Abstract
Background
Lung ultrasound (LUS) may accurately diagnose pneumothorax. However, there is uncertainty about its usefulness in the quantification of pneumothorax size. To determine the ability of LUS in the semi-quantification of pneumothorax volume, we compared the projection of the lung point (LP) with the pneumothorax volume measured by computerized tomography (CT) and the interpleural distance on chest radiography (CXR).
Methods
We performed LUS in patients with pneumothorax and all the LP located on the chest wall were compared to CXR and CT studies. The primary outcome of the study was the ability of LP to grade pneumothorax volumes measured by CT. The secondary outcome was the accuracy of LP to predict small and large pneumothorax according to the societal guidelines based on CXR reading.
Results
A total of 124 patients with pneumothorax were enrolled (76 spontaneous, 20 traumatic and 28 post-procedural). Ninety-four CXR and 58 CT were available for the analysis. An LP posterior to the mid axillary line corresponded to three different CXR criteria for large pneumothorax with sensitivity from 81.4 to 88.2 % and specificity from 64.7 to 72.6 %. The mid axillary line also represented the limit for predicting greater than 15 % of lung collapse when volume is measured at CT, with sensitivity 83.3 % and specificity 82.4 %.
Conclusions
LUS-targeted assessment of LP was a useful predictor of pneumothorax volume in this research study setting. LUS reliably classified pneumothorax size when compared to criteria based on CXR reading, particularly the small sized pneumothorax. However, LUS greatly outperformed conventional CXR reading for a graded quantification of the percentage of lung collapse.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The clinical utility and high sensitivity of bedside lung ultrasound (LUS) in the diagnosis of traumatic and post-procedural pneumothorax have been described [1]. Many studies show that the sensitivity of ultrasound diagnosis for pneumothorax is superior to bedside chest radiography (CXR) and similar to computerized tomography (CT) [2–5]. In a condition of partial collapse of the lung, the ultrasound diagnosis of pneumothorax is based on the visualization of the point where the visceral pleura is again next to the parietal pleura without air interposition, and slides with respiration. The projection of this point on the chest wall is known as the lung point (LP) [6]. Visualization of an LP during LUS rules in pneumothorax with the same high specificity of the interpleural space at CXR [7]. It has been theorized that the projection of the LP on the chest wall may also potentially allow quantification of the volume of pneumothorax [8]. However, while the reliability of ultrasound in the diagnosis of pneumothorax has been widely demonstrated, the possibility to quantify the volume of intrapleural air based on the projection of the LP is still debated [7].
The size of lung collapse represents one of the criteria used to determine treatment and establish prognosis of pneumothorax [9–12]. When a CT scan is not indicated or is not available, pneumothorax is conventionally quantified by measuring the distance between the two pleural layers on CXR [10, 13–15]. However, the limitations of the standard CXR in diagnosing and quantifying pneumothorax and its inferiority to LUS, particularly when the sole supine projection is available, are well known [2–5, 16–18].
In the present paper, we first hypothesize that different projections of the LP on the chest wall indicate corresponding volumes of pneumothorax quantified by CT studies. The second goal is to investigate if LUS may differentiate small and large sized pneumothorax when compared to guideline criteria based on CXR reading.
Materials and methods
This was a prospective, single-blind study with consecutive sampling of patients with pneumothorax at San Luigi Gonzaga University Hospital, Turin, Italy. The study was conducted from April 2012 to July 2013. Patients were enrolled from two units of the hospital: the emergency department (ED) and the interventional radiology unit (IRU). The ED has an annual census of 50,000 patients. Radiologists in the IRU perform approximately 300 CT-guided lung biopsies per year. The study was approved by the institutional review board (Comitato Etico Interaziendale, number 181/2011 Prot.25091) and patients provided written informed consent before enrollment. For unconscious patients, the informed consent was waived. The protocol was registered at ClinicalTrials.gov (NCT01572584).
Patients
Patients with pneumothorax were consecutively enrolled in the study. Enrollment criteria were established diagnosis of pneumothorax based on CXR and/or CT studies and age more than 15 years. Cases recruited from the ED were trauma and spontaneous pneumothorax. We also enrolled consecutive cases of pneumothorax secondary to CT-guided procedures for lung biopsies performed in the IRU. The only exclusion criteria were impossibility to perform LUS because of wounds and bandages, emergency drainage before completion of the imaging studies, or refusal of the patient to participate.
