Abstract
Purpose
The optimal target blood glucose concentration for acute glycemic control remains unclear because few studies have directly compared 144–180 with 110–144 or >180 mg/dL. Accordingly, we performed a network meta-analysis to compare four different target blood glucose levels (<110, 110–144, 144–180, and >180 mg/dL) in terms of the benefit and risk of insulin therapy.
Methods
We included all of the studies from three systematic reviews and searched the PubMed and Cochrane databases for other studies investigating glucose targets among critically ill patients. The primary outcome was hospital mortality, and the secondary outcomes were sepsis or bloodstream infection and the risk of hypoglycemia. Network meta-analysis to identify an optimal target glucose concentration.
Results
The network meta-analysis included 18,098 patients from 35 studies. There were no significant differences in the risk of mortality and infection among the four blood glucose ranges overall or in subgroup analysis. Conversely, target concentrations of <110 and 110–144 mg/dL were associated with a four to ninefold increase in the risk of hypoglycemia compared with 144–180 and >180 mg/dL. However, there were no significant differences between the target concentrations of 144–180 and >180 mg/dL.
Conclusions
This network meta-analysis found no significant difference in the risk of mortality and infection among four target blood glucose ranges in critically ill patients, but indicated that target blood glucose levels of <110 and 110–144 mg/dL were associated with a higher risk of hypoglycemia than target levels of 144–180 and >180 mg/dL. Further studies are required to refute or confirm our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blood glucose management has been considered important in critically ill patients [1], since the pioneering study by van den Berghe et al. reported that intensive insulin therapy (IIT; target blood glycemic range, 80–110 mg/dL) reduced mortality and morbidity in surgical intensive care unit (ICU) patients (63 % were post-cardiac surgical patients) compared with a more liberal target range (180–200 mg/dL) [2]. Subsequently, several studies reported that tight glycemic control reduced mortality and morbidity at certain centers and in selected populations [3, 4]. A previous meta-analysis also reported that IIT decreased mortality and the risk of septicemia in surgical ICU patients [5, 6]. Furthermore, hyperglycemia might be an independent risk factor for ICU-acquired weakness [7]. Therefore, lowering the blood glucose level might be beneficial in critically ill patients.
On the other hand, IIT is frequently reported to increase the risk of hypoglycemia [2–5]. Although there is no evidence to suggest that such acute hypoglycemia would directly worsen patient outcomes, the increased risk of hypoglycemia during tight glycemic control in critically ill patients might result in undesirable adverse effects, as even moderate hypoglycemia has been associated with poor outcomes [8]. In 2009, the landmark Normoglycemia in Intensive Care Evaluation and Surviving Using Glucose Algorithm Regulation (NICE-SUGAR) trial reported that IIT (target blood glycemic range, 80–108 mg/dL) increased mortality among critical ill patients compared with an intermediate level of glycemic control (144–180 mg/dL) [9]. Therefore, several clinical guidelines do not recommend IIT, with some, including the Surviving Sepsis Campaign guidelines, recommending that insulin therapy be commenced for blood glucose levels of >180 mg/dL [10], while others have recommended that insulin therapy be initiated at blood glucose levels of >150 mg/dL [11].
This divergence among guidelines may be due to the lack of a meta-analysis comparing the risks or benefits of diverse glycemic targets, including <110, 110–144, 144–180, and >180 mg/dL. Notably, all prior meta-analyses compared IIT with insulin therapy targeting higher glycemic levels [5, 6, 12]. Recently, the methodology for network meta-analyses was established and it was made statistically possible to compare groups even when a direct comparison was not originally performed [13, 14]. The integration of direct evidence with indirect evidence increases the precision of the estimates and produces a relative ranking of all treatments for the studied outcome [15]. The assumption of consistency underlies the methodology and, if it holds, a network meta-analysis can provide valuable information [15]. Therefore, we considered that a network meta-analysis could provide comparisons among four glycemic targets, which was not possible using a conventional meta-analysis. Accordingly, we performed a network meta-analysis to identify the optimal blood glucose target in terms of balance of the benefit (the reduction of mortality and incidence of infection) and the risk (incidence of hypoglycemia) of insulin therapy among critically ill patients.
Methods
Search strategy
For the network meta-analysis, we considered all studies from three recent systematic reviews [5, 6, 12]. In addition, we searched the PubMed and Cochrane Library databases (6 June 2008 to 25 February 2016) for studies that were published after the meta-analysis by Wiener et al. [5], using the following search terms: “blood glucose” or “insulin” (administration and dosage, adverse effects, therapeutic use, therapy) AND “intensive care unit” or “critical care” or “critical illness” or “postoperative care” or “sepsis” or “myocardial infarction” or “stroke” or “cardiovascular surgical procedures” or “wounds and injuries”. We also evaluated the reference lists of the relevant clinical trials to identify additional studies.
