Abstract
Introduction
To investigate the risks and possible benefits of routine versus intensive insulin therapy, assessed by the frequency of hypoglycemic events defined as a glucose concentration less than 80 mg/dl (<4.44 mmol/l) in patients admitted to the intensive care unit (ICU) after severe traumatic brain injury (TBI).
Methods and Results
Ninety-seven patients admitted after severe TBI, were enrolled and randomly assigned to two groups of target glycemia. Insulin was infused at conventional rates when blood glucose levels exceeded 220 mg/dl (12.22 mmol/l) or at intensive rates, to maintain glycemia at 80–120 mg/dl (4.44–6.66 mmol/l). The following primary and outcome variables were measured during follow-up: hypoglycemic episodes, duration of ICU stay, infection rate, and 6-month mortality and neurologic outcome measured using the Glasgow Outcome Scale (GOS). Episodes of hypoglycemia (defined as blood glucose <80 mg/dl or 4.44 mmol/l) were significantly higher in patients receiving intensive insulin therapy: median (min–max) conventional insulin therapy 7 (range 0–11) vs. intensive insulin therapy 15 (range 6–33); P<0.0001. Duration of ICU stay was shorter in patients receiving intensive insulin therapy (7.3 vs. 10.0 days; P < 0.05); while infection rates during ICU stay (25.0% vs. 38.8%, P = 0.15), and GOS scores and mortality at 6 months were similar in the two groups.
Conclusions
Intensive insulin therapy significantly increases the risk of hypoglycemic episodes. Even though patients receiving intensive insulin therapy have shorter ICU stays and infection rates similar to those receiving conventional insulin therapy, both groups have similar follow-up mortality and neurologic outcome. Hence if intensive insulin therapy is to be used, great effort must be taken to avoid hypoglycemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mortality and morbidity rates are significantly lower in patients treated in a postoperative surgical intensive care unit (ICU) with intensive insulin therapy, titrated to maintain strict blood glucose control (blood glucose levels at 80–110 mg/dl or 4.44–6.11 mmol/l), than in patients receiving conventional therapy [1, 2]. Strict glycemic control with low target ranges invariably carries a risk of inadvertent hypoglycemic episodes. Recently, one study has documented an association between incidental hypoglycemia and an increase in short-term complications or mortality [3]. Several studies have nevertheless reported a potentially higher incidence of hypoglycemia in patients under strict glycemic control [2, 4–8].
Hyperglycemia is frequent during acute brain injury such as ischemic stroke, intracerebral hemorrhage, or head trauma and is associated with increased morbidity and mortality [9–13]. Traumatic brain injury (TBI) also leads to a profound increase in glucose utilization (hyperglycolysis) that can persist for up to one week and alters the ability to use ketone bodies as energetic substrates [14]. Little is known, however, about the optimal glycolytic rate and the influence of intensive insulin therapy on the TBI-induced changes in glucose metabolism [15, 16]. In TBI patients receiving intensive insulin therapy, brain microdialysis monitoring showed lower glucose and increased glutamate concentrations, and an elevated lactate/pyruvate ratio when the blood glucose level was less than or equal to 80 mg/dl (4.44 mmol/l) [17, 18]. An important question is whether episodes of hypoglycemia contribute to worsened long-term neurologic outcome after TBI. More information is also needed to show whether the intensive insulin therapy given to TBI patients in the ICU increases the risk of hypoglycemia.
Our aim in this prospective randomized, single-center study was to investigate the risks and possible benefits of intensive insulin therapy and strict glucose control (target blood glucose 80–120 mg/dl i.e., 4.44–6.66 mmol/l), on the long-term neurologic outcome after TBI and to find out whether intensive insulin therapy increases the risk of hypoglycemia defined as blood glucose levels less than or equal to 80 mg/dl (4.44 mmol/l) in patients admitted to a neurosurgical ICU after severe TBI.
Materials and Methods
The protocol and consent forms were approved by the institutional review board for human research, and written informed consent was obtained. Patients eligible for enrollment were patients who had TBI and Glasgow coma scale (GCS) ≤8 requiring placement of an intracranial pressure (ICP) monitor or evidence of traumatic mass lesion on computed tomographic scan. Patients eligible for this study were expected to stay in the ICU for at least 3 days. Patients deemed to be approaching death based on the following criteria were excluded: (1) GCS of 3 without sedation; (2) systolic BP < 50 or HR < 40 despite vasoactive drugs; (3) oxygen saturation of less than 90% despite FIO2 of 100%.
