortality was even as high as 44.9%. Sepsis is, therefore, a significant global burden of disease and a major challenge in the medical field (Yao et al. 2021). In recent years, researchers have continuously explored the pathological, physiological, and biological mechanisms of sepsis and found that the essence of sepsis is that pathogenic microorganisms infect the body, causing uncontrolled inflammatory reactions and immune disorders, that is, from excessive immune activation to extensive immunosuppression. When the body is infected, bacterial endotoxins activate phagocytes to produce TNF-a and induce pro-inflammatory mediators: TNF-a, IL-1, IL-6, IL-8, PAF, prostaglandins and leukotrienes secrete and activate granulocytes, resulting in endothelial cell injury; platelets adhere to damaged endothelial cells and release many oxygen free radicals and lipid metabolites, further causing tissue injury (Marik 2018).
Studies have shown that the nature of systemic inflammatory response syndrome refers to the uncontrolled release of a variety of inflammatory mediators by the body, resulting in waterfall chain reaction caused by the body, which causes immune loss and immune disorders (Agus 2020). Studies have shown that corticosteroids, as one of the therapies of considerable concern in the treatment of patients with sepsis and septic shock, can not only relieve the severe inflammatory response and organ dysfunction of the body during SIRS, but also down-regulate the uncontrolled pro-inflammatory response, inhibit the excessive consumption of immune factors and cells, and maintain the innate immune capacity of the body. Corticosteroids are divided into two classes: glucocorticoids and mineralocorticoids (Gibbison et al. 2017). General medical hormones refer to glucocorticoids, oral glucocorticoids prednisone, methylprednisone, hydrocortisone, and so on. Liang et al. (2021) grouped 3800 sepsis patients to receive low-dose hydrocortisone and showed no significant differences between the two groups in 28d mortality, ICU length and mortality, length of hospital stay and mortality, and recurrence of shock. In the trial by Fang et al. (2019), 1241 patients were grouped to receive low-dose hydrocortisone plus fludrocortisone, and the results showed that 90-day mortality, ICU length of stay, hospital stay, and mortality were higher in the placebo group, and the time to reach a Sequential Organ Failure Assessment (SOFA) below 6 was shorter in the experimental group than in the placebo group. 9 randomized controlled trials of corticosteroids in sepsis were included, with 2850 subjects in the experimental group (corticosteroids) and 2867 subjects in the controls. Heterogeneity analysis of 28dACM showed I2 = 0%. Corticosteroids could significantly reduce 28d mortality in sepsis patients and heterogeneity was very low (I2 = 0%), and the difference had statistical meaning (P < 0.05). Heterogeneity analysis of ICU mortality showed I2 = 0%, P = 0.96, and meta-analysis comprehensive model analysis showed OR = 0.77, 95% CI 0.63–0.94, Z = 2.60, P = 0.009. The results showed that the ICU mortality of sepsis patients treated with corticosteroids was lower than that of patients adopting placebo (P < 0.05). The heterogeneity analysis results of inpatient mortality showed that I2 = 0%, P = 0.76, and the meta-analysis results showed that OR = 0.80, 95% CI 0.66–0.96, Z = 2.34, P = 0.002. The results showed that the in-hospital mortality of sepsis patients treated with corticosteroids was lower than that of patients treated with placebo (P < 0.05), which was similar to the above findings. In conclusion, this evaluation suggests that corticosteroids reduce 28dACM and significantly reduce ICU and hospital mortality in sepsis. The results showed the significant heterogeneity. Regarding the sepsis disease, there are many studies (Pan and He 2020; Zhang 2020; Zeng et al. 2022; Shen et al. 2022; Zheng et al. 2022; Lv et al. 2022; Chen et al. 2022) in the field of cellular, molecular and bioinformatics.
