Abstract
Purpose
This study was undertaken to investigate the efficacy of red blood cell transfusion (RBCT) at reversing the deleterious effects of moderate anemia in critically ill, non-bleeding patients.
Methods
This was a retrospective, pair-matched (ratio 1:1) cohort study. Non-bleeding critically ill patients with moderate anemia (nadir hemoglobin level between 70 and 95 g/l), admitted to the ICU over a 27-month period, were included. Anemic patients were included upon meeting five matching criteria of having the same nadir hemoglobin (±5 g/l), APACHE II score (±5), SOFA score (±2), admission diagnostic group, and age (±5 years). Outcome events occurring over the whole ICU stay and after RBCT were collected. After hospital discharge, all patients had a 2-year follow-up period.
Results
Two hundred fourteen non-transfused anemic patients (NTAPs) were successfully matched with 214 transfused anemic patients (TAPs). In addition to the matching criteria, at baseline, both groups were homogenous with respect to multiple comorbidities. Compared with TAPs, NTAPs showed significantly lower rates of hospital mortality (21 vs.13 %, respectively; p < 0.05) and ICU re-admission (7.4 vs. 1.9 %, respectively; p < 0.05). Additionally, NTAPs had significantly lower rates of nosocomial infection (12.9 vs. 6.7 %, respectively; p < 0.05) and acute kidney injury (24.8 vs. 16.7 %, respectively; p < 0.05). Similar results were obtained in subgroup analysis where only more anemic patients (68 matched pairs) or patients with cardiovascular comorbidities (63 matched pairs) were considered.
Conclusions
RBCT does not improve the clinical outcome in non-bleeding critically ill patients with moderate anemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogeneic red blood cell transfusion (RBCT) remains a cornerstone therapy for treating anemia in critically ill patients. In fact, nearly 50 % of all critically ill patients receive at least one RBCT during their ICU stay [1–4].
Anemia may be well tolerated by healthy volunteers until they reach hemoglobin (Hb) levels of less than 50 g/l [5]. In contrast, severe anemia (Hb < 70 g/l) may induce tissue hypoxia and worsen the clinical outcome of patients presenting with cerebral dysfunction [6], acute coronary artery disease [7, 8], or sepsis [9], as well as of those undergoing major surgical procedures with significant blood loss [10, 11]. Therefore, as RBCT increases hemoglobin levels and arterial oxygen content [4], alleviating at least in theory oxygen tissue debt, it may benefit patients with severe, acute anemia.
However, at the ICU, more than two-thirds of RBCT are given to patients who presented with mild-to-moderate anemia and without acute blood loss [4]. In addition, although transfusion practices may vary among physicians and clinical settings [12–15], a relatively high transfusion trigger (hemoglobin 8.8 ± 2 g/dl) seems to be consistently applied in most ICUs [1–4], which means that critically ill patients are transfused to maintain hemoglobin levels above 9 g/dl.
In this regard, there is a large body of evidence suggesting that RBCT is in itself associated with increased risks of infectious complications, mortality, and prolonged hospital stays [1–4, 16, 17], whereas it has not been consistently demonstrated that the deleterious effects of moderate anemia may be reverted by RBCT [17]. This paucity of knowledge is at least in part due to the difficulty of separating adverse effects associated with anemia from those associated with RBCT.
We hypothesized that RBTC does not improve clinical outcomes of non-bleeding, moderately anemic critically ill patients. Therefore, this retrospective cohort study was designed to investigate the efficacy of RBCT in two matched populations of non-bleeding, moderately anemic critically ill patients: non-transfused anemic patients (NTAPs) (exposed to the risks of anemia) and transfused anemic patients (TAPs) (theoretically less exposed to the risks of anemia, but exposed to the risks of RBCT).
Materials and methods
Setting
This retrospective study was conducted at the multidisciplinary ICU of the teaching hospital “Virgen del Rocío,” which has a total of 40 beds and over 2,000 admissions per year. The Institutional Ethics and Research Committee approved this study and waived the need for requesting patient’s written informed consent.
At the ICU, all dedicated medical senior staff, trainees in critical care, and trainees from other specialties (such as anesthesiology, surgery, and other medical specialties) are allowed to make decisions on RBCT indications. A general guideline for blood transfusion (not exclusively for critically ill patients) is available at the hospital intranet and may be consulted at any time. This guideline suggests that transfusion decisions should be individualized in patients with nadir hemoglobin levels between 70 and 90 g/l, according to cardiopulmonary reserve, intra-vascular volume, and clinical symptoms. As a consequence, we [14] and other authors [12, 13, 15] have observed a high variability when prescribing RBCT to non-bleeding, moderately anemic critically ill patients.