At baseline, data on demographic and clinical characteristics were obtained, and scored according to the simplified acute physiologic score (SAPS II), with higher values indicating more severe illness [19]. Surgical procedures such as ICP monitoring, decompressive craniectomy, or drainage of intraparenchymal, extra- or sub-dural hematoma were recorded for each patient (Table 1). Of the 106 subjects originally screened 97 consecutive patients were randomly assigned (in computer-derived order) to one of two groups to receive conventional insulin therapy: continuous insulin infusion adjusted to maintain blood glucose below 220 mg/dl (12.22 mmol/l); or intensive insulin therapy, insulin infusion adjusted according to a dynamic scale protocol to maintain blood glucose levels in a range between 80 and 120 mg/dl (4.44 and 6.66 mmol/l). Treatment was assigned by the use of sealed envelopes and balanced in permuted blocks of 10.
Study Protocol
Blood glucose levels were measured at admission to the ICU and at least every 4 h thereafter during the 14 days of the study. Hypoglycemic events were recorded. If upon the patient’s arrival in the ICU, the blood glucose concentration was out of the target range for their assigned group, insulin therapy began and continued throughout the study. In both treatment groups, insulin (50 IU of Actrapid HM, Novo Nordisk, Copenhagen, Denmark) was diluted in 50 ml of 0.9% sodium chloride and infused intravenously with a pump (Perfusor, B-Braun, Melsungen, Germany). These procedures resemble the protocol described by Van den Berghe and coll [1, 2] and a more detailed subsequently released protocol [20, 21] (Appendix 1). In the intensive treatment arm for glucose values ranging from 121 to 130 mg/dl we began with ½ IU/h of insulin. When patients were transferred to the diagnostic suite insulin infusion was discontinued. To minimize sample bias, whole-blood glucose concentration was measured in the ICU on undiluted arterial blood (i-Stat, Abbott corporation, NJ, USA) sampled every 4 h. Additional measurements were obtained whenever the last reading showed a steep rise or fall in blood glucose levels or the nursing staff considered a second measurement necessary. For quality control, the hospital central laboratory checked 10% of all blood glucose measurements on admission to the ICU and daily during the study period. Before the study began, the ICU nurses received a 3-month, run in, training course designed to improve normogram compliance and insulin therapy management. During the study, the nurse:patients ratio in the ICU was 1:2.
Nutrition Protocol
During the first 24 h, all patients received an intravenous infusion of 0.9% sodium chloride solution at a rate of 1 ml/kg/h. Thereafter, parenteral or enteral nutrition or both were initiated according to a standardized schedule: 20–30 kcal/kg of body weight per 24 h of non-protein nutrition and a balanced composition including 0.13–0.26 g of nitrogen per kg per 24 h and 20–40% of non-protein calories in the form of lipids [22]. The patients’ dietician, blinded to the assigned group, used the same nutritional protocol for all patients. The study protocol ended for each patient at discharge from the ICU or on postoperative day 14. At the end of the study, all patients received standard glycemia care to maintain the blood glucose level below 200 mg/dl (11.10 mmol/l).
Treatment of Cerebral Edema
During their stay in the ICU patients with signs of severe cerebral edema on neuroimaging received glucocorticoids in moderate-to-low doses (betamethasone 8 mg once per day for 2–3 days followed by 4 mg once per day for 2–3 days) at the surgeons’ discretion.
Treatment of Hypoglycemia
All patients with blood glucose levels <80 mg/dl (<4.44 mmol/l) received glucose in a bolus (0.1 g/kg at a concentration of 33%) with the insulin infusion stopped. The glucose concentration was then measured within 30 min.