Conclusion
Systematic evaluation of the effects of corticosteroids and placebo on 28dACM, inpatient mortality, and ICU mortality in critically ill sepsis patients showed that corticosteroids were associated with lower mortality than placebo, and corticosteroids could improve survival to some extent. However, only clinical studies were collected for systematic evaluation of corticosteroids for the treatment of critically ill sepsis patients, and the number of articles included in the analysis was too small. More clinical patient data need to be collected in the future to validate the results of this meta-analysis. The results provide some reference for selecting efficient treatment for sepsis patients, and can be used as a reference for clinical treatment.
Availability of data and materials
The datasets used and/or analyzed in the present study are available from the corresponding author upon reasonable request.
References
Agus MSD (2020) Corticosteroids probably reduce sepsis-related 28-day mortality in adults—unclear effect in children. J Pediatr 220:264–267. https://doi.org/10.1016/j.jpeds.2020.02.067
Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, Cariou A, Forceville X, Schwebel C, Martin C, Timsit JF, Misset B, Ali Benali M, Colin G, Souweine B, Asehnoune K, Mercier E, Chimot L, Charpentier C, François B, Boulain T, Petitpas F, Constantin JM, Dhonneur G, Baudin F, Combes A, Bohé J, Loriferne JF, Amathieu R, Cook F, Slama M, Leroy O, Capellier G, Dargent A, Hissem T, Maxime V, Bellissant E, CRICS-TRIGGERSEP Network (2018) Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med 378(9):809–818. https://doi.org/10.1056/NEJMoa1705716
Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troché G, Chaumet-Riffaud P, Bellissant E (2002) Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 288(7): 862-71. https://doi.org/10.1001/jama.288.7.862. Erratum in: JAMA. 2008 Oct 8; 300(14):1652. Chaumet-Riffaut, Philippe [corrected to Chaumet-Riffaud, Philippe]
Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y (2015) Corticosteroids for treating sepsis. Cochrane Database Syst Rev. 2015(12): CD002243. https://doi.org/10.1002/14651858.CD002243.pub3. Update in: Cochrane Database Syst Rev 2019; 12: CD002243.
Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, Knawy BA, Hajeer AH, Tamimi W, Cherfan A (2010) Low-dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. CMAJ 182(18):1971–1977. https://doi.org/10.1503/cmaj.090707
Chen Y, Zhou L, Wang J, Gu T, Li S (2022) Clinical effect of Xuebijing combined with thymosinα1 on patients with severe pneumonia complicated with sepsis and its effect on serum inflammatory factors. Cell Mol Biol (noisy-Le-Grand) 67(6):228–235. https://doi.org/10.14715/cmb/2021.67.6.30
Esposito S, De Simone G, Boccia G, De Caro F, Pagliano P (2017) Sepsis and septic shock: new definitions, new diagnostic and therapeutic approaches. J Glob Antimicrob Resist 10:204–212. https://doi.org/10.1016/j.jgar.2017.06.013
Fang F, Zhang Y, Tang J, Lunsford LD, Li T, Tang R, He J, Xu P, Faramand A, Xu J, You C (2019) Association of corticosteroid treatment with outcomes in adult patients with sepsis: a systematic review and meta-analysis. JAMA Intern Med 179(2):213–223. https://doi.org/10.1001/jamainternmed.2018.5849
Filippone M, Nardo D, Bonadies L, Salvadori S, Baraldi E (2019) Update on postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Am J Perinatol 36(S 02):S58–S62. https://doi.org/10.1055/s-0039-1691802
Font MD, Thyagarajan B, Khanna AK (2020) Sepsis and septic shock—basics of diagnosis, pathophysiology and clinical decision making. Med Clin North Am 104(4):573–585. https://doi.org/10.1016/j.mcna.2020.02.011