Study selection
We included randomized controlled trials (RCTs) that fulfilled the following criteria: (1) full-text publication in English; (2) included adult patients who were treated in the ICU; (3) compared different glycemic targets; and (4) the outcome measures included the incidences of hypoglycemia, sepsis and bloodstream infection, or mortality. Studies were excluded if they did not meet the above four criteria. We also reviewed the full texts of all studies obtained from our search of the PubMed and Cochrane databases and the studies from the three systematic reviews.
Data abstraction and quality assessment
Three reviewers (TY, SI, and ME) independently abstracted the data and assessed the methodological quality of the eligible studies. Disagreements were resolved via consensus after a group discussion. The abstracted data for each study included the first author’s name, year of publication, number of study sites, number of patients, ICU setting, patients’ age, history of diabetes, target blood glucose levels, mean glucose levels during the study period, presence of a protocol, method of glucose measurement, duration of the study, and the mean length of stay in the ICU. Methodological quality was evaluated using the Cochrane risk of bias assessment tool, which assesses randomization, allocation concealment, blinding of the study participants and personnel, blinding of the outcome assessments, incomplete outcome data, selective outcome reporting, and other potential sources of bias [16].
Outcome measures and categories of target blood glucose levels
For the analyses, we defined the primary outcome as hospital mortality. The secondary outcomes were defined as the risk of sepsis or bloodstream infection, and hypoglycemia (defined as blood glucose levels of <40 mg/dL or hypoglycemia-associated symptoms). If hospital mortality data were not available, we substituted the 90-day mortality for hospital mortality. Cases of sepsis or bloodstream infection were defined as patients with sepsis, bacteremia, or positive blood cultures.
Blood glucose levels were categorized on the basis of the upper limit of the blood glucose target: <110, 110–144, 144–180, and >180 mg/dL. For example, if the target blood glucose range was 80–130 mg/dL, it was categorized as 110–144 mg/dL.
Subgroup analyses and additional analysis using actually achieved mean glycemic levels
We conducted subgroup analyses according to the ICU setting, proportion of diabetic patients, and observed mortality. We also performed an additional analysis using achieved mean glycemic levels during the study period.
ICU setting
We divided studies according to the ICU setting. First, we classified the ICU setting in each study into three categories, according to a previous systematic review [5]: (1) surgical ICU, including general-surgical, cardiothoracic-surgical, neurosurgical, and trauma ICUs; (2) medical ICU, including general medical, cardiac, and neurological ICUs; and (3) mixed medical-surgical ICUs. Next, we stratified the mixed medical-surgical ICUs by the proportion of surgical patients. If surgical patients constituted ≤50 % of the total cohort in a mixed medical-surgical ICU, it was defined as a medical ICU; if surgical patients in a mixed medical-surgical ICU constituted >50 % of the total cohort in the ICU, it was defined as a surgical ICU. Finally, we performed a subgroup analysis of the ICU setting for surgical ICUs and medical ICUs separately.
Proportion of diabetic patients
We stratified studies according to the proportion of diabetic patients: ≤50 or >50 %.
Observed mortality
We performed a subgroup analysis of the patients with >20 % observed mortality.
Additional analysis using actual mean glycemic levels
We performed an additional analysis based on the actually achieved mean glycemic levels.
Statistical analysis
We performed the network meta-analysis within a Bayesian framework using JAGS (version 4.1.0), R software (version 3.1.1), and the rjags and gemtc packages [17, 18]. Comparative odds ratios (OR) are reported with their 95 % credible intervals (CrI). Both fixed-effect and random-effect models were used, and the deviation information criterion (DIC) was used to compare the treatment effects of the two models. The DIC measures the fit of the model, while penalizing it for the number of effective parameters; the most appropriate model has the lowest DIC value [19]. We selected the random-effect model based on the DIC values for the two models. Furthermore, a Bayesian framework meta-analysis provided a rank probability for each glycemic target range and outcome. We used these results for our interpretation. In addition, factors such as the total number of trials in a network, the number of trials with more than two comparison arms, heterogeneity (i.e., clinical, methodological, and statistical variability within direct and indirect comparisons), inconsistency (i.e., a discrepancy between direct and indirect comparisons), and bias may influence effect estimates obtained from network meta-analyses [20]. The inconsistency was assessed by using Bayesian P values by the node splitting analysis of rjags the gemtc packages. The value of I 2 ≥ 50 % would be considered substantial heterogeneity [21, 22].