Outcome Measures
The primary outcome measure was rate of hypoglycemia (blood glucose <80 mg/dl or 4.44 mmol/l). Secondary measures were: the number of patients who develop one or more than one episode of hypoglycemia, the incidence of infections, duration of ICU stay, neurologic outcome, and death at 6 months’ follow-up [23, 24]. Recorded infections (wound infections, pneumonia, urinary infections, bacteremia) were defined according to the National Nosocomial Infection Surveillance System [25, 26]. The onset and the type of a new infection in the ICU were assessed by one of two infective disease specialists who were blinded to treatment allocation. Duration of the ICU stay was measured as number of days after arrival in the ICU. To minimize the possibility of bias in measuring the length of the ICU stay, generally caused by delayed transfers of patients from the ICU to a regular ward due to the unavailability of regular ward beds, patients were considered to be discharged from the ICU when they no longer needed vital organ support and were receiving at least two-thirds of their caloric intake by the normal enteral route. Physicians on the general wards to whom the patients were transferred from the ICU had no access to the results of blood glucose testing and were unaware of the study treatment assignment. Neurologic outcome at 6 months’ follow-up was evaluated according to the Glasgow Outcome Scale (GOS) (see Appendix 2) by an expert neurologist blind to the treatment assignment
Statistical Analysis
Data were analyzed according to the intention-to-treat approach. The demographic and clinical characteristics variables, outcome variables, and GOS scores were compared by means of chi-square test or Fisher’s exact test, and by means of Student’s t-test or Kruskal–Wallis test, as appropriate. The effect of the type of insulin therapy on the time to discharge from the ICU was estimated by means of the cumulative incidence curve of discharge from the ICU, considering as competing risk the death during hospitalization, and differences were tested using the Gray test. Unless otherwise indicated, the data are presented as means ± SD or medians. P values equal to or less than 0.05 were considered to indicate statistical significance. All analyses were performed using the SAS software (SAS Institute, Cary, NC).
Results
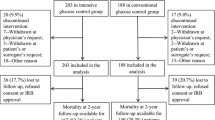
From January 2002 to October 2005, 106 consecutive patients who underwent surgery after TBI and were admitted to the ICU within two to 24 h after surgery were prospectively screened. Of the 106 consecutive patients screened 97 patients (53 men and 44 women, mean age: 53 years, range: 20–74 years) were enrolled and randomly assigned to one of the two treatment groups: 49 received conventional insulin therapy and 48 received intensive insulin therapy. Among the 106 patients screened, 9 patients were excluded, 4 because they were approaching death, 3 because they were enrolled in other studies, and 2 because they had not given informed consent. All 97 patients enrolled in the study remained in the ICU for at least 3 days (Table 2). Preoperative clinical characteristics, including neurologic status and pre-existing diabetes, were similar in the two study groups (Table 1). In all patients, ICP was monitored by a ventricular catheter. This was the sole surgical procedure in 20 patients (40.8%) among the conventional insulin therapy group and in 19 patients (39.6%) among the intensive insulin therapy group. Other surgical procedures included drainage of extradural, subdural, or intraparenchymal hematoma, or decompressive craniotomy. At admission to the ICU, blood glucose values were similar in both groups (Table 1).
Study Feasibility
The number of blood glucose concentration measurements obtained in each single patient is similar in the two study groups: median of 72 (range 58–119) in the conventional insulin therapy group vs. median of 69 (range: 49–139) in the intensive insulin therapy group (P = 0.6803). We have also analyzed the difference in the number of episodes of hypoglycemia per patient (defined as blood glucose levels less than or equal to 80 mg/dl) with the Kruskal–Wallis test, and we found a statistically significant difference between the two study groups: median of 7 (range 0–11) in the conventional insulin therapy group vs. median of 15 (range 6–33) in the intensive insulin therapy group (P < 0.0001) (Table 2).
