Abstract
Purpose
Guidelines recommend teaching of lung ultrasound for critical care, though little information exists on how much training is required for independent practice, especially for non-physician trainees. We thus aimed to elucidate a threshold number of cases above which competency for independent practice may be attained for respiratory therapists (RTs).
Methods
We conducted a prospective audit of lung ultrasound training between July 2014 and April 2015 in our 20-bed medical intensive care unit. Following theoretical instruction and self-learning, trainees acquired images from 12 lung zones under direct supervision and classified images into six patterns. Assistance during image acquisition and correct interpretation of ultrasound images were recorded.
Results
Eleven ultrasound-naïve RTs scanned an average of 15 patients each (170 patients in total). Among supervisor-adjudicated lung ultrasound findings, 35.5 % were abnormal. Blinded verification of the adjudicated findings was done for the first 92 patients (1104 images), with an agreement of 95.4 %. As RTs scanned more patients, there was a significant decrease in the proportion of images requiring supervisor assistance (Cuzick’s P < 0.001), and a significant increase in the proportion of correctly identified images (Cuzick’s P = 0.008). After trainees performed at least ten scans, less than 2 % of images required assistance with acquisition and less than 5 % were wrongly interpreted.
Conclusions
Our training method allowed RTs to independently perform lung ultrasound after at least ten directly supervised scans. Given that RTs are likely to have less ultrasound knowledge and less clinical know-how compared to physicians, we believe that the same threshold number of scans may be also safely applied to the latter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lung ultrasound, as part of the critical care ultrasound armamentarium, has been shown to be useful in uncovering the etiology of respiratory failure, monitoring alveolar recruitment, managing circulatory failure, monitoring hemodynamics, guiding pleural procedures, confirming endotracheal tube positioning, and predicting post-extubation distress [1–8]. However, ultrasound is an operator-dependent skill, and adequate training is required for effective clinical use [9]. Also, training should be structured and be taught by experts who have amassed the necessary knowledge and experience. Guidelines recommend the inclusion of lung ultrasound within the critical care medicine curriculum, though little information exists on how much training is required for independent practice [10–12].
While physicians conventionally perform most procedures including ultrasound in the intensive care unit (ICU), non-physician healthcare professionals can be trained in skills traditionally employed by physicians, such as central venous catheter insertions [13]. On the same note, just as medical technologists now perform echocardiography, respiratory therapists (RTs) can also perform lung ultrasound. In our institution, RTs manage all invasive and non-invasive ventilation for adults, see all patients on admission, and are available on a 24-h basis. As such, with the added competency of lung ultrasound, they can assist physicians in screening all ventilated patients for pathology and in providing additional information for extubation decisions.
We hypothesized that lung ultrasound can be readily learnt by ultrasound-naïve trainees, and that RTs can be trained to perform lung ultrasound. We thus aimed to devise a training curriculum for RTs, to study the learning trajectory (image acquisition and interpretation), and to elucidate a threshold number of cases above which competency for independent practice may be attained. Given that RTs are likely to have less ultrasound knowledge and less clinical know-how compared to physicians, we believe that our study results could also be extrapolated to ultrasound-naïve ICU physician-trainees.
Methods
Settings and participants
We conducted a prospective audit of lung ultrasound training instituted between July 2014 and April 2015 in our 20-bed medical ICU, located within a 1228-bed university hospital. Our ethics review board permitted waiver of informed consent (DSRB B/2015/00560). Lung ultrasound screening was to be done for all mechanically ventilated patients or patients with respiratory failure (requiring at least 40 % inspired oxygen fraction to maintain an oxygen saturation of 90 %), with the results entered into the computerized ICU documentation system (IntelliSpace Critical Care and Anesthesia, Philips Singapore). The first author (KCS) uses general critical care ultrasonography regularly. KCS has undergone multiple courses in whole-body critical care ultrasound, has 8 years of ultrasound experience (150–200 thoracic/lung scans per year), and is a trainer for his hospital in thoracic ultrasound. The last author (CMT) is a pulmonologist competent in thoracic ultrasound, who did not work in the medical ICU. CMT has completed training in thoracic ultrasound to the Royal College of Radiologists level 2 standard [14], has 11 years of ultrasound experience (30–50 thoracic/lung scans per year), was a member of the Pleural Procedures and Thoracic Ultrasound Subgroup of the British Thoracic Society’s Pleural Disease Guideline Committee, and is a trainer for his hospital in thoracic ultrasound. We shared the services of 22 RTs, who also covered other non-medical ICUs.