Fujii T, Deane AM, Nair P (2020) Metabolic support in sepsis: corticosteroids and vitamins: the why, the when, the how. CurrOpin Crit Care 26(4):363–368. https://doi.org/10.1097/MCC.0000000000000736
Gaballa SA, Kompella UB, Elgarhy O, Alqahtani AM, Pierscionek B, Alany RG, Abdelkader H (2021) Corticosteroids in ophthalmology: drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv Transl Res 11(3):866–893. https://doi.org/10.1007/s13346-020-00843-z
Gibbison B, López-López JA, Higgins JP, Miller T, Angelini GD, Lightman SL, Annane D (2017) Corticosteroids in septic shock: a systematic review and network meta-analysis. Crit Care 21(1):78. https://doi.org/10.1186/s13054-017-1659-4
Gordon AC, Mason AJ, Perkins GD, Stotz M, Terblanche M, Ashby D, Brett SJ (2014) The interaction of vasopressin and corticosteroids in septic shock: a pilot randomized controlled trial. Crit Care Med 42(6):1325–1333. https://doi.org/10.1097/CCM.0000000000000212
Greer O, Shah NM, Sriskandan S, Johnson MR (2019) Sepsis: precision-based medicine for pregnancy and the puerperium. Int J Mol Sci 20(21):5388. https://doi.org/10.3390/ijms20215388
Hecker A, Reichert M, Reuß CJ, Schmoch T, Riedel JG, Schneck E, Padberg W, Weigand MA, Hecker M (2019) Intra-abdominal sepsis: new definitions and current clinical standards. Langenbecks Arch Surg 404(3):257–271. https://doi.org/10.1007/s00423-019-01752-7
Heming N, Sivanandamoorthy S, Meng P, Bounab R, Annane D (2018) Immune effects of corticosteroids in sepsis. Front Immunol 30(9):1736. https://doi.org/10.3389/fimmu.2018.01736
Liang H, Song H, Zhai R, Song G, Li H, Ding X, Kan Q, Sun T (2021) Corticosteroids for treating sepsis in adult patients: a systematic review and meta-analysis. Front Immunol. 12: 709155. https://doi.org/10.3389/fimmu.2021.709155. Erratum in: Front Immunol 2021 Nov 05; 12: 771779
Liyanage CK, Galappatthy P, Seneviratne SL (2017) Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol 49(5):196–207. https://doi.org/10.23822/EurAnnACI.1764-1489.15
Lv QQ, Gu XH, Chen QH, Yu JQ, Zheng RQ (2017) Early initiation of low-dose hydrocortisone treatment for septic shock in adults: a randomized clinical trial. Am J Emerg Med 35(12):1810–1814. https://doi.org/10.1016/j.ajem.2017.06.004
Lv M, Xie D, Long X (2022) The effect of fenofibrate, a peroxisome proliferator-activated receptor α agonist, on cardiac damage from sepsis in BALB/c mice. Cell Mol Biol (noisy-Le-Grand) 67(6):260–266. https://doi.org/10.14715/cmb/2021.67.6.34
Marik PE (2018) Vitamin C for the treatment of sepsis: the scientific rationale. PharmacolTher 189:63–70. https://doi.org/10.1016/j.pharmthera.2018.04.007
Menon K, McNally D, O’Hearn K, Acharya A, Wong HR, Lawson M, Ramsay T, McIntyre L, Gilfoyle E, Tucci M, Wensley D, Gottesman R, Morrison G, Choong K, Canadian Critical Care Trials Group (2017) A randomized controlled trial of corticosteroids in pediatric septic shock: a pilot feasibility study. Pediatr Crit Care Med 18(6):505–512. https://doi.org/10.1097/PCC.0000000000001121
MOCORSEP Study Group (2021) Monocyte distribution width as a biomarker of resistance to corticosteroids in patients with sepsis: the MOCORSEP observational study. Intensive Care Med 47(10):1161–1164. https://doi.org/10.1007/s00134-021-06478-z
Osborn TM (2017) Severe sepsis and septic shock trials (ProCESS, ARISE, ProMISe): what is optimal resuscitation? Crit Care Clin 33(2):323–344. https://doi.org/10.1016/j.ccc.2016.12.004
Pan X, He L (2020) LncRNA MEG3 expression in sepsis and its effect on LPS-induced macrophage function. Cell Mol Biol (noisy-Le-Grand) 66(5):131–136