Lung ultrasound
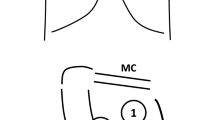
In the ED, LUS was performed by specifically trained operators, no more than 10 min following CXR and/or CT scan. The operator knew the diagnosis, but was unaware of the side and size of pneumothorax. In the IRU, LUS was performed by an operator who was on call when pneumothorax was detected following the CT-guided lung biopsy. In post-procedural cases when the side was evident, the operator was only blinded to the size of pneumothorax. Examination was performed using one Esaote MyLab 40 and one Esaote MyLab 50 Gold Vinco ultrasound system (Esaote Italia, Milan, Italy), equipped with linear 7–12 MHz transducers. In all patients, the ultrasound examination was performed in the supine position by scanning the anterior-inferior region of the chest bilaterally to confirm absence of lung sliding, B-lines, and lung pulse [1, 19]. The probe was then moved to the lateral chest to visualize the LP, which is the projection on the chest wall of the point where a lung sliding is again visualized (Fig. 1 and Video 1 in the electronic supplementary material, ESM) [1, 7]. The LP corresponds to the lateral edge of the pneumothorax in a condition of partial collapse of the lung. In cases where the lung is totally collapsed, the LP cannot be visualized [1, 8]. When an LP was detected, its exact location on the chest wall was recorded with respect to the parasternal, mid-clavicle, anterior-axillary, mid-axillary, and posterior-axillary lines. On occasion, the possibility of a double LP as a sign of loculated pneumothorax was also investigated and the most lateral LP considered in our analyses [20]. All the ultrasound examinations were recorded and stored. The operators were board certified emergency physicians (GV, GF), board certified radiologist (MB), residents in emergency medicine (AL, MT, VS, EB) and radiology (FB), all with specific competency and training in LUS, performing a minimum of 400 emergency ultrasound studies per year.
Chest radiography
In the ED, all the enrolled collaborating patients underwent expiratory posterior-anterior CXR. Even if CXR in expiration adds little to the first diagnosis and is not indicated as a routine investigation, it may be more accurate than regular CXR for the evaluation of the size of pneumothorax [21]. Unconscious and critically ill patients underwent portable, supine anterior-posterior CXR. An independent radiologist (LC), blinded to the ultrasound exam, retrospectively read the digital image and measured the distance between the pleural layers at apex and base. The erect CXR studies were then separately classified as large or small pneumothorax, according to each of three societal criteria published in the literature [10, 13, 14]. The radiologic cut-offs for diagnosing large pneumothorax were a visible interpleural rim of air of at least 2 cm between the lung and chest wall [10], or at least 3 cm apex-to-cupola distance of pleural rim [13], or a pleural gap along the entire length of the lateral chest wall [14]. In the supine radiographs, the interpleural gap was measured at the base and the average distance was converted to the corresponding values of erect CXR by using a simplified diagram published in the literature [22].
CT scan
In the ED, a thoracic CT scan was performed only for clinical reasons at the discretion of the attending physician, independent from the study protocol. In spontaneous pneumothorax, a CT scan was performed for two main clinical reasons: evaluation of recurrences or evaluation of the real distribution, size, and associated conditions to decide on the most appropriate treatment. In the IRU, CT was used to guide the thoracic procedure for lung biopsy. A 64-row multi-detector CT (Philips Brilliant MX80001DT, Koninklijke Philips Medical Systems, Eindhoven, the Netherlands) was used to volumetrically scan the whole lung. No contrast medium was used. Images were reconstructed at lung setting (width = 1,550 HU, level = −550 HU) and transferred to a personal computer running specialized software (MevisPULMO, version 1.4; Fraunhofer MEVIS, Bremen, Germany) for the quantification of pneumothorax. This procedure was performed by one operator (NS) with 9 years’ experience in chest imaging. Briefly, this software allows the application of high-precision 3D image analysis tools to volumetric CT data, providing automated lung segmentation. The operator manually defined one or more seed points within the pneumothorax area. Then, the software generated a segmentation using a threshold-based 3D region growing algorithm and starting from these seed points. On the basis of visual inspection, seed points and thresholds could be interactively adjusted by the operator. After these adjustments and final approval, the volume of the segmentation was computed.