Results
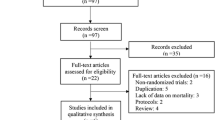
We identified 39 studies through the re-analysis of the three systematic reviews [5, 6, 12], 431 references through the PubMed search, and 539 references through the Cochrane search. Fifty-seven studies were considered potentially eligible (Supplementary appendix 1). We excluded 22 studies after the full-text screening (4 conference abstracts, 7 non-English studies, and 11 studies that did not fulfill the eligibility criteria) (Supplementary appendix 2). Accordingly, the current network meta-analysis included 18,098 patients from 35 studies [2, 4, 9, 23–54].
As Table 1 shows, there was a large heterogeneity in the characteristics of the included studies. The mean age of the patients varied from 38.5-years-old to 74.9-years-old. The proportion of patients with diabetes varied from 1.3 to 100 %. Table 1 also provides the mean glucose levels during the study period for each group of studies. The risk of bias for each study is shown in the Supplementary appendix 3.
The network of eligible comparisons for the meta-analysis is shown in Supplementary appendix 4. The Bayesian P value and I 2 value in each comparison are shown in Supplementary appendix 5. The forest plot for hospital mortality is shown in Fig. 1a. Although the target glucose concentration of 144–180 mg/dL was associated with a significantly lower hospital mortality compared with the target concentration of >180 mg/dL in a direct comparison (OR 0.1; 95 % CrI 0.01–0.71), there were no significant differences among all comparisons of the network meta-analysis (<110 vs. 110–144 mg/dL, <110 vs. 144–180 mg/dL, <110 vs. >180 mg/dL, 110–144 vs. 144–180 mg/dL, 110–144 vs. >180 mg/dL, and 144–180 vs. >180 mg/dL) (Fig. 1a; Table 2). The rank probabilities for the four blood glucose targets are shown in Fig. 1b. This analysis suggested that target blood glucose levels of 110–144 and 144–180 mg/dL had a 48.4 and 43.6 % probability, respectively, of being the best treatment in view of reducing hospital mortality. In contrast, <110 and >180 mg/dL had a 7.0 and 68.4 % probability, respectively, of being the worst treatment in view of reducing hospital mortality. The Bayesian P value between 144–180 and >180 mg/dL was 0.034 (Supplementary appendix 5).
a Forest plot for hospital mortality. b Rank probabilities among all blood glucose targets for hospital mortality based on the network meta-analysis. a The upper row indicates the pairwise meta-analysis result (blue) and the lower row indicates the network meta-analysis result (black). OR odds ratio, 95 % CrI 95 % credible intervals, no direct study there were no studies directly comparing these blood glucose targets, number of patients the number of patients per target category
The forest plot for the risk of sepsis or bloodstream infection is shown in Fig. 2a. There were no significant differences among all comparisons of the network meta-analysis (Fig. 2a; Table 2). The rank probabilities indicated that target blood glucose levels of 110–144 and 144–180 mg/dL had a 61.6 and 31.7 % probability, respectively, of being the best treatment in view of lowering the risk of sepsis or bloodstream infection. In contrast, a target blood glucose concentration of >180 mg/dL had an 83.3 % probability of being the worst treatment in view of reducing the risk of infection (Fig. 2b).
a Forest plot for sepsis or bloodstream infection. b Rank probabilities among all blood glucose targets for sepsis or bloodstream infection based on the network meta-analysis. a The upper row indicates the pairwise meta-analysis result (blue) and the lower row indicates the network meta-analysis result (black). OR odds ratio, 95 % CrI 95 % credible intervals, no direct study there were no studies directly comparing these blood glucose targets, number of patients the number of patients per target category
The forest plot for the risk of hypoglycemia is shown in Fig. 3a. Target blood glucose levels of <110 and 110–144 mg/dL were associated with a significantly higher risk of hypoglycemia compared with target blood glucose levels of 144–180 and >180 mg/dL. There were no significant differences in the risk of hypoglycemia between the 144–180 and >180 mg/dL target blood glucose levels (Fig. 3a; Table 2). The rank probability indicated that target blood glucose levels of >180 mg/dL had a 91.9 % probability of being the best treatment in view of lowering the risk of hypoglycemia. Furthermore, target blood glucose levels of <110 and 110–144 mg/dL had a 36.9 and 63.1 % probability, respectively, of being the worst treatment in view of reducing the risk of hypoglycemia (Fig. 3b).
a Forest plot for hypoglycemia. b Rank probabilities among all blood glucose targets for the risk of hypoglycemia based on the network meta-analysis. a The upper row indicates the pairwise meta-analysis result (blue) and the lower row indicates the network meta-analysis result (black). OR odds ratio, 95 % CrI 95 % credible intervals, no direct study there were no studies directly comparing these blood glucose targets, number of patients the number of patients per target category
Subgroup analyses
ICU setting
There were no significant differences in the risk of hospital mortality and infection for all comparisons in the network meta-analysis performed for studies in both surgical and medical ICUs (Table 2). In terms of the risk of hypoglycemia, target blood glucose levels of <110 mg/dL were associated with a significantly higher risk compared with target blood glucose levels of 144–180 and >180 mg/dL, irrespective of the ICU setting. In addition, a target level of 110–144 mg/dL was associated with a significantly higher risk of hypoglycemia compared with target levels of 144–180 and >180 mg/dL in medical ICUs.