For lung ultrasound screening to be implemented successfully, we adopted a three-phase approach: the first phase (training phase 1) was for KCS to directly supervise 11 RTs in lung ultrasound for as many eligible patients as possible during office hours (i.e., convenience sampling); the second phase (training phase 2) was for KCS to train the remaining 11 RTs; the third phase (roll-out phase) was full service implementation. This study focused on the first phase.
Curriculum creation and training implementation
The ultrasound curriculum was created by the authors, who identified several key learning concepts (Table 1). These concepts were directed at allowing training to occur within a busy clinical environment (building rapport, self-directed learning), motivating trainees to learn lung ultrasound theory (triggering prior knowledge, test-enhanced learning, goal-directed learning, providing feedback), avoiding cognitive overload (scaffolding of skill acquisition, compartmentalization of learning), and ensuring patient safety (training audit and verification) [15–17]. To achieve the training objective of independent lung ultrasound practice, we included the following subtopics in the curriculum: basic ultrasound physics, use of ultrasound equipment, probe positioning, and lung ultrasound interpretation (A-lines, B-lines, consolidation, lung sliding, lung pulse, miscellaneous artifacts). We excluded the learning of M-mode and Doppler ultrasound as these were not essential for lung imaging [11, 12, 18].
KCS, JT, and CMT organized a briefing for all 11 RTs to introduce lung ultrasound in July 2014. This was immediately followed by pre-testing (supplementary material—lung ultrasound test), release of electronic/on-line materials for self-learning over 1 month, and post-testing (Table 1). Trainees recorded the time spent on self-learning. The result of the post-test was not used to determine progression to the practical training, as KCS would also quiz the trainees about the same theoretical subtopics during the latter period. Actual patient contact for lung ultrasound occurred from August 2014. During weekday afternoons, excluding public holidays, the trainees would scan newly admitted and mechanically ventilated patients under direct supervision. We focused on image acquisition while scanning each patient, and shifted our emphasis to image interpretation after leaving the patient’s room.
Image acquisition and assistance
While blinding of the supervisor and trainees was not possible, we avoided looking at the clinical notes till ultrasound image acquisition and interpretation were completed. We used a Sparq Ultrasound System (Philips Healthcare, Andover, MA) equipped with a 2–4 MHz broadband sector phased array transducer. The relatively small transducer footprint allowed comfortable probe positioning between ribs. We focused on 12 lung zones as described by Soummer et al. so that we could apply the findings to predict post-extubation distress [4]. Adapting the same technique would also allow diagnosis as described by Lichtenstein and Meziere in their BLUE protocol [19], and includes the eight zones for the first diagnosis of interstitial syndrome recommended by an international expert panel [12]. We adhered to the following sequence of lung zones: (1) right anterior upper, (2) right anterior lower, (3) right lateral upper, (4) right lateral lower, (5) right posterior upper, (6) right posterior lower, (7) left anterior upper, (8) left anterior lower, (9) left lateral upper, (10) left lateral lower, (11) left posterior upper, (12) left posterior lower. The upper and lower zones were separated by the fourth rib, which was palpated two ribs below the sternal angle. The anterior and lateral zones were separated by the anterior axillary line, while the lateral and posterior zones were separated by the posterior axillary line. In addition, zones 1, 2, 4, 7, 8, and 10 would correspond to the BLUE points [20].
Under direct supervision, trainees first scanned lung zones 1–4 while the patient was in the semirecumbent position, zones 5–6 in the left decubitus position, zones 7–10 in the semirecumbent position, and zones 11–12 in the right decubitus position. Particular attention was paid to move the probe away from the scapula at lung zones 5 and 11, and away from the heart at lung zone 8 (no patient had dextrocardia). Image depth was adjusted such that the pleural line was positioned one-third of the distance from the probe marker to the opposite side of the monitor screen, which allowed at least two A lines to be visible. Image acquisition was deemed acceptable when the pleural line was visible between two rib shadows. The supervisor rendered assistance for image acquisition whenever the trainee struggled for more than a minute at any scanning zone, and helped record the total scanning time. A cine image was saved for every lung zone in our standard sequence, and no image labelling was needed.
