Abstract
Purpose
The aim of this study was to explore the effect of red blood cell (RBC) transfusion on microdialysis-assessed interstitial fluid metabolic parameters in septic patients.
Methods
We conducted a retrospective study of 37 patients with severe sepsis/septic shock requiring transfusion of one to two RBC units. Interstitial fluid metabolic alterations were monitored by a microdialysis catheter inserted in the subcutaneous adipose tissue. Samples were collected before (T0) and after transfusion at two time-points: T1a and T1b; median post-transfusion times of 120 [interquartile range (IQR); 45–180] and 360 (IQR; 285–320) min. Lactate, pyruvate, glycerol and glucose concentrations were measured with a bedside analyzer, and the lactate/pyruvate (LP) ratio was calculated automatically.
Results
RBC transfusions decreased the LP ratio from (T0) 18.80 [interquartile range (IQR); 14.85–27.45] to (T1a) 17.80 (IQR; 14.35–25.20; P < 0.05) and (T1b) 17.90 (IQR; 14.45–22.75; P < 0.001), while there was also significant interindividual variation. Post-transfusion LP ratio changes at T1a [r = −0.42; 95 % confidence interval (CI), −0.66 to −0.098; P = 0.01] and T1b (r = −0.68; 95 % [CI], −0.82 to −0.44; P < 0.001) were significantly correlated with the pre-transfusion LP ratio, but not with baseline demographic characteristics, vital signs, severity scores, hemoglobin level and blood lactate. RBC storage time and leukocyte reduction had no influence on the tissue metabolic response to transfusion.
Conclusions
Tissue oxygenation is affected by RBC transfusion in critically ill septic patients. Monitoring of tissue LP ratio by microdialysis may represent a useful method for individual clinical management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia is very common among critically ill patients [1], and more significant in septic than non-septic subjects [2]. It is therefore not surprising that septic patients frequently receive red blood cell (RBC) transfusions during their intensive care unit (ICU) stays.
Since the publication of the TRICC trial [3], transfusion practices have shifted away [4] from the “10/30 rule” [5]. This trend was reinforced by numerous observational studies showing a worrisome association of RBC transfusion with mortality and other relevant clinical outcomes [6–9]. Undoubtedly, the ultimate goal of blood transfusion is the correction of tissue hypoxia, not the restoration of systemic hemodynamics or the achievement of an arbitrary hemoglobin value. Therefore, and despite the endorsement of RBC transfusion by either early-goal directed sepsis therapy [10] or the Surviving Sepsis Campaign Guidelines [11], it is obvious that there is no “one size fits all” hemoglobin trigger, and the decision to transfuse should take into consideration the tissue oxygenation status. Various markers of impaired global oxygenation (such as lactate, oxygen extraction ratio and venous oxygen saturation) have been studied as transfusion triggers [5, 12–14]. Unfortunately, in sepsis global oxygenation markers do not accurately reflect regional oxygenation because of microcirculation abnormalities and shunting [15].
Until the last decade, the lack of appropriate noninvasive techniques to monitor tissue hypoxia in vivo had hampered the study of the RBC transfusion effect on tissue oxygenation. Recently, orthogonal polarization spectral imaging (OPSI) and near-infrared spectroscopy (NIRS) techniques have been employed to evaluate the effect of RBC transfusion on the microcirculation [16] and tissue oxygenation [17] of septic patients. Neither of them showed a consistent effect of RBC transfusion despite remarkable interindividual variation. In addition, even if the total vascular density, perfused capillary density and tissue hemoglobin index had been significantly altered by RBC transfusion, this would not necessarily equate to improved cellular metabolism since sepsis is also characterized by marked mitochondrial dysfunction and cytopathic hypoxia [18].
Microdialysis (MD) is a minimally invasive and quite easy to perform bedside technique allowing continuous study of the tissue metabolic status. The measured metabolites provide information regarding the tissue redox status (lactate/pyruvate ratio, LP ratio), substrate availability (glucose) and degradation of glycerophospholipids in cell membranes (glycerol) [19]. Our research group has previously shown that MD can detect tissue metabolic abnormalities that differ in relation to the sepsis stage and hold prognostic potential [20, 21].