Proportion of diabetes patients
We could not perform a subgroup network meta-analysis for the >50 % diabetic patients subgroup, as there were only six such studies. In the <50 % diabetic patients subgroup, there were no significant differences in the risk of hospital mortality and infection in all comparisons (Table 2). Target blood glucose levels of <110 and 110–144 mg/dL were associated with significantly higher risks of hypoglycemia compared with 144–180 and >180 mg/dL.
Observed mortality >20 %
We performed a subgroup network meta-analysis for studies wherein the observed mortality was >20 %. There were no significant differences in the risk of hospital mortality and infection in all comparisons (Table 2). Target blood glucose levels of <110 and 110–144 mg/dL were associated with significantly higher risks of hypoglycemia compared with 144–180 and >180 mg/dL.
Actually achieved mean glycemic levels
We performed an additional network meta-analysis based on the actual mean glycemic levels. Although the actually achieved glucose concentration of <110 mg/dL was associated with a significantly higher hospital mortality compared with the concentration of 110–144 mg/dL in a direct comparison (OR 4.17; 95 % CrI 1.04–17.86), there were no significant differences among all comparisons of the network meta-analysis (Table 2; Supplementary appendix 6). Actually achieved blood glucose levels of <110 and 110–144 mg/dL were associated with significantly higher risks of hypoglycemia compared with 144–180 mg/dL (OR 7.4; 95 % CrI 2.3–25, OR 6.3; 95 % CrI 3.9–10, respectively).
Summary of findings regarding comparison between target blood glucose levels of 110–144 and 144–180 mg/dL
Although several guidelines do not recommend target blood glucose levels of both <110 and >180 mg/dL, it remains ambiguous whether target blood glucose levels of 110–144 or 144–180 mg/dL are superior because there were few studies which compared these two ranges directly [11, 55]. Therefore, we summarized the findings from the network meta-analysis for each outcome and subgroup for target glucose concentrations of 110–144 and 144–180 mg/dL in Supplementary appendix 7. There was no significant difference in the risk of death and infection between the two target concentrations. However, the risk of hypoglycemia was significantly higher in the 110–144 mg/dL group compared with the 144–180 mg/dL group, except for the surgical ICU subgroup.
Discussion
We conducted the current network meta-analysis to compare four different targets of acute glycemic levels in critically ill patients. In critically ill patients, we found that there were no significant differences in the risks of hospital mortality and infection for all comparisons of the four target glucose levels. However, target blood glucose levels of <110 and 110–144 mg/dL were associated with a four to ninefold increase in the risk of hypoglycemia compared with the targets of 144–180 and >180 mg/dL. There was no significant difference in the risk of hypoglycemia between the 144–180 and >180 mg/dL target glucose levels. Although this is a hypothesis-generating study, the current study is the first network meta-analysis to compare different target glucose levels for acute glycemic control. Therefore, our findings may be novel and, thus, require a detailed discussion.
Comparisons with prior studies
The Surviving Sepsis Campaign guidelines recommend that target blood glucose concentrations should be <180 mg/dL, rather than <110 mg/dL, on the basis of the results of the NICE-SUGAR study [10]. Although the NICE-SUGAR study reported that IIT increased mortality and the risk of hypoglycemia compared with targeting blood glucose levels between 144 and 180 mg/dL, there were few direct comparisons of 144–180 with 110–144 or >180 mg/dL as the target glycemic range. Therefore, our network meta-analysis provided the first evidence for comparisons between these targets.
Interpretation
Lowering blood glucose levels to <110 mg/dL was reported to reduce the mortality in a single-center study [2]; however, this finding was not reproduced in multicenter studies [9]. Our network meta-analysis also revealed that targeting blood glucose levels to <110 mg/dL was associated with no reduction in the risk of mortality and infection and, therefore, it may not be considered the best treatment for reducing the risk of mortality and infection. Furthermore, targeting blood glucose levels to <110 mg/dL was associated with a four to eightfold higher risk of hypoglycemia. This finding is consistent with a previous meta-analysis [5], and with the recent recommendation reported in international guidelines [10, 11].