Image interpretation, verification, and audit
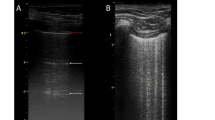
After scanning, the supervisor and trainee brought the machine away from the patient and performed off-line interpretation of the cine images. Trainees had to identify the presence of lung sliding, lung pulse, A lines, b lines (only one B line), bb lines (only two B lines), lung rockets (more than two B lines, indicating interstitial syndrome), septal rockets (3–4 B lines, indicating septal interstitial syndrome), ground-glass rockets (multiple coalescent B lines, indicating ground-glass areas), and consolidation [5]. Examples of lung rockets, lung sliding, and lung consolidation can be found in the supplementary material. Examples of images for which skills are quickly reached and of images for which more time is required to get the skills can be found in Fig. 1. Trainees classified their findings into six patterns: (1) A lines, lung sliding, 0–2 B lines (A profile, suggestive of normal lungs); (2) A lines, no lung sliding, positive lung pulse (suggestive of collapsed lung); (3) A lines, no lung sliding, no lung pulse (A’ profile, suggestive of pneumothorax); (4) B lines (3 or more), separated (B profile, suggestive of minor interstitial edema); (5) B lines (3 or more), coalesced (B’ profile, suggestive of major interstitial edema); (6) consolidation (C profile) [5]. The supervisor noted and provided feedback when trainees incorrectly interpreted the lung images. We did not specifically study the interpretation of pleural effusion because, in our experience, this finding was very easily noted by trainees seeing it for the first time.
a “Difficult” image: trainee may wrongly identify a horizontal artifact (red arrow) as consolidation. b “Difficult” image: trainee may wrongly identify a Z line (red arrow) as a B line. c “Difficult” image: trainee may miss a small consolidation (red arrow). d “Easy” image: A line (blue arrow). e “Easy” image: B line (blue arrow). f “Easy” image: consolidation (blue arrow), with overlying pleural effusion
For patients scanned in the first 5 months of this audit, verification was done by the last author (CMT) on a separate day, without seeing the patient or viewing the medical records. Using only the saved images, CMT noted his own interpretation, which was compared to the supervisor-adjudicated findings at the end of the study. Agreement was taken as concordance of classification into the above six patterns. For the audit, patient details were also collected from the computerized clinical database.
Data analysis
Pre-test and post-test scores were compared with the paired t test. Supervisor-adjudicated findings, proportion of assisted ultrasound images, and proportion of correctly identified ultrasound images were evaluated in blocks of three patients (i.e., 36 image views) to even out performance fluctuations, yet maintaining enough granularity to demonstrate trends. We used Cuzick’s nonparametric test for trend to analyze our results across the patient blocks [21]. For each patient encounter, the number of correctly identified image views was summed to give an overall performance score (score range 0–12). Linear regression of this performance score was done against lung ultrasound case count, adjusting for individual trainees as random effects. This regression was similarly done for scanning duration. Statistical significance was taken as P < 0.05.
Results
Our trainees were clinically experienced RTs with no prior ultrasound experience (Table 2). For theoretical learning, they spent a median of only 3 h on self-study, and had significantly higher post-test scores compared to pre-test scores. For practical learning, they scanned an average of 15 patients each, and 170 patients in total (Table 3). Each patient was scanned only once. Among supervisor-adjudicated lung ultrasound findings, 64.7 % showed a normal pattern. The other 35.3 % were abnormal (22.9 % consolidation, 6.9 % coalesced B lines, 4.9 % separated B lines, 0.5 % A lines with absent lung sliding). Additional findings included 53 (31.2 %) right-sided pleural effusions and 41 (24.1 %) left-sided pleural effusions. Two (1.2 %) patients had palpable subcutaneous emphysema and ultrasound was done with subcutaneous tissue compression (supplementary Table 1). Verification of the adjudicated findings was done for the first 92 patients (1104 images), with an agreement of 95.4 % by the last author (supplementary Table 2).