Even though the effect of transfusion on the MD-assessed LP ratio has been studied in patients with traumatic brain injury [22], to our knowledge, no clinical study has examined whether RBC transfusions affect tissue metabolism in septic patients. The aims of this study including septic, critically ill patients were: (1) to evaluate the effects of RBC transfusion on tissue metabolism, especially as judged by alterations of the LP ratio, using the MD technique, and (2) to search for possible correlation of these effects with the RBC storage time and prior leukocyte reduction.
Methods
Study population
The protocol was approved by the hospital’s Ethics Committee, and informed consent was obtained from patients’ relatives. In this retrospective study, we reviewed prospectively collected data from a convenience sample of critically ill, septic patients admitted to the 25-bed mixed adult ICU of a tertiary university hospital between March 2008 and July 2011. Critically ill patients analyzed in the current study are partly shared with other studies/publications by our research group [20, 21].
A patient was specifically included if he/she met the following criteria: (1) diagnosis of severe sepsis/septic shock and (2) indication for RBC transfusion as established by the attending physician. Sepsis presence and stages were diagnosed and defined, respectively, according to the criteria exemplified by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Committee [23].
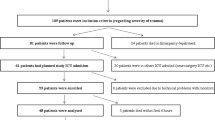
According to the research protocol, MD sampling was performed every 4 h for 6 days after sepsis onset. We defined as observation period of the relevant MD parameters the nearest 4-h time-point before (T0) and the two—spaced 4 h apart—sampling points (T1a and T1b) after the transfusion (details of the study time-line are presented in Fig. 1). We chose this post-transfusion monitoring period because, even though the required time for the transfusion of each RBC unit was the same (approximately 1 h), the exact time points were not standardized. According to our study design, MD monitoring in our ICU should not impose any interference with usual patient care; the exact standardization of transfusion in relation to the assessment time-points could be considered as a type of therapeutic interference.
In order to minimize the effect of other concurrently performed therapeutic interventions on our patients’ tissue metabolism and microcirculation and make our results comparable to similar research projects, we adopted the design and exclusion criteria of the previous studies in the field [16, 17]: a patient was excluded from our study if the doses of inotropic/vasopressor drugs and analgesic/sedative agents were modified during the 4-h period preceding transfusion and the 8-h period that included the time necessary for the transfusion and for extended monitoring. In addition, the presence of shock other than septic (i.e., hemorrhagic shock and active bleeding), the administration of a fluid challenge during the monitoring period and the lack either of a pre- or a post-transfusion MD sample represented exclusion criteria.
Microdialysis measurements and analysis
The details and potential applications of this technique have been detailed elsewhere [19]. Briefly, a microdialysis catheter (CMA 60, CMA Microdialysis AB, Stockholm, Sweden) is inserted upon sepsis onset under sterile conditions into the upper thigh’s subcutaneous adipose tissue, and the collected samples are analyzed for lactate, pyruvate, glycerol and glucose by an automatic analyzer (CMA 600 Microdialysis Analyzer, CMA Microdialysis AB, Stockholm, Sweden).
The relative MD LP ratio change was defined by the equation [(MD LP ratio post-transfusion − MD LP ratio pre-transfusion)/MD LP ratio pre-transfusion] × 100 %, where MD LP ratio post-transfusion was the measurement after transfusion at the time points T1a or T1b, and MD LP ratio pre-transfusion was the measurement at the time point T0 before transfusion.
Statistical analysis
Descriptive statistics were computed for all study variables. Data were tested for normal distribution by the Kolmogorov-Smirnov normality test. Results are presented as mean ± standard deviation (SD), medians (interquartile range: IQR) and counts (percentages), as appropriate, whereas differences between groups were assessed using a chi-square, Fisher’s exact test and Mann-Whitney U test, as indicated. Spearman (ρ) and Pearson (r) correlation coefficients assessed the associations between variables. In addition, where applicable, a linear regression line was computed with a 95 % confidence interval (CI). We employed the Friedman test with Dunn test correction and repeated-measures analysis of variance with Bonferroni’s correction to compare the evolution of non-normally and normally distributed variables in time.