Design
All patients admitted to the ICU from 1 January 2008 to 30 March 2010 were initially evaluated for inclusion in this study. Most ICU patients were admitted following general, cardiothoracic, transplant, vascular, or oncological surgeries. Non-surgical admissions included patients presenting with coronary disease, sepsis or decompensated chronic obstructive pulmonary disease.
Demographic, laboratory, and clinical data, including primary ICU admission diagnosis, morbid complications, length of ICU stay, and clinical outcome (during a 2-year follow-up period), were prospectively collected and retrospectively drawn from our database (GESTUCI: GESTion de enfermos de UCI), which has been described elsewhere [14]. GESTUCI incorporates a hemovigilance module, which enables users to continuously check the number of RBCTs, the pre-transfusion hemoglobin level, and multiple RBCT-related variables. Hemoglobin levels were measured daily throughout the patients’ ICU stay. GESTUCI was used to check all patients’ nadir hemoglobin levels.
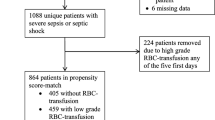
Inclusion criteria. Moderately anemic (nadir hemoglobin levels between 70 and 95 g/l), non-bleeding patients were initially assessed to be included in this retrospective study. Exclusion criteria. The following patients were excluded: (1) those with nadir hemoglobin <70 g/l since our clinicians believe that these severely anemic, critically ill patients should always be transfused; (2) patients with nadir hemoglobin >95 g/l, since there is a large body of evidence showing no benefit of RBCT for non-bleeding patients with nadir hemoglobin >95 g/l; (3) patients with active severe bleeding at the moment of the possible inclusion. Severe bleeding was defined by overt hemorrhage, hemorrhagic shock (bleeding patient with systolic arterial pressure of less than 90 mmHg) or having received more than five RBCTs within the previous 24 h prior to patient inclusion, and (4) patients with any restriction for receiving medical support.
Included patients were classified into two groups: TAP if they received at least one RBC transfusion while they presented with a nadir hemoglobin level between 70 and 95 g/l; NTAP if they did not receive RBCT while they presented with a nadir hemoglobin level between 70 and 95 g/l. For the propose of this study, included patients were further classified into five subgroups based on their admitting diagnoses: (1) postoperative period following non-cardiac surgery, including digestive, oncologic, thoracic, vascular, or solid organ transplant surgeries; (2) postoperative period following coronary artery bypass grafting, valve replacement, or cardiac transplantation; (3) sepsis or septic shock, including mainly patients presenting with community-acquired pneumonia, sepsis from surgical or urological causes, acute pancreatitis (Balthazar classification, grade “E”), or infective endocarditis; (4) coronary artery disease, including acute myocardial infarction, angina pectoris, or congestive heart failure; (5) other medical diagnosis, including patients presenting with re-exacerbation of chronic obstructive pulmonary disease and asthma, metabolic disturbances, medical intoxications, acute pulmonary embolisms, or acute hepatic failure.
Each NTAP was matched by investigators (SRLN and MJS) with a TAP, based on the fulfillment of all of the following five criteria of presenting with the same: (1) nadir hemoglobin level recorded throughout the ICU stay and pre-transfusion hemoglobin level (±5 g/l); (2) admission diagnostic group; (3) severity of illness at admission, as assessed by APACHE II score (±5); (4) severity of illness at the moment of matching, as assessed by SOFA score (±2); (5) age (±5 years). In cases where two or more matched TAPs were found, the subject with the same gender and the closest date to the NTAP admission date was chosen.
In addition to the matching variables, a set of clinical and laboratory baseline variables (Table 1) were gathered. After hospital discharge, all patients underwent a 2-year follow-up period by consulting the hospital database for patients’ clinical status or by phoning the patients or their relatives when it was not recorded.
The primary outcome measure was clinical outcome, including mortality and morbidity. Crude mortality rates in the ICU, during the hospital stay, and after 1 and 2 years of follow-up were considered. Morbidity based on the rate of hypoxemia, nosocomial infectious diseases, acute renal failure, ischemic cerebral accidents, and cardiac events was assessed. The length of ICU stay and the rate of readmission to the ICU were also considered. Readmission to the ICU was defined as admission occurring within 24 h from discharge. All these outcome variables were selected because they have been shown to correlate with both anemia and RBCT in a number of previous studies [1–4]. Only leukoreduced packed red cell units were given to all TAPs.