Salluh JI, Póvoa P (2017) Corticosteroids in severe sepsis and septic shock: a concise review. Shock 47(1S Suppl 1):47–51. https://doi.org/10.1097/SHK.0000000000000704
Salluh JI, Bozza FA, Japiassú AM, Castro Faria Neto HC, Bozza PT, Póvoa P (2010) Corticosteroids in sepsis: pathophysiological rationale and the selection of patients. Endocr Metab Immune Disord Drug Targets 10(3):266–273. https://doi.org/10.2174/187153010791936865
Shen L, Huang M, Xie N (2022) Experimental study on streptococcus agalactiae genotype and erythromycin resistance in neonatal sepsis. Cell Mol Biol (noisy-Le-Grand) 67(6):100–106. https://doi.org/10.14715/cmb/2021.67.6.14
Taeb AM, Hooper MH, Marik PE (2017) Sepsis: current definition, pathophysiology, diagnosis, and management. Nutr Clin Pract 32(3):296–308. https://doi.org/10.1177/0884533617695243
Thompson K, Venkatesh B, Finfer S (2019) Sepsis and septic shock: current approaches to management. Intern Med J 49(2):160–170. https://doi.org/10.1111/imj.14199
Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, Gabarrús A, Sellarés J, Restrepo MI, Anzueto A, Niederman MS, Agustí C (2015) Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 313(7):677–686. https://doi.org/10.1001/jama.2015.88
Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, Billot L, Correa M, Glass P, Harward M, Joyce C, Li Q, McArthur C, Perner A, Rhodes A, Thompson K, Webb S, Myburgh J, ADRENAL Trial Investigators and the Australian–New Zealand Intensive Care Society Clinical Trials Group (2018) Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med 378(9):797–808. https://doi.org/10.1056/NEJMoa1705835
Wan Z, Dong Y, Yu Z, Lv H, Lv Z (2021) Semi-supervised support vector machine for digital twins based brain image fusion. Front Neurosci 9(15):705323. https://doi.org/10.3389/fnins.2021.705323
Yao TC, Wang JY, Chang SM, Chang YC, Tsai YF, Wu AC, Huang JL, Tsai HJ. (2021) Association of oral corticosteroid bursts with severe adverse events in children. JAMA Pediatr. 175(7): 723-729. https://doi.org/10.1001/jamapediatrics.2021.0433. Erratum in: JAMA Pediatr. 2021; 175(7): 751.
Yildiz O, Doganay M, Aygen B, Güven M, Keleştimur F, Tutuû A (2002) Physiological-dose steroid therapy in sepsis [ISRCTN36253388]. Crit Care 6(3):251–259. https://doi.org/10.1186/cc1498
Zeng Y, Wu D, Zhuo X, Song J (2022) Effects of continuous blood purification without heparin on strem-1, NSE, and IL-10 levels in patients with sepsis. Cell Mol Biol (noisy-Le-Grand) 68(4):178–187. https://doi.org/10.14715/cmb/2022.68.4.21
Zhang F (2020) Efficacy of cefotaxime combined with gamma globulins on C-reactive protein and procalcitonin in neonatal sepsis. Cell Mol Biol (noisy-Le-Grand) 66(2):172–176
Zheng Y, Peng L, He Z, Zou Z, Li F, Huang C, Li W (2022) Identification of differentially expressed genes, transcription factors, microRNAs and pathways in neutrophils of sepsis patients through bioinformatics analysis. Cell Mol Biol (noisy-Le-Grand) 67(5):405–420. https://doi.org/10.14715/cmb/2021.67.5.53
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
YSL, JH and YL participated the design, supervision and editing, and resources, writing of original draft, experimental implementation, and data statistics and analysis. All authors read and approved final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, Y., Hao, J. & Liu, Y. Role of corticosteroids in the treatment of critically ill sepsis patients: a meta-analysis review. Inflammopharmacol 32, 965–974 (2024). https://doi.org/10.1007/s10787-023-01426-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01426-3