All statistics were two-tailed, and P < 0.05 was considered statistically significant in all analyses. Statistical analysis was performed using GraphPad Prism 5.00 for Windows (GraphPad Software, San Diego, CA, USA) and IBM SPSS Statistics 20.0 for Mac Os.
In the electronic supplementary material (ESM), we provide additional data on data collection and measurements, microdialysis technique, RBC transfusion characteristics and statistical analysis [24–28].
Results
Study population
We initially screened 143 septic, critically ill patients. Among them, 63 (44.0 %) were transfused. We excluded data from 15 patients because doses of inotropic/vasopressor drugs and analgesic/sedative agents had been modified during the observation period, and 11 patients for technical reasons (insufficient sample, accidental catheter removal, etc.). Therefore, we finally recruited 37 patients. The details of their baseline characteristics are presented in Table 1.
Transfusion characteristics
The mean time elapsed from sepsis onset to transfusion was 5.8 ± 1.5 days. Five patients had received transfusions more than 48 h prior to protocol enrollment. Four patients (10.8 % of the whole cohort) received two RBC units. The median post-transfusion times until the first (T1a) and second (T1b) MD measurement were 120 (IQR; 45–180) and 360 (IQR; 285–320) min, respectively.
After transfusion, mean arterial pressure (MAP) increased from 79 ± 9 to 82 ± 10 (T1a vs. T0: P < 0.05) and 83 ± 10 mmHg (T1b vs. T0: P < 0.001), and hemoglobin concentration increased from 7.17 ± 0.99 to 8.27 ± 1.13 (T1a vs. T0: P < 0.001) and 8.20 ± 1.14 g/dl (T1b vs. T0: P < 0.001). We observed no other statistically significant change in arterial blood gases and vital signs (Table 2).
Transfusion effect on microdialysis-assessed LP ratio
Overall, RBC transfusion did not alter any of the MD-assessed parameters (i.e., lactate, pyruvate, glycerol and glucose) or blood lactate (Table 2). However, it was associated with a statistically significant decrease of the MD LP ratio from T0 [18.80 (IQR); 14.85–27.45] to T1a [17.80 (IQR); 14.35–25.20) (T1a vs. T0: P < 0.05) that persisted 4 h later at T1b [17.90 (IQR); 14.45–22.75] (P < 0.001) (T1b vs. T0: P < 0.001).
We observed interindividual variation (Fig. 2); 25 (67.6 %) patients showed a MD LP ratio decrease between time points T0 and T1a, and 32 (86.4 %) patients showed a MD LP ratio decrease between time points T0 and T1b. In only three (8.1 %) transfusion episodes, we found an initial drop of MD LP ratio followed by a later rebound to higher values.
There was no significant correlation between the relative change in MD LP ratio (T1a–T0) and age (Spearman ρ = 0.14; P = 0.41), gender (ρ = −0.28; P = 0.09), sepsis day on transfusion (ρ = 0.27; P = 0.10), SOFA score (ρ = −0.28; P = 0.09), pre-transfusion MAP (ρ = 0.16; P = 0.35), pre-transfusion heart rate (ρ = −0.26; P = 0.12), vasopressor dose (ρ = −0.19; P = 0.27), blood lactate (ρ = −0.30; P = 0.07) and post-transfusion Hb level increment (ρ = 0.01; P = 0.94). Similar results were obtained from the correlation analysis of the relative change in the MD LP ratio (T1b–T0) and the previously mentioned parameters.