Data analysis
Descriptive analysis was carried out using common measures of synthesis. Values are expressed as means ± standard deviation (SD). Continuous variables (percentage of pulmonary collapse) were transformed into dichotomous variables to build a receiver operating characteristic (ROC) curve. Statistical significance was set at 5 % for every test used and 95 % confidence intervals (CI) were reported. Concordance between dichotomous variables was calculated by Cohen’s k. Statistical analysis was performed with MedCalc v12.3.0.
Results
We consecutively enrolled 124 patients with pneumothorax. All patients were in spontaneous breathing at the moment of enrollment and imaging. Of these, 96 were outpatients admitted to the ED and 28 were patients undergoing a thoracic invasive procedure in the IRU. Characteristics of the population studied are outlined in Table 1 and a flow chart showing enrollment and analyses of the study is shown in Fig. 2 of the ESM. LUS was feasible in all patients and in all cases the main sonographic signs of pneumothorax, absence of lung sliding, B-lines, and lung pulse were confirmed. In 102 cases a single LP was detected. In 17 patients the LP was not visualized, 13 of them had large pneumothorax on CXR, three had small pneumothorax on CXR, and one showed over 50 % lung collapse on CT. In five patients a double LP was detected.
LUS vs chest radiography
We performed the analysis on 94 CXR (Fig. 2 in the ESM), 82 performed in the erect position and 12 in the supine position. For each societal guideline criterion, the ROC curve analysis identified the corresponding LP projections as shown in Table 2.
LUS vs CT scan
CT studies were performed on 59 cases of pneumothorax, of which 20 were spontaneous, 16 traumatic, and 23 post-procedural. Quantification of pneumothorax volume was possible in 58 studies, because the low quality of the images of one patient with major chest trauma did not allow reliable measurement (Fig. 2 in the ESM). A graphic representation of volume distribution in the three etiology groups of pneumothorax is reported in a box-and-whisker plot (Fig. 3 in the ESM).
Twenty-four pneumothoraxes had a volume greater than 15 %. ROC curve analysis showed that the best LP projection to predict greater than 15 % of lung collapse was on the mid axillary line or posterior, with sensitivity 83.3 % (CI 62.6–95.3 %), specificity 82.4 % (CI 65.5–93.2 %), positive predictive value 76.9 % (CI 56.4–91.0 %), negative predictive value 87.5 % (CI 70.7–96.6 %), positive likelihood ratio 4.72 (CI 2.2–10.0), and negative likelihood ratio 0.2 (CI 0.08–0.5). Area under the curve was 0.87 (CI 0.74–0.95), with significance level P < 0.0001. Table 3 shows the concordance between ultrasound and CXR criteria for differentiating small and large pneumothorax, when the cut-off was fixed at 15 % of lung collapse measured at CT.
In a second step we analyzed more deeply the possibility of semi-quantifying pneumothorax volumes by grading percentage of collapse in progressive classes, and performing the ROC curve analysis for each LP projection. Results are reported in Tables 4 and 5.
Discussion
For the very first time in humans, our study indicates that LUS may be used to reliably grade pneumothorax size on patients in spontaneous breathing with traumatic, post-procedural, and spontaneous pneumothorax. When compared to societal criteria that classify the size of pneumothorax based on CXR reading, LP projection reliably indicates the size, particularly the small pneumothorax. However, LUS outperforms conventional CXR reading when volumetric CT measurements are considered. In our study, the mid axillary line represented the most accurate anatomic boundary between large and small pneumothorax at LUS, which coincided also with a cut-off set at 15 % of lung collapse. Moreover, we showed that the increase in class of pneumothorax volume corresponds to progression of the laterality of the LP, as different locations of the LP from the anterior to the lateral chest progressively indicate three different classes of pneumothorax volume, from less than 10 % to more than 30 % of lung collapse.
The interpleural distance at CXR reading is indicated as the practical method to classify two grades of pneumothorax size. In spontaneous pneumothorax, a large sized pneumothorax is an objective indication for drainage, as pointed out in three important societal guidelines [10, 13, 14]. However, the volume of lung collapse that corresponds respectively to small and large is not clear [23]. Differences between the three guidelines may be striking, and volume cut-offs corresponding to the three radiologic criteria may vary from 15 to 49 % of lung collapse [15]. An interpleural rim greater than 2 cm, which is the cut-off to predict large sized pneumothorax recommended by the British Thoracic Society, should correspond to at least 50 % of collapse, but only when measurement is performed at lung basis [10]. However, the method that the experts used to calculate the corresponding volume is considered inaccurate [15, 17]. The volume of 15 % lung collapse that we used in our study is indicated in the literature as a safe cut-off to differentiate pneumothorax that may be treated by conservative management. When pneumothoraxes with less than 15 % of lung collapse are not drained, they have no persistent air leak and very low recurrence rate, far inferior to cases treated with drainage [10, 24]. Also, the time to resolve fully is relatively short, whereas in cases with at least 16 % collapse it increases up to 3.2 weeks [10, 24, 25].