Our analysis included 23 RCTs with >180 mg/dL as the upper limit of the blood glucose target. Although all direct and indirect comparisons did not show statistically significant differences in terms of hospital mortality and the incidence of infection, our rank probability suggested that a target glucose level of >180 mg/dL was the worst treatment in view of these two outcomes. This finding is consistent with the recent recommendation reported in international guidelines [10, 11]. However, we should note that only two RCTs achieved actual glycemic levels of >180 mg/dL. Therefore, our findings with regard to the >180 mg/dL target might not be truly reflective of the impact of this glycemic target. Furthermore, we should note that, as we categorized all studies in which the upper limit of their target was >180 as “>180 mg/dL”, the risk or benefit of a more liberal target (e.g., <200 mg/dL) could not be assessed in our analysis and, thus, requires further investigation.
Although the current guidelines do not recommend target blood glucose levels of both <110 and >180 mg/dL, it remains ambiguous whether target blood glucose levels of 110–144 or 144–180 mg/dL are superior [11, 55]. In our network meta-analysis, there were no significant differences in the reduction of hospital mortality and infection between the target blood glucose levels of 110–144 and 144–180 mg/dL, which is summarized in Supplementary appendix 7. Furthermore, the probability of being the best treatment for 110–144 and 144–180 mg/dL was similar, especially in view of reducing hospital mortality. Therefore, we were unable to deduce whether target blood glucose levels of 110–144 or 144–180 mg/dL were superior, as the order of 110–144 and 144–180 mg/dL in the rank probability was highly imprecise in terms of reducing hospital mortality and the incidence of infection. However, our results also showed that target blood glucose levels of 110–144 mg/dL were associated with a greater risk of hypoglycemia compared with targets of 144–180 mg/dL in critically ill patients, medical ICUs, the non-diabetic cohort, and overall mortality >20 % cohort.
Limitations
Although this is the first network meta-analysis on this issue and to study heterogeneous populations of critically ill patients, there are several limitations of this study. First, the majority of the included studies were retrieved from three recent systematic reviews [5, 6, 12]. However, we did also search the PubMed and Cochrane databases for additional studies that had been published after the meta-analysis by Wiener et al. (which used the same search terms) [5].
Second, we limited the included studies to full-text English publications, as we assumed that conference abstracts and non-English reports would not provide sufficient methodological or outcome data. Nevertheless, we carefully searched for full-text publications that were based on conference abstracts, and considered these studies.
Third, we performed a network meta-analysis in several subgroups to identify the impact of heterogeneity among the included RCTs. Notably, the proportion of surgical patients influenced the result in terms of the risk of hypoglycemia. Therefore, it should be noted that, as well as the different glycemic targets, there were other differences among the studies, including the glycemic control methodology, the glucose monitoring tools used, the feeding regimen, and the duration of intervention. This heterogeneity could also have influenced our results. Therefore, future studies are required to refute or confirm our results.
Fourth, our study was conducted using predefined four categories of blood glucose targets. Thus, we could not assess the impact of other blood glucose thresholds, such as 130, 160, 200, and 220 mg/dL. Accordingly, future studies are necessary to assess different blood glucose bands.
Fifth, the results of our network meta-analysis had some heterogeneity and inconsistency: in particular, the comparison between 144–180 and >180 mg/dL showed inconsistency in terms of the hospital mortality (Bayesian P value = 0.034). In addition, the comparison between <110 and 144–180 mg/dL in terms of the risk of hypoglycemia analysis showed heterogeneity (I 2 = 85.95). Therefore, it is necessary to consider this point in the interpretation of our results.
Finally, we used target blood glucose levels according to the methodology of previous systematic reviews [5, 6, 12]. However, this methodology did not consider the quality of blood glucose control during the study period. Therefore, we performed an additional network meta-analysis based on the actual mean glycemic levels, as we hypothesized that our results might be influenced by how accurately blood glucose control was achieved. Although these results were the same as those based on the target blood glucose level; the 95 % CrI in the comparison of >180 mg/dL was large because there were only two studies in which the actual blood glucose level of >180 mg/dL was reached. In this regards, future studies should consider the influence of the method of blood glucose level control when designing their own insulin protocol.
Conclusions
The current network meta-analysis revealed that there was no significant difference in the risk of mortality and infection among the four blood glucose ranges in critically ill patients. Our results also indicated that targeting blood glucose levels to <110 and 110–144 mg/dL was associated with a higher risk of hypoglycemia compared with the blood glucose targets of 144–180 and >180 mg/dL. These findings may shed light on the optimal blood glucose target in critically ill patients. To refute or confirm our findings, further studies should be conducted.