Definitions
Information about baseline comorbidities was obtained from the electronic records of the hospital, which are coded according to C.I.E.9. New onset (i.e., not present upon patient’s admission to the ICU) of the following events was recorded: (1) cardiac event, which includes myocardial infarction, angina pectoris, and congestive heart failure (chest radiograph interpreted as a new congestive heart failure in combination with compatible echocardiography and treatment with diuretics, angiotensin-converting enzyme inhibitors, and/or parenteral administration of vasoactive amines); (2) cerebral stroke, defined as persistent or transient ischemic neurologic events; (3) nosocomial infection and septic shock, including ventilator-associated nosocomial pneumonia, catheter-related bloodstream infection, primary bacteremia, sepsis, and septic shock, according to the definitions described elsewhere [9]; (4) hypoxemia, defined as a peripheral hemoglobin saturation, as assessed by pulse oximetry, of less than 90 % with a facial mask; (5) acute kidney injury, defined according to the RIFLE criteria [18].
All of these outcome variables were recorded for the whole ICU stay, independently of whether a specific outcome preceded the RBCT or not. Subsequently, the analysis was repeated only for those events that occurred after RBCT (Tables 1, 2, 3).
Statistical analysis
Sample size The null hypothesis that the proportion of 1 of the discordant paired results is equal to 0.50 was assessed by using McNemar’s test. In accordance with McNemar’s test of equality of paired proportions, a sample size of 200 pairs was needed to detect a difference in proportions of 0.10, with a 90 % power and a 0.05 two-sided significance level, when the proportion of discordant paired results is expected to be 0.20. Discordant paired results in excess of 20 % should be sufficient to conclude that the groups are not equivalent [19].
Analysis of variables Most variables were non-normally distributed, so the data are reported as median [interquartile range (IQR) 25–75 %] and percentages. Continuous variables were compared using Wilcoxon’s test. Dichotomous variables were analyzed by McNemar’s test. All statistical analyses were performed using a licensed computer software package [SPSS (Statistical Package for the Social Sciences) 18, SPSS, Inc., Chicago, IL], and a p value of less than 0.05 was considered significant.
Results
A flow chart of the matching process, including the reasons for patients’ exclusion, is depicted in Fig. 1. We obtained 214 pairs of patients who fulfilled the five matching criteria. No TAP matches were found for 81 NTAPs who were excluded from the study. No significant differences were observed between successfully matched and non-matched anemic patients (data not shown).
Baseline characteristics of both groups (NTAPs and TAPs) are shown in Table 1. In particular, all conditions that may be worsened by anemia (previous history of ischemic cardiomyopathy, respiratory insufficiency, and scorings of severity, as assessed by APACHE II at admission and SOFA at the time of matching, were homogeneously distributed between the two groups (Table 1). Moreover, the pH and base excess values, measured within a 12-h period before RBCT, were similar in both groups (Table 1).
TAPs were given two [1, 3] units of leukoreduced packed red blood cells, and most of them were transfused on the 2nd [1, 4] day of their ICU stay. For NTAPs, hemoglobin levels reach a nadir on the 4th day [3, 5] of their ICU stay. Interestingly, both groups showed an almost identical nadir hemoglobin level on the 2nd day of evolution [NTAP 84.1 (80, 89) g/l vs. TAP 83.3 (80, 88) g/l], when most TPAs received RBCT.
Most patients (317/428; 74.2 %) were admitted to the ICU on mechanical ventilation (Table 1). A total of 182 (42.5 %) of patients were admitted following general, non-cardiac surgery, and 88 [20.6 %] following cardiac surgery. Non-surgical patients composed the remaining 36.9 % (158/428) of ICU admissions.
Crude mortality was evaluated over different follow-up periods. When compared with NTAPS, in-hospital mortality was higher in TAPs, although this difference was no longer significant during the follow-up (Table 1). Similarly, TAPs also had higher rates of nosocomial infections and new onset of acute kidney injury, both over the entire length of ICU stay and when considering only the period after RBCT (Table 1). Finally, the length of the ICU stay and the rate of readmission were also higher in TAPs.
Given that the NTAPs with more severe anemia (Hb ≤ 80 g/l) might have worse clinical results than the matched TAPs, the whole analysis was repeated for these subgroups of patients (68 pairs), but the results were not significantly different from those obtained with the global sample (Table 2).