The measurement of the interpleural distance in a single expiratory posterior/anterior chest view is imprecise and assumes patient collaboration. It is also noteworthy that CXR in the supine position has low sensitivity for the diagnosis of pneumothorax and this limitation may be irrespective of pneumothorax size [26–29]. LUS is far more sensitive than CXR in the diagnosis of traumatic, spontaneous, and post-procedural pneumothoraxes, having a similar high specificity [2–5, 18, 30–33]. Using LUS for pneumothorax quantification is largely intuitive, because the chest wall area where the lung sliding is not visualized is a measure of the superficial extension of pneumothorax [1, 6]. Lichtenstein hypothesized that the laterality of the LP is proportional to the dimension of pneumothorax, and that the anterior axillary line represents the spatial limit to differentiate large and small pneumothoraxes [8]. However, the superficial extension of pneumothorax does not necessarily coincide with its volume. Blaivas et al. found that in traumatic pneumothorax the surface area in which lung sliding was absent increased significantly from the small to the medium pneumothoraxes, but did not differentiate medium from large pneumothoraxes [2]. The proposed explanation was that the increase in pneumothorax volume only initially manifests as absence of sliding in an increasing arc from the anterior to the lateral portion of the chest. As the volume of intrapleural air further increases from medium to large pneumothorax, the lung is pushed even more away from the chest wall, but the surface in which lung sliding is absent increases less significantly. For these reasons, and as a result also of lack of evidence in human studies, the main experts at the recent international consensus conference on point-of-care LUS did not agree that ultrasound is a reliable method to assess the volume of pneumothorax as compared to CT, and concluded the need for additional evidence [7].
Some previous studies evaluated the ability of LUS to estimate the size of pneumothorax, with conflicting conclusions. Sistrom et al. concluded that sonography is of no use in estimating the volume of pneumothorax [34]. However, they used only CXR for the diagnosis and estimation of pneumothorax size, which represented a great limitation. Studying an elegant porcine model, recently Oveland et al. showed that lateral movement of the LP is an indicator of pneumothorax progression during invasive ventilation [35]. The conclusion of the study was that serial LUS examinations may be useful to monitor the size of a pneumothorax and to decide on the patient’s needs for chest drain.
Our data show that ultrasonography may be a potential replacement of CXR to predict pneumothorax size in spontaneous pneumothorax. This potential may be useful also in monitoring progression in size during observation of pneumothorax, which seems to be more related than absolute size to the need for a chest drain [36]. We also speculate that LUS may be useful to follow resolution of pneumothorax and decide when to remove chest drains [37]. We also know that in trauma patients on invasive ventilation, there is uncertainty on whether to drain occult pneumothorax [38, 39]. Some studies show that conservative treatment may be safe when an immediate response to deterioration may be provided at the bedside and for those patients predicted to require intensive care for less than 1 week [40, 41]. The new possibility of monitoring progression of pneumothorax and assessing its size at the bedside by LUS may be crucial to improve the safety of the treatment decision process.
Our study has many limitations. The operator performing LUS was aware of the diagnosis of pneumothorax, which may be considered a study bias. However, the main aim of the study was not to investigate the accuracy of LUS for the first diagnosis of pneumothorax, which was extensively studied by others. Rather, the original purpose was to investigate the potential of LUS for the quantification of pneumothorax size. To this aim, the operator was only blinded to CXR and CT images for pneumothorax size. Indeed, it is more correct to conclude that the results of our study may be applied to situations where the operator already knows the diagnosis of a pneumothorax.
A further limitation is the lack of a systematic comparison with expiratory CXR in the upright position, which is the ideal view to evaluate pneumothorax [23]. The difference between supine anterior/posterior CXR in a non-collaborating patient may be striking [21, 42, 43]. However, we used a normogram published in the literature to compare supine CXR with erect CXR, which should minimize the differences [17]. Indeed, in trauma patients supine CXR is still accepted and largely used as a tool to monitor pneumothorax progression, even if the limitations of this latter method have been described [36].