References
Egi M, Finfer S, Bellomo R (2011) Glycemic control in the ICU. Chest 140:212–220. doi:10.1378/chest.10-1478
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345:1359–1367
Krinsley JS (2004) Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc 79:992–1000
Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R (2006) Intensive insulin therapy in the medical ICU. N Engl J Med 354:449–461
Wiener RS, Wiener DC, Larson RJ (2008) Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA 300:933–944. doi:10.1001/jama.300.8.933
Friedrich JO, Chant C, Adhikari NK (2010) Does intensive insulin therapy really reduce mortality in critically ill surgical patients? A reanalysis of meta-analytic data. Crit Care 14:324. doi:10.1186/cc9240
Hermans G, Van den Berghe G (2015) Clinical review: intensive care unit acquired weakness. Crit Care 19:274. doi:10.1186/s13054-015-0993-7
NICE-SUGAR Study Investigators, Finfer S, Liu B, Chittock DR et al (2012) Hypoglycemia and risk of death in critically ill patients. N Engl J Med 367:1108–1118
NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY et al (2009) Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360:1283–1297. doi:10.1056/NEJMoa0810625
Dellinger RP, Levy MM, Rhodes A et al (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637. doi:10.1097/CCM.0b013e31827e83af
Jacobi J, Bircher N, Krinsley J et al (2012) Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 40:3251–3276. doi:10.1097/CCM.0b013e3182653269
Song F, Zhong LJ, Han L, Xie GH, Xiao C, Zhao B, Hu YQ, Wang SY, Qin CJ, Zhang Y, Lai DM, Cui P, Fang XM (2014) Intensive insulin therapy for septic patients: a meta-analysis of randomized controlled trials. Biomed Res Int 2014:698265. doi:10.1155/2014/698265
Skapinakis P, Caldwell DM, Hollingworth W, Bryden P, Fineberg NA, Salkovskis P, Welton NJ, Baxter H, Kessler D, Churchill R, Lewis G (2016) Pharmacological and psychotherapeutic interventions for management of obsessive–compulsive disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatry. doi:10.1016/S2215-0366(16)30069-4
Singh JA, Hossain A, Tanjong Ghogomu E, Kotb A, Christensen R, Mudano AS, Maxwell LJ, Shah NP, Tugwell P, Wells G (2016) Biologics or tofacitinib for rheumatoid arthritis in incomplete responders to methotrexate or other traditional disease-modifying anti-rheumatic drugs: a systematic review and network meta-analysis. Cochrane Database Syst Rev 13:CD012183. doi:10.1002/14651858.CD012183
Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G (2013) Graphical tools for network meta-analysis in STATA. PLoS One 8:e76654. doi:10.1371/journal.pone.0076654.eCollection
Higgins JPT, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org. Accessed March 2011
Plummer M (2016) rjags: Bayesian graphical models using MCMC. R package version 4–6. http://CRAN.R-project.org/package=rjags
van Valkenhoef G, Kuiper J (2016) gemtc: network meta-analysis using Bayesian methods. R package version 0.8. http://CRAN.R-project.org/package=gemtc
Shyangdan DS, Uthman OA, Waugh N (2016) SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. BMJ Open 6:e009417. doi:10.1136/bmjopen-2015-009417
Li T, Puhan MA, Vedula SS, Singh S, Dickersin K, Ad Hoc Network Meta-analysis Methods Meeting Working Group (2011) Network meta-analysis-highly attractive but more methodological research is needed. BMC Med 9:79. doi:10.1186/1741-7015-9-79
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Yin S, Zhang D, Du H, Du H, Yin Z, Qiu Y (2015) Is there any difference in survivorship of total hip arthroplasty with different bearing surfaces? A systematic review and network meta-analysis. Int J Clin Exp Med 8:21871–21885
Grey NJ, Perdrizet GA (2004) Reduction of nosocomial infections in the surgical intensive-care unit by strict glycemic control. Endocr Pract 10(Suppl 2):46–52
Bilotta F, Spinelli A, Giovannini F, Doronzio A, Delfini R, Rosa G (2007) The effect of intensive insulin therapy on infection rate, vasospasm, neurologic outcome, and mortality in neurointensive care unit after intracranial aneurysm clipping in patients with acute subarachnoid hemorrhage: a randomized prospective pilot trial. J Neurosurg Anesthesiol 19:156–160
Bilotta F, Caramia R, Cernak I, Paoloni FP, Doronzio A, Cuzzone V, Santoro A, Rosa G (2008) Intensive insulin therapy after severe traumatic brain injury: a randomized clinical trial. Neurocrit Care 9:159–166. doi:10.1007/s12028-008-9084-9