In the conventional insulin therapy group 48 out of 49 patients (98%) developed one or more episodes of hypoglycemia; in the intensive insulin therapy group 48 out of 48 patients (100%) developed one or more episodes of hypoglycemia. There was no difference in the number of patients who developed one or more episodes of hypoglycemia between the two treatment groups. All the patients who developed one episode of hypoglycemia had at least a second episode. Mean blood glucose concentration was significantly different in the two treatment groups (147 mg/dl in the conventional insulin therapy vs. 92 mg/dl in the intensive insulin therapy, P < 0.0001 with Kruskal–Wallis test). The lowest glycemia value (38 mg/dl) was recorded in a patient receiving intensive insulin therapy. In the overall study sample tested in parallel at the hospital central laboratory, 92% of whole-blood glucose concentrations in undiluted arterial blood measured in the ICU were within 10% of central laboratory measurements, whereas the remaining 8% were within 30% of laboratory measurements. During the study period, few episodes of hypoglycemia were associated with typical signs or symptoms of hypoglycemia, including seizures and hemodynamic instability. Although the current study was not designed to establish a relationship between hypoglycemia and complications or outcome, no evident clinical differences were found in patients in whom hypoglycemia did or did not develop. The infection rate was non-significantly lower in patients receiving intensive insulin therapy (12/48, 25.0% vs. 19/49, 38.8%; P = 0.15) (Table 2). The ICU stay was longer in patients treated with conventional insulin therapy than in patients receiving intensive insulin therapy (7.3 vs. 10.0 days, P < 0.05). At 6 months’ follow-up neurological outcome as scored with GOS and mortality was similar in the two treatment groups (Table 2). We found no statistically significant difference between in-hospital mortality in the two groups (8.2%, four patients in the conventional and 8.3%, four patients in the intensive insulin therapy group). Of the 97 patients, 10 (20.4%) in the conventional insulin therapy group and 9 (18.8%) in the intensive group were considered by the neurologist to be in a persistent vegetative state.
Discussion
In this prospective randomized, single-center study, we show that in patients admitted to a neurosurgical ICU after severe TBI, intensive insulin therapy titrated to maintain strict blood glucose control (blood glucose levels at 80–120 mg/dl or 4.44–6.66 mmol/l) has no significant benefits on the long-term neurologic outcome and increases the risk of hypoglycemia.
Our primary outcome variable, the number of glycemic measurements below the hypoglycemia threshold (<80 mg/dl or <4.44 mmol/l), was significantly higher in the group of patients treated with intensive insulin therapy than in those receiving conventional insulin therapy (median of 7 (range: 0–11) in the conventional insulin therapy group vs. median of 15 (range: 6–33) in the intensive insulin therapy group; P < 0.0001).
We found no difference in the number of patients who had only one hypoglycemic event in the two groups, but there was a significant difference between the total number of hypoglycemic events in the two groups (Table 2). To limit the number of hypoglycemic events we began with a low insulin dose, ½ IU (121–130 mg/dl) and increased the dose stepwise to 1 IU (131–140 mg/dl). As expected, the frequency of hypoglycemia was evenly spread over all the patients. The frequency of hypoglycemia had no association with secondary outcomes (6-month mortality and GOS). Additionally, the ratio between the measurements within target values and those values below the target was significantly higher in patients receiving intensive insulin therapy, although the total number of measurements was higher because hypoglycemia lasted longer in this group than in the other.
We found no relationship between hypoglycemia and worsened outcome, but in neurocritical care patients controversy surrounds the glycemic thresholds that potentially induce hypoglycemic symptoms and worsen central nervous system damage [3–5, 17, 27]. Many of these events can be explained by the numerous variables that alter glucose metabolism such as glycemic control, oxygen arterial tension, mean arterial pressure, and serum hemoglobin concentrations [28]. Indeed, in the non-diabetic patient, the arterialized venous threshold for hypoglycemic symptoms is about 53 ± 2 mg/dl (2.94 ± 0.11 mmol/l), whereas in poorly controlled diabetics it reaches 78 ± 5 mg/dl (4.33 ± 0.28 mmol/l) [29]. Hence we assume that blood glucose levels below 80 mg/dl (4.44 mmol/l) in patients with severe TBI are potentially associated with detrimental effects on the brain. Although direct comparison of these results is difficult due to a lack of unified and well-defined study designs, a recent meta-analysis, combining prevalence data on hypoglycemia from 10 studies, found that hypoglycemia was three times more likely to develop in patients receiving insulin therapy than in those without insulin therapy [27]. Neuronal damage related to hyperglycemia does not develop progressively, but seems to have a threshold at 170 mg/dl (9.44 mmol/l). Experimental studies show this plasma glucose level as critical for ischemia-induced brain damage [30]. Several studies have described the deleterious effects of both hypo- and hyperglycemia in patients with acute brain damage [6, 31, 32]. In patients admitted to the ICU after TBI, to minimize the risk of additional brain damage due to hypoglycemia, we suggest widening the upper values of the target range of glycemia. Monitoring to maintain tight glycemic control with an upper limit of 155 mg/dl, as suggested over 10 years ago by Wass and Lanier and more recently on the basis of a large literature revision [32, 33], would avoid hypoglycemia and maintain plasma glucose below the critical value of 9.44 mmol/l (170 mg/dl). An international consensus study [34] has defined hyperglycemia as a blood glucose level of 126 mg/dl (7 mmol/l). In most of the patients we studied, hypoglycemic episodes were not associated with clinically relevant signs, and were detected only by blood glucose measurement, probably because most of the enrolled patients were either comatose or receiving sedation.