CT scan is the gold standard for estimating the volume of a pneumothorax. Comparing LUS with CXR may be considered of little scientific value, as we know that CXR performs poorly compared to CT. However, a CT study is not always indicated as standard of care in patients with pneumothorax. Moreover, while the main societal guidelines indicate CXR reading criteria for differentiating large and small sized pneumothorax, they do not clearly indicate cut-off volumes. Hence, comparison with CXR is realistic because it is still considered a clinical reference.
There was a 10-min window between the radiologic studies and LUS, which occasionally may be enough to have a change in the pneumothorax volume. However, we used this limit as the maximum delay to perform LUS. In most cases, LUS was performed immediately after CXR or even in the same radiology unit where the CT scan was performed.
One should also consider the possibility of facing complex cases of pneumothorax. Loculated and septated pneumothoraxes may be encountered in clinical practice [20, 44, 45]. In complex cases where pleural adherences and septa cause fragmented air collection, LUS has some limitations because the laterality of the LP does not necessarily correspond to the extension of pneumothorax [20]. Moreover, we did not encounter hydropneumothorax. Sometimes, this condition may be detected in trauma patients with both pneumothorax and hemothorax, and in case of pneumothorax complicating a procedure for draining pleural effusion. In these cases the “hydro-point” is the ultrasound visualization of the boundary between the effusion (or blood) and the air of the pneumothorax [4, 20, 46]. The significance of this sign is likely the same as that of the LP, both in terms of specificity for pneumothorax and for the estimation of its size [20]. We also did not find any significant subcutaneous emphysema in our trauma population. Air between the probe and the parietal pleura undoubtedly represents a potential great limitation for the ultrasound evaluation of the pneumothorax size. In all these complex cases, CT still remains the only method to reliably quantify pneumothorax.
Finally, we did not assess interoperator variability for LUS. We cannot exclude that ultrasound operators with different levels of expertise may be highly variable in visualizing the LP and indicating its exact location. However, in our study we propose a semi-quantification method based on the approximate location of the LP with respect to the longitudinal anatomic lines, which is a very basic technique.
Conclusion
In spontaneous breathing patients with mixed cases of previously known pneumothorax, the LP projection detected at LUS reliably indicates the size of pneumothorax in comparison with the interpleural distance measured at CXR, particularly for diagnosing small pneumothorax. When LUS is compared to CT to estimate the volume of a pneumothorax, the LP in the mid axillary line was shown to discriminate 15 % of lung collapse. Finally, the lateral progression of LP on the chest wall corresponds to increase in the entity of pneumothorax volumes. With further validation in practice, these findings may contribute to open up new perspectives for the practical use of LUS in the decision-making process of the management of spontaneous, post-procedural, and traumatic pneumothoraxes.
References
Volpicelli G (2011) Sonographic diagnosis of pneumothorax. Intensive Care Med 37:224–232
Blaivas M, Lyon M, Duggal S (2005) A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med 12:844–849
Lichtenstein DA, Meziere G, Lascols N, Biderman P, Courret JP, Gepner A, Goldstein I, Tenoudji-Cohen M (2005) Ultrasound diagnosis of occult pneumothorax. Crit Care Med 33:1231–1238
Reissig A, Kroegel C (2005) Accuracy of transthoracic sonography in excluding post-interventional pneumothorax and hydropneumothorax. Comparison to chest radiography. Eur J Radiol 53:463–470
Soldati G, Testa A, Sher S, Pignataro G, La Sala M, Silveri NG (2008) Occult traumatic pneumothorax: diagnostic accuracy of lung ultrasonography in the emergency department. Chest 133:204–211