The relative change in the MD-assessed LP ratio was negatively correlated to the MD LP ratio (T0) at both post-transfusion time points (at T1a: r = −0.42 [−0.66 to −0.098]; P = 0.01 and at T1b: r = −0.68 [−0.82 to −0.44]; P < 0.001 (Fig. 3 and ESM Fig. 1). The strength of correlation did not differ significantly between the two time points (z statistic = −0.94, P = 0.35). We repeated the analysis by plotting the absolute MD LP ratio changes versus baseline MD LP ratio, and the results stayed materially unchanged: the delta MD LP ratio (T1a–T0) versus the MD LP ratio: r = −0.52; P = 0.001 and the delta MD LP ratio (T1b–T0) versus the MD LP ratio: r = −0.80; P < 0.0001. After transfusion, patients with a baseline tissue LP ratio higher versus lower than 25 showed a larger relative LP ratio decrease (at T1a: −10.9 ± 8.7 % vs. −1.2 ± 15.2 %; P = 0.02 and at T1b: −20.4 ± 7.8 % vs. −3.4 ± 16.9 %; P < 0.001) (ESM Fig. 2). Of note, patients with baseline LP ratio higher versus lower than 25 did not differ in their demographic characteristics, vital signs, arterial blood gases, SOFA score, vasopressor dose, blood lactate and hemoglobin level.
Scatter plot representing the baseline MD LP ratio (T0) (x-axis) and the relative change in MD LP ratio (T1a–T0) [delta MD LP ratio (T1a–T0), %, y-axis]. Linear regression (solid line) with 95 % confidence intervals (CI; dashed lines) are fitted, where Y = 7.57 − 0.53X (P = 0.028) and Pearson r = −0.36 (95 % CI, −0.61 to −0.04; P < 0.05)
We conducted a multivariate analysis with the (T1a–T0) change of the MD LP ratio as the dependent outcome and (1) the post-transfusion change of the MAP, (2) the post-transfusion change of the Hb level and (3) the baseline MD LP ratio as the independent variables. We found that only the baseline MD LP ratio remained a statistically significant predictor (P = 0.002) of the MD LP ratio change.
Effect of RBC storage time and leukoreduction status
In ESM, we provide details regarding the insignificant effect of RBC storage time and leukoreduction status on the LP ratio.
Discussion
The principal findings of our study were that (1) as expressed by a significant drop in the microdialysis-assessed adipose tissue lactate/pyruvate ratio, RBC transfusion had a significant discernible effect on tissue metabolism in a group of critically ill septic patients and (2) there was a considerable interindividual response to RBC transfusion with several patients showing unchanged LP ratios, some improved and some deteriorated. The RBC storage time and leukoreduction status did not seem to be additional modifiers of this effect.
Another important study finding was that patients experiencing the greatest LP ratio change/decrease after transfusion were those with the largest impairment in their tissue oxygenation status—the highest LP ratios—before transfusion. This is in accordance with previous clinical data where, despite significant interindividual variability and an overall lack of observed association, improvements in microvascular reactivity and sublingual microvascular perfusion were noticed in patients with baseline altered microvascular perfusion [16] and reactivity [17], respectively. In addition, we found that patients with a large impairment in their tissue oxygenation had similar baseline demographic characteristics, vital signs, severity scores, hemoglobin and blood lactate levels to other patients. This lends further credence to the notion that tissue metabolism—similar to microcirculation—is often dissociated from the global macrocirculatory indices [29–31] and thus should be directly studied.
As previously mentioned, the tissue effect of RBC transfusion is largely unexplored, especially among septic patients. Recently, two studies failed to show a positive effect of RBC transfusion on tissue oxygenation markers [16, 17], despite the implementation of two separate techniques, namely OPSI and NIRS. Both techniques have limitations, such as intra- or inter-observer variability, semi-quantitative analysis, lack of specificity, etc. We used the LP ratio for technical as well as biochemical reasons. From the biochemical point of view, the tissue LP ratio is a well-established index of alterations taking place in the redox state of cells. From the technical point of view, the use of the ratio of two metabolites may ameliorate the effect of possible changes in MD catheter recovery as this would be expected to affect both lactate and pyruvate to a similar degree [19]. On the other hand, it is quite possible that the septic cohorts under study were not at the same metabolic/microcirculatory status during the sepsis episode and thus were not supposed to benefit equally from blood transfusion.