Perhaps owing to our small study sample from a single center we were unable to confirm the previously reported reduced mortality among critically ill patients in the surgical ICU. A future study would need to enroll about 2,000 patients with TBI, no easy task even in a multicenter study, to detect a 10% absolute difference in mortality. In our patient population, an unusually high proportion of our patients (20%) remained in GOS 2; differentiating a persistent vegetative state from severe disability can be a hard task even for an expert examiner. All our patients’ neurological outcomes were assessed by a single expert neurologist.
Our study has several limitations. For example, the lack of blinding for insulin therapy is a possible though unavoidable limitation. Despite the resources necessary to complete this single center trial being relatively limited, a run-in period was designed for the nursing staff to optimize their experience in providing intensive insulin therapy. Since staff turnover might be a problem in clinical studies, our nursing staff’s work was organized to limit turnover (10% new entries per year). New entrants always worked together with more experienced staff. We therefore consider protocol fatigue and poor protocol compliance unlikely or at least similar to these problems in experienced centers with high standards of clinical practice. Second, the extrapolation of the obtained results to the settings of other studies is limited by the single-center study, and although our preliminary results indicate an increased risk of hypoglycemia they leave inconclusive a potential beneficial role of intensive insulin therapy and a neuroprotective role of this treatment in patients with severe TBI if it could be accomplished without increasing the risk of hypoglycemia (broader blood glucose concentration ranges and improved infusion algorithms). Third, some minor differences in the severity of the clinical conditions between the two groups may partially account for the differing length of ICU stay or infection rate, or both, even though we found no clinically or statistically significant differences in the SAPS II score. Hence, we agree that a large, prospective, multi-center controlled clinical study is required to clarify the neuroprotective potential of intensive insulin therapy in patients with acute severe TBI. Another possible limitation is that the ICU setting (including sedation and mechanical ventilation) might mask the clinical signs and symptoms of hypoglycemia. Finally, most of our patients, with similar figures in the two study groups, received corticosteroids. Although the use of corticosteroids in patients hospitalized for brain injury is currently discouraged, because most of our patients were enrolled before the CRASH study [35] was published, they received corticosteroids at low doses (8 mg betamethasone for the first 2–3 days and 4 mg for the next 2–3 days).
Conclusions
Hypoglycemic episodes are more frequent in critically ill patients with TBI receiving intensive insulin therapy than in those who receive conventional insulin therapy. The use of intensive insulin therapy and strict glucose monitoring in these patients may be a useful strategy for preventing abnormalities of oxidative stress and brain glucose metabolism after TBI. Until future studies clarify how intensive insulin therapy affects the clinical outcome after TBI and other acute brain injuries, the practical message for physicians in charge of an ICU is that greater caution is needed to avoid hypoglycemia. Either glucose concentrations need to be monitored more carefully, or slightly “looser” glucose control permitted.
References
Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–67.
Van den Berghe G, Schoonheydt K, Becx P, et al. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64:1348–53.
Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35(10):2262–7.
Turina M, Christ-Crain M, Polk HC Jr. Diabetes and hyperglycemia: strict glycemic control. Crit Care Med. 2006;34(9 Suppl):291–300.
Van den Berghe G, Wilmer A, Milants I, et al. Intensive insulin therapy in mixed medical/surgical intensive care units. Diabetes. 2006;55(11):3151–9.
Yamada K, Milbrandt EB, Moore J. Intensive insulin therapy in the medical ICU – not so sweet? Crit Care. 2007;11(4):311.