Lichtenstein D, Meziere G, Biderman P, Gepner A (2000) The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med 26:1434–1440
Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T, International Liaison Committee on Lung Ultrasound for International Consensus Conference on Lung Ultrasound (2012) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 38:577–591
Lichtenstein DA (2007) Ultrasound in the management of thoracic disease. Crit Care Med 35:S250–S261
Baumann MH, Noppen M (2004) Pneumothorax. Respirology 9:157–164
Henry M, Arnold T, Harvey J, Pleural Diseases Group SoCCBTS (2003) BTS guidelines for the management of spontaneous pneumothorax. Thorax Suppl 2:ii39–ii52
de Moya MA, Seaver C, Spaniolas K, Inaba K, Nguyen M, Veltman Y, Shatz D, Alam HB, Pizano L (2007) Occult pneumothorax in trauma patients: development of an objective scoring system. J Trauma 63:13–17
Wolfman NT, Gilpin JW, Bechtold RE, Meredith JW, Ditesheim JA (1993) Occult pneumothorax in patients with abdominal trauma: CT studies. J Comput Assist Tomogr 17:56–59
Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J, Luketich JD, Panacek EA, Sahn SA, Group APC (2001) Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 119:590–602
De Leyn P, Lismonde M, Ninane V, Noppen M, Slabbynck H, Van Meerhaeghe A, Van Schil P, Vermassen F (2005) Guidelines Belgian Society of Pneumology. Guidelines on the management of spontaneous pneumothorax. Acta Chir Belg 105:265–267
Kelly AM, Druda D (2008) Comparison of size classification of primary spontaneous pneumothorax by three international guidelines: a case for international consensus? Respir Med 102:1830–1832
Engdahl O, Toft T, Boe J (1993) Chest radiograph–a poor method for determining the size of a pneumothorax. Chest 103:26–29
Hoi K, Turchin B, Kelly AM (2007) How accurate is the Light index for estimating pneumothorax size? Australas Radiol 51:196–198
Wilkerson RG, Stone MB (2010) Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 17:11–17
Kiley S, Tighe P, Hajibrahim O, Deitte L, Gravenstein N, Robinson A 3rd (2013) Retrospective computed tomography mapping of intrapleural air may demonstrate optimal window for ultrasound diagnosis of pneumothorax. J Intensive Care Med. doi:10.1177/0885066613488735
Volpicelli G, Boero E, Stefanone V, Storti E (2013) Unusual new signs of pneumothorax at lung ultrasound. Crit Ultrasound J 5:10. doi:10.1186/2036-7902-5-10
Druda D, Kelly AM (2009) What is the difference in size of spontaneous pneumothorax between inspiratory and expiratory X-rays? Emerg Med J 26:861–863
Choi BG, Park SH, Yun EH, Chae KO, Shinn KS (1998) Pneumothorax size: correlation of supine anteroposterior with erect posteroanterior chest radiographs. Radiology 209:567–569
Collins CD, Lopez A, Mathie A, Wood V, Jackson JE, Roddie ME (1995) Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. AJR Am J Roentgenol 165:1127–1130
O’Rourke JP, Yee ES (1989) Civilian spontaneous pneumothorax. Treatment options and long-term results. Chest 96:1302–1306
Flint K, Al Hilawi AH, Johnson NM (1984) Conservative management of spontaneous pneumothorax. Lancet ii:687–688
Ball CG, Kirkpatrick AW, Feliciano DV (2009) The occult pneumothorax: what have we learned? Can J Surg 52:E173–E179
Ball CG, Kirkpatrick AW, Fox DL, Laupland KB, Louis LJ, Andrews GD, Dunlop MP, Kortbeek JB, Nicolaou S (2006) Are occult pneumothoraces truly occult or simply missed? J Trauma 60:294–298
Ball CG, Kirkpatrick AW, Laupland KB, Fox DL, Litvinchuk S, Dyer DM, Anderson IB, Hameed SM, Kortbeek JB, Mulloy R (2005) Factors related to the failure of radiographic recognition of occult posttraumatic pneumothoraces. Am J Surg 189:541–546
Roberts DJ, Ball CG, Tiruta C, Kirkpatrick AW (2011) Image of the month. Tension occult pneumothorax. Arch Surg 146:1211–1212
Volpicelli G, Cardinale L, Berchialla P, Mussa A, Bar F, Frascisco MF (2012) A comparison of different diagnostic tests in the bedside evaluation of pleuritic pain in the ED. Am J Emerg Med 30:317–324
Agricola E, Arbelot C, Blaivas M, Bouhemad B, Copetti R, Dean A, Dulchavsky S, Elbarbary M, Gargani L, Hoppmann R, Kirkpatrick AW, Lichtenstein D, Liteplo A, Mathis G, Melniker L, Neri L, Noble VE, Petrovic T, Reissig A, Rouby JJ, Seibel A, Soldati G, Storti E, Tsung JW, Via G, Volpicelli G (2011) Ultrasound performs better than radiographs. Thorax 66:828–829