To rule out the possibility that having heart illness influences the results in the NTAPs, data from TAPs and NTAPs who were admitted to the ICU with diagnoses of coronary artery disease or after cardiac surgery (63 pairs) were separately analyzed. Once again, the results were not significantly different from those obtained with the global sample (Table 3).
Discussion
We compared the clinical outcomes of two populations of non-bleeding, moderately anemic critically ill patients fulfilling five matching criteria, which included two severity scores, nadir hemoglobin level, admitting diagnosis, and age. The first group consisted of patients exposed to anemia risks throughout their ICU stay (NTAPs). The second group comprised transfused patients who were, at least theoretically, less exposed to anemia risks, but exposed to RBCT risks (TAPs). Our results suggest that for non-bleeding, moderately anemic critically ill patients, RBCT does not confer any advantage in terms of reduced mortality and morbidity. Moreover, TAPs had poorer clinical outcomes than matched NTAPs, thus suggesting the potential adverse effects of RBCT.
Current clinical guidelines suggest that transfusion decisions should be influenced by data suggestive of tissue hypoxia and not solely by a pre-fixed cutoff hemoglobin value [3, 4]. Data on tissue hypoxia can be obtained from hemodynamic parameters, pulse oxymetry, lactate, and mixed venous oxygen saturation. In everyday practice, the vast majority of intensivists rarely measure these variables prior to prescribing a RBCT, and prospective observational surveys on blood use at the ICU reveal that ‘a low hemoglobin level’ is by far the most commonly reported reason for RBCT [1–4, 20].
However, the critical hemoglobin level that is considered harmful for patients and triggers RBCT varies widely among hospitals, disciplines, and physicians, leading to a great variability in RBCT practice [12–15]. Recently, an international multidisciplinary panel of experts reviewed the appropriateness of RBCTs in moderately anemic, non-bleeding patients and rated most indications (90 %) as inappropriate or uncertain [21], thus confirming our recent observations [14].
In addition, a hemoglobin level increment, as opposed to an oxygen utilization improvement, is generally regarded as RBCTsuccess. It is obvious that RBCTs may be life-saving in the context of acute anemia or severe bleeding [6–8], but there is little evidence of a benefit for non-bleeding medical or surgical patients with moderate anemia (Hb > 70 g/l) [1, 2, 15, 17], who actually receive a high percentage of all RBCTs [11, 17, 22, 23]. In fact, the available evidence suggests that the benefits of RBCT in these patient populations do not outweigh the risks [16, 17, 24].
We assessed the effects of anemia and RBCT in patients from five diagnostic groups. Classically, cardiac function has been thought to dictate the patient’s ability to tolerate anemia. However, a recent RCT documented that octogenarian patients who had either a history of or risk factors for cardiovascular disease, and whose hemoglobin level was below 100 g/dl after hip-fracture surgery, did not benefit from RBCT [23]. In cardiac surgery, recent guidelines suggest that postoperative RBCT is reasonable in most patients whose hemoglobin level is less than 70 g/l [8]. RBCT in low-risk cardiac surgery patients without complications may lead to increased rates of postoperative complications [3, 13, 14, 22]. Our data seem to corroborate these conclusions, as significantly longer length of ICU stay and higher rates of nosocomial infections, acute renal failure, and readmission to the ICU were observed in TAPs when compared with paired NTAPs (Table 1). The reasons why RBCT does not benefit these patients remain largely elusive, although they might include a decrease in cardiac output because of the increase in blood viscosity, the absence of acute anemia or severe bleeding, supply-independent oxygen consumption [25], and reduced ability of stored RBCs to upload oxygen [26].
The concept of blood transfusion is continuously evolving, from being part of the solution to being part of the problem. Two observational, multicenter studies, which included patients admitted to 198 European ICUs and were conducted 6 years apart, showed that the percentage of transfused patients was close to 35 % and did not vary greatly over the time [1, 27]. These data strongly suggest that the well-documented association between RBCT and poorer clinical outcomes has not significantly influenced transfusion practices. Therefore, appropriate training, education, and awareness are needed to improve decisions on RBCT, thus limiting the exposure to RBCT and RBCT-related risks.