Across patient blocks, there was a significant decrease in the proportion of images requiring supervisor assistance (Cuzick’s z = −7.76, P < 0.001) (Fig. 2a; supplementary Table 3), and a significant increase in the proportion of correctly identified images (Cuzick’s z = 2.63, P = 0.008) (Fig. 2b; supplementary Table 4). From Fig. 2a, it appears that after performing six scans, trainees required very little assistance (less than 2 % of the images). Median scanning duration was 12 min (interquartile range 8–16 min, range 4–45 min). Using linear regression, scanning duration did not significantly decrease with more training cases (coefficient −0.006, P = 0.444), adjusting for individual trainees as random effects. From Fig. 2b, it appears that at least ten scans were required before trainees achieved 95 % accuracy in image interpretation. Using linear regression, performance score (log-transformed for normality) was associated with more training cases (coefficient 0.003, P = 0.047), adjusting for individual trainees as random effects.
a Line chart showing percentage of assisted views during lung ultrasound scanning. b Line chart showing percentage of correct identification of lung ultrasound findings (excluding identification of pleural effusion), with a 2nd-order polynomial trend line (2nd-order polynomial trend line added to demonstrate the overall trend)
Discussion
We showed that ultrasound-naïve RTs could be readily trained to perform lung ultrasound independently and competently. Low levels of assistance (less than 2 %) were required after six cases, while high levels of accuracy (greater than 95 %) could be attained with at least ten cases. This has important implications in guiding training curricula and processes.
To achieve these results, a conceptual framework based on educational pedagogy was critical to sustaining the training effort for 11 RTs over 10 months and optimizing the effect of training. Additionally, we endeavored to maintain consistency in practice by adhering to a fixed scanning method which helped to maintain quality. A small dip in the proportion of correctly identified lung images occurred between seven and nine scanned cases, which could be the result of trainee overconfidence in image interpretation, leading to over-reading or under-reading of lung scan abnormalities. Nonetheless, interpretative accuracy increased from ten scanned cases onwards. As expected for novices, nearly half of the image acquisitions required assistance early in training, though this need rapidly decreased with experience. However, we were unable to show a significant decrease in scanning time with more cases scanned. This might be due to two reasons: firstly, trainees were extra careful as supervisor assistance waned; and secondly, we allowed a trainee to spend at least 1 min per image before any supervisor interruption.
Our study has several strengths. Firstly, our curriculum was pragmatic and could be implemented even when resources are limited. We encouraged self-learning using pre-tests, post-tests, and online materials, which allowed supervisor time to be reserved for practical learning. Secondly, to assess for supervisor bias in scoring the trainees, we employed a blinded observer who read the images of more than half of the patients off-line. Thirdly, we used an imaging protocol extending to 12 lung zones, which would allow enough data to be collected for a variety of existing protocols. Fourthly, by training ultrasound-naïve RTs, we were able to derive a learning trajectory that yielded a conservative—and therefore relatively safe—threshold for the minimum number of practice cases required for competency.
Our study has to be interpreted with some limitations. Firstly, our patient cohort had a low prevalence of lung collapse or pneumothorax, which meant that only a minority of trainees saw these conditions during practical training. Nonetheless, in practice, they were also able to accurately detect a normal A profile, which would be an important skill for excluding the presence of lung collapse or pneumothorax. Secondly, determination of supervisor assistance for image interpretation was subjective, which could lead to excessive assistance earlier in training. This means that we could overestimate the number of scans required for competency based on supervisor assistance required. Thirdly, our trainees were non-physicians, which could potentially limit the generalizability of our results to physicians, who may have received greater educational grounding in human pathology and may have had ultrasound experience from elsewhere (e.g., for central venous catheter placement). Again, this means that our estimate for the number of scans required for competency could be conservative. Fourthly, we did not employ high-fidelity simulation models, which could have allowed more frequent practice and more rapid attainment of competency. Lastly, we did not systematically study how our lung ultrasound findings were used by the managing clinicians and therefore would not be able to demonstrate clinical impact. However, the clinical utility of lung ultrasound has been previously shown [1, 22, 23].