Thomas G, Rojas MC, Epstein SK, Balk EM, Liangos O, Jaber BL. Insulin therapy and acute kidney injury in critically ill patients a systematic review. Nephrol Dial Transplant. 2007;22(10):2849–55.
Mechanick JI, Handelsman Y, Bloomgarden ZT. Hypoglycemia in the intensive care unit. Curr Opin Clin Nutr Metab Care. 2007;10(2):193–6.
Pulsinelli WA, Levy DE, Sigsbee B, et al. Increased damage after ischemic stroke in patients with hyperglycemia with or without established diabetes mellitus. Am J Med. 1983;74:540–4.
Scott JF, Robinson GM, French JM, et al. Glucose potassium insulin infusions in the treatment of acute stroke patients with mild to moderate hyperglycemia. Stroke. 1999;30:793–9.
Bhalla A, Tilling K, Kolominsky-Rabas P, et al. Variation in the management of acute physiological parameters after ischemic stroke: a European perspective. Eur J Neurol. 2003;10(1):25–33.
Bilotta F, Spinelli A, Giovannini F, et al. Effects of intensive insulin infusion on infection rates, vasospasm and neurologic outcome in patients with acute subarachnoid hemorrhage admitted to a postoperative neurosurgical intensive care unit after surgical clipping of intracranial aneurysms a prospective randomized trial. J Neurosurg Anesth. 2007;19(3):156–60.
Walia S, Sutcliffe AJ. The relationship between blood glucose, mean arterial pressure and outcome after head injury: an observational study. Injury 2002;33:339–44.
Robertson CS, Goodman JC, Narayan RK, Contant CF, Grossman RG. The effect of glucose administration on carbohydrate metabolism after head injury. J Neurosurg. 1991;74(1):43–50.
Bergsneider MA, Hovda DA, Shalmon E, et al. Cerebral hyperglycolysis following severe human traumatic brain inyury: a positron emission tomography study. J Neurosurg. 1997;86:241–51.
Hamlin GP, Cernak I, Wixey JA, et al. Increased expression of neuronal glucose transporter 3 but not glial glucose transporter 1 following severe diffuse traumatic brain injury in rats. J Neurotrauma. 2001;18(10):1011–8.
Vespa P, Boonyaputthinkul R, McArthur DL, et al. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. 2006;34(3):850–6.
Vespa P, O’Phelan K, McArthur D, Miller C, Eliseo M, Hirt D, Glenn T, Hovda DA. Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Crit Care Med. 2007;35(4):1153–60.
Le Galle JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1994;271(17):1321.
University of Minnesota Medical Center Continuous Intravenous Insulin Infusion Orders Protocol. APSF Newsletter Summer 2006. apsf.org.
Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79(8):992–1000.
Souba WW. Nutritional support. N Engl J Med. 1997;336:41–8.
Himanen L, Portin R, Isoniemi H, Helenius H, Kurki T, Tenovuo O. Longitudinal cognitive changes in traumatic brain injury: a 30-year follow-up study. Neurology. 2006;66(2):187–92.
Luukinen H, Viramo P, Koski K, Laippala P, Kivela SL. Head injuries and cognitive decline among older adults: a population-based study. Neurology. 1999;52(3):557–62.
Horan TC, Gaynes RP. Surveillance of nosocomial infections In: Mayhall CG, editor. Hospital and infection control, 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 1659–702.
Weinstein MP, Towns ML, Quartey SM, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584–602.
Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a metanalysis of randomized controlled trials. Arch Intern Med. 2004;164(18):2005–11.
Widom B, Simonson DC. Glycemic control and neuropsychologic function during hypoglycemia in patients with insulin-dependent diabetes mellitus. Ann Intern Med. 1990;112(12):904–12.
Boyle PJ, Schwartz NS, Shah SD, et al. Plasma glucose concentrations at the onset of hypoglycemic symptoms in patients with poorly controlled diabetes and in nondiabetics. N Engl J Med. 1988;318:1487–92.
Li PA, Shamloo M, Smith ML, et al. The influence of plasma glucose concentrations on ischemic brain damage is a threshold function. Neurosci Lett. 1994;177:63–5.
Sieber FE, Traystman RJ. Special issues: glucose and brain. Crit Care Med. 1992;20(1):104–14.