Finally, it is worth noting that our study has limitations and strengths. Among its several limitations, the possible selection bias that may have occurred when comparing TAP versus NTAP could be the most important one. The need for RBCT might have selected a group of individuals at greater risk for adverse outcomes, and TAP could have been perceived as being sicker than NTAPS. Although TAP and NTAP presented the same nadir hemoglobin level at different days over their evolution (2nd vs. 4th day, respectively), the time course of daily hemoglobin concentrations was similar (at admission and the 2nd day) in both groups. These data are in agreement with a study including non-bleeding ICU patients that clearly showed that hemoglobin levels typically decline by >5 g/l/day during the first 2 days of the ICU stay [28]. However, as we compared two carefully matched populations of moderately anemic, non-bleeding patients, the risk for selection bias was greatly diminished, although not completely excluded. In this regard, it is important to stress that enormous variability in intra- and inter-center transfusion practices exits [12–15]. It is therefore conceivable that in our study this variability, rather than patient’s severity (as assessed by APACHE and SOFA scores), was responsible for giving RBCT or not to patients with similar clinical characteristics.
Among the strengths, both the study design and the study population should be quoted. Matching ensured that both groups were similar in terms of severity, admission diagnosis, and nadir hemoglobin, which are the most important confounding variables. Moreover, this research focused on non-bleeding, moderately anemic patients, a critically ill population that consumes a significant percentage of all transfusion resources without clear evidence of benefit. But, above all, our patient management database (GESTUCI) enabled us for documenting a temporal relation between RBCT and clinical outcome.
Conclusions
The results of this study suggest that using RBCT to increase Hb concentration in an attempt to improve tissue oxygen delivery in critically ill patients with moderate anemia is not associated with a clear clinical benefit and could result in adverse effects. Therefore, our data add to the evidence provided by the TRICC trial [29] and seem to further support the use of restrictive transfusion protocols in critically ill patients, including those with cardiovascular diseases.
Further research is needed to determine the role of RBCT in non-bleeding, moderately anemic patients. Meanwhile, prescribing RBCT to this patient population to merely increase hemoglobin levels should be strongly discouraged.
References
Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellman A, Nollet G, Peres-Bota D, ABC Investigators (2002) Anemia and blood transfusion in critically ill patients. JAMA 288:1499–1507
Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabott MM, Duh MS, Shapiro MJ (2004) The CRIT study: Anemia and blood transfusion in the critically ill—current clinical practices in the United States. Crit Care Med 32:39–52
Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, Hebert PC, Anderson GL, Bard MR, Bromberg W, Chiu WC, Cipolle MD, Clancy KD, Diebel L, Hoff WS, Hughes KM, Munshi I, Nayduch D, Sandhu R, Yelon JA, American College of Critical Care Medicine of the Society of Critical Care Medicine, Eastern Association for the Surgery of Trauma Practice Management Workgroup, American College of Critical Care Medicine of the Society of Critical Care Medicine, Eastern Association for the Surgery of Trauma Practice Management Workgroup (2009) Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med 37:3124–3157
Wang JK, Klein HG (2010) Red blood cell transfusion in the treatment and management of anaemia: the search for the elusive transfusion trigger. Vox Sang 98:2–10
Weiskopf RB, FeinerJ Hopf H, Lieberman J, Finlay HE, Quah C, Kramer JH, Bostrom A, Toy P (2006) Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology 104:911–920
Kramer AH, Zygun DA (2009) Anemia and red blood cell transfusion in neurocritical care. Crit Care 13:R89
Gerber DR (2008) Transfusion of packed red blood cells in patients with ischemic heart disease. Crit Care Med 36:1068–1074
Society of Thoracic Surgeons Blood Conservation Guideline Task Force, Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, Song HK, Clough ER, Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion, Shore-Lesserson LJ, Goodnough LT, Mazer CD, Shander A, Stafford-Smith M, Waters J, International Consortium for Evidence Based Perfusion, Baker RA, Dickinson TA, FitzGerald DJ, Likosky DS, Shann KG (2011) Update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Society of Thoracic Surgeons Blood Conservation Guideline Task Force; Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion. International Consortium for Evidence Based Perfusion. Ann Thorac Surg 91:944–982