Bland DK, Fankhanel Y, Langford E, Lee M, Lee SW, Maloney C, Rogers M, Zimmerman G (2005) Intensive versus modified conventional control of blood glucose level in medical intensive care patients: a pilot study. Am J Crit Care 14:370–376
Walters MR, Weir CJ, Lees KR (2006) A randomised, controlled pilot study to investigate the potential benefit of intervention with insulin in hyperglycaemic acute ischaemic stroke patients. Cerebrovasc Dis 22:116–122
Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, Kissela BM, Williams LS (2008) Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke 39:384–389
Mitchell I, Knight E, Gissane J, Tamhane R, Kolli R, Leditschke IA, Bellomo R, Finfer S (2006) Australian and New Zealand Intensive Care Society Clinical Trials Group. A phase II randomised controlled trial of intensive insulin therapy in general intensive care patients. Crit Care Resusc 8:289–293
Brunkhorst FM, Engel C, Bloos F et al (2008) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 358:125–139. doi:10.1056/NEJMoa070716
Iapichino G, Albicini M, Umbrello M, Sacconi F, Fermo I, Pavlovich R, Paroni R, Bellani G, Mistraletti G, Cugno M, Pesenti A, Gattinoni L (2008) Tight glycemic control does not affect asymmetric-dimethylarginine in septic patients. Intensive Care Med 34:1843–1850. doi:10.1007/s00134-008-1158-9
De La Rosa Gdel C, Donado JH, Restrepo AH et al (2008) Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Crit Care 12:R120. doi:10.1186/cc7017
Arabi YM, Dabbagh OC, Tamim HM, Al-Shimemeri AA, Memish ZA, Haddad SH, Syed SJ, Giridhar HR, Rishu AH, Al-Daker MO, Kahoul SH, Britts RJ, Sakkijha MH (2008) Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med 36:3190–3197. doi:10.1097/CCM.0b013e31818f21aa
Chan RP, Galas FR, Hajjar LA, Bello CN, Piccioni MA, Auler JO Jr (2009) Intensive perioperative glucose control does not improve outcomes of patients submitted to open-heart surgery: a randomized controlled trial. Clinics (Sao Paulo) 64:51–60
Farah R, Samokhvalov A, Zviebel F, Makhoul N (2007) Insulin therapy of hyperglycemia in intensive care. Isr Med Assoc J 9:140–142
Henderson WR, Dhingra V, Chittock D, Foster D, Hebert P, Cook D, Heyland D, Dodek P, Griesdale D, Schulzer M, Ronco JJ, Canadian Critical Trials Group (2009) The efficacy and safety of glucose control algorithms in intensive care: a pilot study of the Survival Using Glucose Algorithm Regulation (SUGAR) trial. Pol Arch Med Wewn 119:439–446
COIITSS Study Investigators, Annane D, Cariou A, Maxime V et al (2010) Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA 303:341–348. doi:10.1001/jama.2010.2
Savioli M, Cugno M, Polli F, Taccone P, Bellani G, Spanu P, Pesenti A, Iapichino G, Gattinoni L (2009) Tight glycemic control may favor fibrinolysis in patients with sepsis. Crit Care Med 37:424–431. doi:10.1097/CCM.0b013e31819542da
Cappi SB, Noritomi DT, Velasco IT, Curi R, Loureiro TC, Soriano FG (2012) Dyslipidemia: a prospective controlled randomized trial of intensive glycemic control in sepsis. Intensive Care Med 38:634–641. doi:10.1007/s00134-011-2458-z
Preiser JC, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chioléro R (2009) A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 35:1738–1748. doi:10.1007/s00134-009-1585-2
McMullin J, Brozek J, McDonald E, Clarke F, Jaeschke R, Heels-Ansdell D, Leppert R, Foss A, Cook D (2007) Lowering of glucose in critical care: a randomized pilot trial. J Crit Care 22:112–118 (discussion 118–119)
Oksanen T, Skrifvars MB, Varpula T, Kuitunen A, Pettilä V, Nurmi J, Castrén M (2007) Strict versus moderate glucose control after resuscitation from ventricular fibrillation. Intensive Care Med 33:2093–2100
de Azevedo JR, de Araujo LO, da Silva WS, de Azevedo RP (2010) A carbohydrate-restrictive strategy is safer and as efficient as intensive insulin therapy in critically ill patients. J Crit Care 25:84–89. doi:10.1016/j.jcrc.2008.10.011
Mackenzie IM, Ercole A, Ingle S, Palmer CR (2008) Glycaemic control and outcome in general intensive care: the East Anglian GLYCOGENIC study. Br J Intensive Care 18:121–126
Davies RR, Newton RW, McNeill GP, Fisher BM, Kesson CM, Pearson D (1991) Metabolic control in diabetic subjects following myocardial infarction: difficulties in improving blood glucose levels by intravenous insulin infusion. Scott Med J 36:74–76
Arabi YM, Tamim HM, Dhar GS, Al-Dawood A, Al-Sultan M, Sakkijha MH, Kahoul SH, Brits R (2011) Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr 93:569–577. doi:10.3945/ajcn.110.005074