Even though we found a relationship between the changes in the tissue LP ratio and RBC transfusion, this did not seem to be affected by the RBC storage time. This finding should be viewed with caution since our study was probably not powered for detecting such significant differences, and a type II error is possible. Nevertheless, it is in line with similar studies [16, 17]. On the other hand, an earlier study [26] had shown gastric tonometry-assessed intra-mucosal pH dropping with the transfusion of RBC units stored for more than 2 weeks. The seemingly contradictory results can probably be explained by the heterogeneity of both the quality of the transfused RBCs, whose age is just one parameter, and the microvascular/metabolic status of the transfused patient, of which we have only a rudimentary understanding.
The delay in the institution of universal RBC leukoreduction in our hospital that took place during the conductance of this study gave us the opportunity to analyze the effect of leukoreduction on tissue oxygenation. Other studies in the field [16, 17] had not provided any data on this association since they were performed in a hospital employing universal leukoreduction. Our findings seem to support the notion that the clinical benefit of leukoreduction remains at least uncertain for the critically ill septic patients [32].
Our study has a number of limitations to consider. First, it is a single-center pilot study with a relatively small (albeit comparable to similar investigations [16, 17]), highly-selected sample of critically ill septic patients. Therefore, the extrapolation of our results to other clinical settings should be considered extremely premature. Second, we cannot provide any mechanistic explanation for the observed change in tissue oxygenation since we did not perform cardiac output or central/mixed venous blood saturation monitoring, and we did not measure the microcirculatory blood flow or hematocrit. In addition, capillary perfusion has been repeatedly shown to be independent of systemic hemodynamic effects [29–31], while the tissue hemoglobin index was not related to blood hemoglobin concentration in septic patients [17, 33]. Third, one might reasonably argue that, since both pre- and post-transfusion MD LP ratios were within the normal range, the small effect of RBC transfusion upon them is of questionable clinical significance. However, these are median values, and certainly among our patients there were many with MD LP ratios higher than the values considered “normal” (LP ratio >20–25). Therefore, changes in LP ratios in individual patients may be quite significant and clinically important. In addition, recent data suggest that, at least as far as blood lactate levels are concerned, the currently accepted normal range may need to be re-adjusted downwards [34]. Along the same lines, the increment of interstitial pyruvate in our study may be considered clinically irrelevant. However, in a number of diverse clinical settings, such as off-pump cardiac surgery [35] and traumatic brain injury [36], pyruvate has been shown to have beneficial effects and be a favorable prognostic factor. Fourth, despite being among our predetermined analyses, we did not collect any data regarding a possible differential effect of RBC transfusion on the MD LP ratio in early as opposed to late sepsis. Finally, and most importantly, outside the design of a randomized controlled trial and the presence of a control group, we cannot exclude the possibility that the observed metabolic alterations were due to either spontaneous changes in the patients’ clinical condition over time or to other therapeutic interventions [16, 17]. In light of the previously discussed limitations, we believe our study findings should be viewed as hypothesis-generating and preliminary awaiting further confirmation [37].
Conclusions
Transfusion of RBCs has a discernible effect on microdialysis-assessed tissue lactate/pyruvate ratio. This effect cannot be forecasted by patient’s clinical characteristics or conventional laboratory parameters and—with the caveat of possible type II error—it does not seem to be further modified by the RBC storage time or leukoreduction status. However, it is strongly associated with the baseline tissue redox status. Future studies may provide additional insight regarding the potential role of the LP ratio in guiding transfusion practices and tailoring them to the individual needs of critically ill patients.