We hope that our findings can encourage more health professionals, both physicians and non-physicians, to undertake lung ultrasound training. Apart from ultrasound equipment and time, no further resources or expenditure were required. Trainees could expect to quickly achieve competency after theoretical self-study, followed by at least ten directly supervised scans. This information would be a useful addition to lung ultrasound training guidelines, and would help establish lung ultrasound as standard of care [24]. Nonetheless, our findings should be validated in other settings and with other personnel. Similar studies using high-fidelity ultrasound models may also be done to investigate if appropriate simulation training would improve the learning trajectory. Furthermore, once competency in lung ultrasound image acquisition and pattern recognition is accomplished, one should use these skills to enhance diagnostic accuracy. Lung ultrasound findings should be integrated with history taking, clinical examination, and laboratory testing for the clinical reasoning process. Unlike basic image acquisition and pattern recognition demonstrated in our study, the latter clinical reasoning process is more challenging. It requires deliberate practice, real-life experience, appropriate feedback, metacognition, and self-regulation to attain mastery [25–27].
In conclusion, we devised a pragmatic lung ultrasound curriculum, which involved building rapport, stimulating self-directed learning, and avoiding cognitive overload. We also instituted direct supervision and audit to ensure patient safety. Our training method allowed RTs to acquire the ability to independently perform lung ultrasound after at least ten directly supervised scans. Being a conservative estimate, the same threshold number of scans may be safely applied to physician-trainees.
References
Xirouchaki N, Kondili E, Prinianakis G, Malliotakis P, Georgopoulos D (2014) Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med 40:57–65
Havelock T, Teoh R, Laws D, Gleeson F, British Thoracic Society Pleural Disease Guideline Group (2010) Pleural procedures and thoracic ultrasound: British Thoracic Society pleural disease guideline 2010. Thorax 65(Suppl 2):ii61–ii76
Sim SS, Lien WC, Chou HC, Chong KM, Liu SH, Wang CH, Chen SY, Hsu CY, Yen ZS, Chang WT, Huang CH, Ma MH, Chen SC (2012) Ultrasonographic lung sliding sign in confirming proper endotracheal intubation during emergency intubation. Resuscitation 83:307–312
Soummer A, Perbet S, Brisson H, Arbelot C, Constantin JM, Lu Q, Rouby JJ, Lung Ultrasound Study Group (2012) Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med 40:2064–2072
Lichtenstein DA (2015) BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 147:1659–1670
Volpicelli G, Skurzak S, Boero E, Carpinteri G, Tengattini M, Stefanone V, Luberto L, Anile A, Cerutti E, Radeschi G, Frascisco MF (2014) Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology 121:320–327
Bouhemad B, Brisson H, Le-Guen M, Arbelot C, Lu Q, Rouby JJ (2011) Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med 183:341–347
Via G, Storti E, Gulati G, Neri L, Mojoli F, Braschi A (2012) Lung ultrasound in the ICU: from diagnostic instrument to respiratory monitoring tool. Minerva Anestesiol 78:1282–1296
See KC, Ong V, Ng J, Tan RA, Phua J (2014) Basic critical care echocardiography by pulmonary fellows: learning trajectory and prognostic impact using a minimally resourced training model. Crit Care Med 42:2169–2177
Neri L, Storti E, Lichtenstein D (2007) Toward an ultrasound curriculum for critical care medicine. Crit Care Med 35:S290–S304
Cholley BP, Mayo PH, Poelaert J, Vieillard-Baron A, Vignon P, Alhamid S, Balik M, Beaulieu Y, Breitkreutz R, Canivet JL, Doelken P, Flaatten H, Frankel H, Haney M, Hilton A, Maury E, McDermid RC, McLean AS, Mendes C, Pinsky MR, Price S, Schmidlin D, Slama M, Talmor D, Teles JM, Via G, Voga G, Wouters P, Yamamoto T, Expert Round Table on Ultrasound in ICU (2011) International expert statement on training standards for critical care ultrasonography. Intensive Care Med 37:1077–1083
Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T, International Liaison Committee on Lung Ultrasound for International Consensus Conference on Lung Ultrasound (2012) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 38:577–591
Alexandrou E, Spencer TR, Frost SA, Mifflin N, Davidson PM, Hillman KM (2014) Central venous catheter placement by advanced practice nurses demonstrates low procedural complication and infection rates—a report from 13 years of service. Crit Care Med 42:536–543
Royal College of Radiologists (2012) Ultrasound training recommendations for medical and surgical specialities. RCR, London
Ambrose SA (2010) How learning works: seven research-based principles for smart teaching. Jossey-Bass, San Francisco
See KC, Ong V, Teoh CM, Ooi OC, Widjaja LS, Mujumdar S, Phua J, Khoo KL, Lee P, Lim TK (2014) Bedside pleural procedures by pulmonologists and non-pulmonologists: a 3-year safety audit. Respirology 19:396–402
Larsen DP, Butler AC, Roediger HL 3rd (2008) Test-enhanced learning in medical education. Med Educ 42:959–966
Lichtenstein DA (2014) Lung ultrasound in the critically ill. Ann Intensive Care 4:1
Lichtenstein DA, Meziere GA (2008) Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 134:117–125
Lichtenstein D, Meziere GA (2011) The BLUE-points: three standardized points used in the BLUE-protocol for ultrasound assessment of the lung in acute respiratory failure. Crit Ultrasound J 3:109–110
Cuzick J (1985) A Wilcoxon-type test for trend. Stat Med 4:87–90
Volpicelli G, Boero E, Sverzellati N, Cardinale L, Busso M, Boccuzzi F, Tullio M, Lamorte A, Stefanone V, Ferrari G, Veltri A, Frascisco MF (2014) Semi-quantification of pneumothorax volume by lung ultrasound. Intensive Care Med 40:1460–1467
Leblanc D, Bouvet C, Degiovanni F, Nedelcu C, Bouhours G, Rineau E, Ridereau-Zins C, Beydon L, Lasocki S (2014) Early lung ultrasonography predicts the occurrence of acute respiratory distress syndrome in blunt trauma patients. Intensive Care Med 40:1468–1474
Mayo PH, Beaulieu Y, Doelken P, Feller-Kopman D, Harrod C, Kaplan A, Oropello J, Vieillard-Baron A, Axler O, Lichtenstein D, Maury E, Slama M, Vignon P (2009) American College of Chest Physicians/La Societe de Reanimation de Langue Francaise statement on competence in critical care ultrasonography. Chest 135:1050–1060
Ericsson KA (2004) Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med 79:S70–S81
Patel R, Sandars J, Carr S (2015) Clinical diagnostic decision-making in real life contexts: a trans-theoretical approach for teaching: AMEE Guide No. 95. Med Teach 37:211–227
Spencer J (2003) Learning and teaching in the clinical environment. BMJ 326:591–594
Acknowledgments
All the authors jointly conceived the study and prepared the manuscript. Dr. See, Ms. Wong, Mr. Leanda, Ms. Santos, and Mr. Taculod organized and implemented the training curriculum. Ms. Ong, and Ms. Wong performed the data extraction. Dr. See performed the data analysis. Dr. Phua and Dr. Teoh supervised the analysis and edited the article. Dr. See had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have disclosed that they do not have any potential conflicts of interest.
Additional information
This work was performed at the National University Health System, Singapore.
Take-home message: Ultrasound-naïve respiratory therapists (RTs) can be readily trained to perform lung ultrasound independently and competently: low levels of assistance (less than 2 %) were required after six cases, while high levels of accuracy (greater than 95 %) can be attained with at least ten cases. This has important implications in guiding training curricula and processes: (1) Given that RTs are likely to have less ultrasound knowledge and less clinical know-how compared to physicians, our study results could be extrapolated to ultrasound-naïve physician-trainees; (2) RTs may help physicians perform lung ultrasound, potentially improving the array of ICU services without further burdening physicians.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
See, K.C., Ong, V., Wong, S.H. et al. Lung ultrasound training: curriculum implementation and learning trajectory among respiratory therapists. Intensive Care Med 42, 63–71 (2016). https://doi.org/10.1007/s00134-015-4102-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-4102-9