Bilotta F, Caramia R, Paoloni FP, Delfini R, Rosa G (2009) Safety and efficacy of intensive insulin therapy in critical neurosurgical patients. Anesthesiology 110:611–619. doi:10.1097/ALN.0b013e318198004b
Kalfon P, Giraudeau B, Ichai C, Guerrini A, Brechot N, Cinotti R, Dequin PF, Riu-Poulenc B, Montravers P, Annane D, Dupont H, Sorine M, Riou B, CGAO-REA Study Group (2014) Tight computerized versus conventional glucose control in the ICU: a randomized controlled trial. Intensive Care Med 40:171–181. doi:10.1007/s00134-013-3189-0
Hsu CW, Sun SF, Lin SL, Huang HH, Wong KF (2012) Moderate glucose control results in less negative nitrogen balances in medical intensive care unit patients: a randomized, controlled study. Crit Care 16:R56. doi:10.1186/cc11299
Coester A, Neumann CR, Schmidt MI (2010) Intensive insulin therapy in severe traumatic brain injury: a randomized trial. J Trauma 68:904–911. doi:10.1097/TA.0b013e3181c9afc2
Mahmoodpoor A, Ali-Asgharzadeh A, Parish M, Amir-Aslanzadeh Z, Abedini A (2011) A comparative study of efficacy of intensive insulin therapy versus conventional method on mortality and morbidity of critically ill patients. Pak J Med Sci 27:496–499
Green DM, O’Phelan KH, Bassin SL, Chang CW, Stern TS, Asai SM (2010) Intensive versus conventional insulin therapy in critically ill neurologic patients. Neurocrit Care 13:299–306. doi:10.1007/s12028-010-9417-3
Giakoumidakis K, Eltheni R, Patelarou E, Theologou S, Patris V, Michopanou N, Mikropoulos T, Brokalaki H (2013) Effects of intensive glycemic control on outcomes of cardiac surgery. Heart Lung 42:146–151. doi:10.1016/j.hrtlng.2012.12.007
Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Tokumaru T, Iiyama T, Sugimoto T, Kobayashi M, Yokoyama M, Hanazaki K (2014) Intensive versus intermediate glucose control in surgical intensive care unit patients. Diabetes Care 37:1516–1524. doi:10.2337/dc13-1771
American Diabetes Association (2012) Standards of medical care in diabetes–2012. Diabetes Care 35(Suppl 1):S11–S63. doi:10.2337/dc12-s011
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Take-home message: This network meta-analysis suggested (a) that there was no significant difference in the risk of mortality and infection among the four blood glucose ranges evaluated and (b) that targets of <110 and 110–144 mg/dL were associated with a four to ninefold increase in the risk of hypoglycemia compared with 144–180 and >180 mg/dL. There were no significant differences in the risk of hypoglycemia between the 144–180 and >180 mg/dL target blood glucose levels.
Electronic supplementary material
Below is the link to the electronic supplementary material.
134_2016_4558_MOESM3_ESM.tiff
Supplementary material 3 Risk of bias in the included randomized controlled trials. The Cochrane Collaboration tool was used to assess the risk of bias. The authors’ judgments were used to assign the risk of bias for each included study. +, low risk of bias; −, high risk of bias; empty space, unclear risk of bias. Eight studies with a high risk of “other bias” did not provide financial support and/or conflict of interest statements (TIFF 2456 kb)
134_2016_4558_MOESM4_ESM.tiff
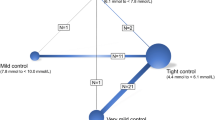
Supplementary material 4 The network of all eligible comparisons for the meta-analysis. a In-hospital mortality, b incidences of sepsis or bloodstream infection and c incidences of hypoglycemia. Each node (circle) corresponds to a target blood glucose range. Each line represents a direct comparison between the target ranges, and the width of the line is proportional to the number of studies that compared each target pair. N number of studies (TIFF 3199 kb)
134_2016_4558_MOESM6_ESM.tif
Supplementary material 6 Forest plot for network meta-analysis based on the actual achieved mean glycemic levels. The upper row indicates the pairwise meta-analysis result (blue) and the lower row indicates the network meta-analysis result (black) (TIFF 1541 kb)
134_2016_4558_MOESM7_ESM.tiff
Supplementary material 7 Results of the network analysis comparing the 110–144 and 144–180 mg/dL target blood glucose levels. The circles correspond to the odds ratio and the black horizontal lines correspond to the 95 % credible intervals (TIFF 984 kb)
Rights and permissions
About this article
Cite this article
Yatabe, T., Inoue, S., Sakaguchi, M. et al. The optimal target for acute glycemic control in critically ill patients: a network meta-analysis. Intensive Care Med 43, 16–28 (2017). https://doi.org/10.1007/s00134-016-4558-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4558-2