Wass CT, Lanier WL. Glucose modulation of ischemic brain injury: review and clinical recommendations. Mayo Clin Proc. 1996;71:801–12.
Preiser JC, Devos P. Clinical experience with tight glucose control by intensive insulin therapy. Crit Care Med. 2007;35:S503–7.
Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97.
CRASH Trial Collaborators. Effect of intravenous corticosteroids on death within 14 days in 10,008 adults with clinically significant head injury (MRC CRASH Trial): randomised, placebo-controlled trial. Lancet. 2004;364:1321–8.
Acknowledgments
We are indebted to all the intensive care staff for their active cooperation and excellent compliance with the study protocol. We also thank Drs Fabio Araimo, Floriana Baisi, Giovanna Branca, Donato Colagiovanni, Nicola DeBlasis, Carmela Imperiale, Giuseppina Magni, Filomena Musolino, Marina Pennacchia, Letizia Pennacchiotti, Francesca Rinaldi, Paolo Tordiglione for patient care; Dr Carolina Maurizio for collecting data; Dr Alessandro Laviano for advice on patients’ nutrition; and Drs Laura Scorsolini and Mario Venditti for their continuous assistance in the clinical and laboratory diagnosis of infections.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was done in the Department of Neuroanaesthesia, University of Rome “La Sapienza”, Rome, Italy, and was in part presented at the Euroanaesthesia 2006 meeting Madrid, Spain.
Appendices
Appendix 1: ICU Protocol for Insulin Infusion
Glucose value (mg/dl) | Insulin dose |
|---|---|
Intensive insulin therapy | |
For first blood glucose measurement | |
121–130 | ½ unit/h |
131–140 | 1 unit/h |
141–160 | 2 units/h |
161–181 | Give 2 units IV bolus and 2.5 units/h |
181–200 | Give 2 units IV bolus and 3 units/h |
201–220 | Give 2 units IV bolus and 3.5 units/h |
>220 | Give 2 units IV bolus and 4 units/h |
For successive blood glucose measurements | |
80–120 | Continue current infusion rate |
121–141 | Increase insulin infusion by 0.5 unit/h |
141–160 | Increase insulin infusion by 1 unit/h |
161–180 | Increase insulin infusion by 1.5 unit/h |
181–200 | Increase insulin infusion by 2 units/h |
201–220 | Increase insulin infusion by 2.5 units/h |
>220 | Increase insulin infusion by 3 units/h |
Conventional insulin therapy | |
For first blood glucose measurement | |
220–240 | Give 2 units IV bolus and 2 units/h |
241–260 | Give 2 units IV bolus and 3.5 units/h |
261–280 | Give 2 units IV bolus and 4 units/h |
>280 | Give 4 units IV bolus and 4 units/h |
For successive blood glucose measurements | |
<220 | Continue current infusion rate |
220–240 | Increase insulin infusion by 1 unit/h |
241–260 | Increase insulin infusion by 2 units/h |
261–280 | Increase insulin infusion by 3 units/h |
>280 | Increase insulin infusion by 4–5 units/h |
Appendix Legend
All patients with blood glucose levels <4.44 mmol/l (<80 mg/dl) received glucose in a bolus (0.1 g/kg at a concentration of 33%) with the insulin infusion stopped. The glucose concentration was then measured within 30 min.
All patients receiving continuous insulin must receive a continuous surge of enteral or parenteral nutrition.
The insulin infusion is discontinued if the patient has to leave the ICU for a diagnostic test as well as upon discharge from the ICU.
Appendix 2: Glasgow Outcome Score
Score | Rating | Definition |
|---|---|---|
5 | Good recovery | Resumption of normal life despite minor deficits |
4 | Moderate disability | Disabled but independent. Can work in sheltered setting |
3 | Severe disability | Conscious but disabled. Dependent for daily support |
2 | Persistent vegetative | Minimal responsiveness |
1 | Death | Non-survival |
Rights and permissions
About this article
Cite this article
Bilotta, F., Caramia, R., Cernak, I. et al. Intensive Insulin Therapy After Severe Traumatic Brain Injury: A Randomized Clinical Trial. Neurocrit Care 9, 159–166 (2008). https://doi.org/10.1007/s12028-008-9084-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-008-9084-9