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2008) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Med 34:17–60
Ranucci M, Conti D, Castelvecchio S, Menicanti L, Frigiola A, Ballotta A, Pelissero G (2010) Hematocrit on cardiopulmonary bypass and outcome after coronary surgery in nontransfused patients. Ann Thorac Surg 9:11–17
Carson JL, Noveck H, Berlin JA, Gould SA (2002) Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion 42:812–818
Shehata N, Burns LA, Nathan H, Hebert P, Hare GM, Fergusson D, Mazer CD (2012) A randomized controlled pilot study on adherence to transfusion strategies in cardiac surgery. Transfusion 52:91–99
Gombotz H, Rehak PH, Shander A, Hofmann A (2007) Blood use in elective surgery: the Austrian benchmark study. Transfusion 47:1468–1480
Leal-Noval SR, Arellano-Orden V, Maestre-Romero A, Múñoz-Gómez M, Fernández-Cisneros V, Ferrándiz-Millón C, Corcia Y (2011) Impact of national transfusion indicators on appropriate blood usage in critically ill patients. Transfusion 51:1957–1965
Sena MJ, Rivers RM, Muizelaar JP, Battistella FD, Utter GH (2009) Transfusion practices for acute traumatic brain injury: a survey of physicians at US trauma centers. Intensive Care Med 35:480–488
Leal-Noval SR, Rincón-Ferrari MD, García-Curiel A, Herruzo-Avilés A, Camacho-Laraña P, Garnacho-Montero J, Amaya-Villar R (2001) Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest 119:1461–1468
Marik PE, Corwin HL (2008) Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med 36:2667–2674
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup (2004) Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative [ADQI] Group. Crit Care 8:R204–R212
Elashoff JD. nQuery Advisor Version 4.0 User’s Guide 2008. Los Angeles, CA: Statistical Solutions Ltd
Istaphanous GK, Wheeler DS, Lisco SJ, Shander A (2011) Red blood cell transfusion in critically ill children: a narrative review. Pediatr Crit Care Med 12:174–183
Shander A, Fink A, Javidroozi M, Jochen E, Farmer SL, Corwin H, Goodnough LT, Hofmann A, Isbister J, Ozawa S, Spahn, for the International Consensus Conference on Transfusion Outcomes Group (2011) Appropriateness of allogeneic red blood cell transfusion: the international consensus conference of transfusion outcome. Transf Med Rev 25:232–246
Hébert PC, Yetisir E, Martin C, Blajchman MA, Wells G, Marshall J, Tweeddale M, Pagliarello G, Schweitzer I (2001) Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med 29:227–234
Carson J, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, Nemo G, Dragert K, Beaupre L, Hildebrand K, Macaulay W, Lewis C, Cook DR, Dobbin G, Zakriya KJ, Apple FS, Horney RA, Magaziner J, FOCUS Investigators (2011) Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 365:2453–2462
Möhnle P, Snyder-Ramos SA, Miao Y, Kulier A, Böttiger BW, Levin J, Mangano DT, Multicenter Study of Perioperative Ischemia (McSPI) Research Group (2011) Multicenter study of perioperative ischemia (McSPI) research group. Intensive Care Med 37:97–109
Madjdpour C, Spahn D, Weiskopf RB (2006) Anemia and perioperative red blood cell transfusion: a matter of tolerance. Crit Care Med 34(5 Suppl):S102–S108
Leal-Noval SR, Muñoz-Gómez M, Arellano-Orden V, Marín-Caballos A, Amaya-Villar R, Marín A, Puppo-Moreno A, Ferrándiz-Millón C, Flores-Cordero JM, Murillo-Cabezas F (2008) Impact of age of transfused blood on cerebral oxygenation in male patients with severe traumatic brain injury. Crit Care Med 36:1290–1296
Vincent JL, Sakr Y, Sprung C, Harboe S, Damas P, Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators (2008) Are blood transfusions associated with greater mortality rates? Results of the sepsis occurrence in acutely ill patients study. Anesthesiology 108:31–39
Ba VN, Peres-Bota D, Mélot C, Vincent J-L (2003) Time course of hemoglobin concentrations in nonbleeding intensive care unit patients. Crit Care Med 31:406–410
Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E (1999) A multicenter, randomized, controlled clinical trial on transfusion requirement in critical care. Transfusion requirements in critical care investigators. Canadian critical care trials group. N Engl J Med 340:409–417
Acknowledgments
This study was partially supported by funds from the Spanish Government (Fondo Investigación Sanitaria, FIS PI 08/1069, Consejería de Salud de la Junta de Andalucía, Proyectos de Investigación, PI 0367/2007 and PI 0320/2010) and the Fundación MAPFRE 2008–2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leal-Noval, S.R., Muñoz-Gómez, M., Jiménez-Sánchez, M. et al. Red blood cell transfusion in non-bleeding critically ill patients with moderate anemia: is there a benefit?. Intensive Care Med 39, 445–453 (2013). https://doi.org/10.1007/s00134-012-2757-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2757-z