Blondeau B, Delour P, Bedon-Carte S, Leger MS, Chimot L (2012) Lung ultrasound to avoid catastrophic care for false pneumothorax. Intensive Care Med 38:1410–1411
Xirouchaki N, Kondili E, Prinianakis G, Malliotakis P, Georgopoulos D (2014) Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med 40:57–65
Sistrom CL, Reiheld CT, Gay SB, Wallace KK (1996) Detection and estimation of the volume of pneumothorax using real-time sonography: efficacy determined by receiver operating characteristic analysis. AJR Am J Roentgenol 166:317–321
Oveland NP, Lossius HM, Wemmelund K, Stokkeland PJ, Knudsen L, Sloth E (2013) Using thoracic ultrasonography to accurately assess pneumothorax progression during positive pressure ventilation: a comparison with CT scanning. Chest 143:415–422
Moore FO, Goslar PW, Coimbra R, Velmahos G, Brown CV, Coopwood TB Jr, Lottenberg L, Phelan HA, Bruns BR, Sherck JP, Norwood SH, Barnes SL, Matthews MR, Hoff WS, de Moya MA, Bansal V, Hu CK, Karmy-Jones RC, Vinces F, Pembaur K, Notrica DM, Haan JM (2011) Blunt traumatic occult pneumothorax: is observation safe?–results of a prospective, AAST multicenter study. J Trauma 70:1019–1023
Galbois A, Ait-Oufella H, Baudel JL, Kofman T, Bottero J, Viennot S, Rabate C, Jabbouri S, Bouzeman A, Guidet B, Offenstadt G, Maury E (2010) Pleural ultrasound compared with chest radiographic detection of pneumothorax resolution after drainage. Chest 138:648–655
Enderson BL, Abdalla R, Frame SB, Casey MT, Gould H, Maull KI (1993) Tube thoracostomy for occult pneumothorax: a prospective randomized study of its use. J Trauma 35:726–729
Brasel KJ, Stafford RE, Weigelt JA, Tenquist JE, Borgstrom DC (1999) Treatment of occult pneumothoraces from blunt trauma. J Trauma 46:987–990
Kirkpatrick AW, Rizoli S, Ouellet JF, Roberts DJ, Sirois M, Ball CG, Xiao ZJ, Tiruta C, Meade M, Trottier V, Zhu G, Chagnon F, Tien H, Canadian Trauma Trials Collaborative and the Research Committee of Trauma Association of Canada (2013) Occult pneumothoraces in critical care: a prospective multicenter randomized controlled trial of pleural drainage for mechanically ventilated trauma patients with occult pneumothoraces. J Trauma Acute Care Surg 74:747–754
Ouellet JF, Trottier V, Kmet L, Rizoli S, Laupland K, Ball CG, Sirois M, Kirkpatrick AW (2009) The OPTICC trial: a multi-institutional study of occult pneumothoraces in critical care. Am J Surg 197:581–586
O’Connor AR, Morgan WE (2005) Radiological review of pneumothorax. BMJ 330:1493–1497
Seow A, Kazerooni EA, Pernicano PG, Neary M (1996) Comparison of upright inspiratory and expiratory chest radiographs for detecting pneumothoraces. AJR Am J Roentgenol 166:313–316
Volpicelli G, Audino B (2011) The double lung point: an unusual sonographic sign of juvenile spontaneous pneumothorax. Am J Emerg Med 29:355.e1–355.e2
Volpicelli G, Garofalo G, Lamorte A, Frascisco MF (2012) Images in emergency medicine. Young man with left thoracic pain. Recurrent pneumothorax after failed pleurodesis. Ann Emerg Med 60:e3–e4
Volpicelli G, Lamorte A, Tullio M, Boero E, Stefanone V (2013) Worsening dyspnea and cough following thoracentesis. Chest 144:e1–e3
Acknowledgments
Support was provided solely from institutional and/or departmental sources. No sponsor funded the study.
Conflicts of interest
The authors have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: Lung ultrasound is useful to semi-quantify the size of pneumothorax at bedside by the localization of the lung point on the chest wall. The lateral progression of the lung point corresponds to significant increase in the entity of pneumothorax volumes.
Trial registry: Clinicaltrials.gov no. NCT01572584 (http://www.clinicaltrials.gov).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 4 (MPG 3502 kb)
Rights and permissions
About this article
Cite this article
Volpicelli, G., Boero, E., Sverzellati, N. et al. Semi-quantification of pneumothorax volume by lung ultrasound. Intensive Care Med 40, 1460–1467 (2014). https://doi.org/10.1007/s00134-014-3402-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3402-9