References
Corwin HL, Krantz SB (2000) Anemia of the critically ill: acute anemia of chronic disease. Crit Care Med 28:3098–3099
Nguyen BV, Bota DP, Melot C, Vincent JL (2003) Time course of hemoglobin concentrations in nonbleeding intensive care unit patients. Crit Care Med 31:406–410
Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E (1999) A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care: Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 340:409–417
Blood Observational Study Investigators of ANZICS-Clinical Trials Group, Westbrook A, Pettilä V, Nichol A, Bailey MJ, Syres G, Murray L, Bellomo R, Wood E, Phillips LE, Street A, French C, Orford N, Santamaria J, Cooper DJ (2010) Transfusion practice and guidelines in Australian and New Zealand intensive care units. Intensive Care Med 36:1138–1146
Wang JK, Klein HG (2010) Red blood cell transfusion in the treatment and management of anaemia: the search for the elusive transfusion trigger. Vox Sang 98:2–11
Vincent J-L, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D, ABC (Anemia and Blood Transfusion in Critical Care) Investigators (2002) Anemia and blood transfusion in critically ill patients. JAMA 288:1499–1507
Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabot MM, Duh MS, Shapiro MJ (2004) The CRIT Study: anemia and blood transfusion in the critically ill—current clinical practice in the United States. Crit Care Med 32:39–52
Oliver E, Carrio ML, Rodriguez-Castro D, Javierre C, Farrero E, Torrado H, Castells E, Ventura JL (2009) Relationships among haemoglobin level, packed red cell transfusion and clinical outcomes in patients after cardiac surgery. Intensive Care Med 35:1548–1555
Möhnle P, Snyder-Ramos SA, Miao Y, Kulier A, Böttiger BW, Levin J, Mangano DT, Multicenter Study of Perioperative Ischemia (McSPI) Research Group (2011) Postoperative red blood cell transfusion and morbid outcome in uncomplicated cardiac surgery patients. Intensive Care Med 37:97–109
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative Group (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL, International Surviving Sepsis Campaign Guidelines Committee, American Association of Critical-Care Nurses, American College of Chest Physicians, American College of Emergency Physicians, Canadian Critical Care Society, European Society of Clinical Microbiology and Infectious Diseases, European Society of Intensive Care Medicine, European Respiratory Society, International Sepsis Forum, Japanese Association for Acute Medicine, Japanese Society of Intensive Care Medicine, Society of Critical Care Medicine, Society of Hospital Medicine, Surgical Infection Society, World Federation of Societies of Intensive and Critical Care Medicine (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
Orlov D, O’Farrell R, McCluskey SA, Carroll J, Poonawala H, Hozhabri S, Karkouti K (2009) The clinical utility of an index of global oxygenation for guiding red blood cell transfusion in cardiac surgery. Transfusion 49:682–688
Vallet B, Adamczyk S, Barreau O, Lebuffe G (2007) Physiologic transfusion triggers. Best Pract Res Clin Anaesthesiol 21:173–181
Vallet B, Robin E, Lebuffe G (2010) Venous oxygen saturation as a physiologic transfusion trigger. Crit Care 14:213
De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL (2002) Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 166:98–104
Sakr Y, Chierego M, Piagnerelli M, Verdant C, Dubois MJ, Koch M, Creteur J, Gullo A, Vincent JL, De Backer D (2007) Microvascular response to red blood cell transfusion in patients with severe sepsis. Crit Care Med 35:1639–1644
Creteur J, Neves AP, Vincent JL (2009) Near-infrared spectroscopy technique to evaluate the effects of red blood cell transfusion on tissue oxygenation. Crit Care 13:S11
Ruggieri AJ, Levy RJ, Deutschman CS (2010) Mitochondrial dysfunction and resuscitation in sepsis. Crit Care Clin 26:567–575
Ungerstedt U, Rostami E (2004) Microdialysis in neurointensive care. Curr Pharm Des 10:2145–2152
Kopterides P, Nikitas N, Vassiliadi D, Orfanos SE, Theodorakopoulou M, Ilias I, Boutati E, Dimitriadis G, Maratou E, Diamantakis A, Armaganidis A, Ungerstedt U, Dimopoulou I (2011) Microdialysis-assessed interstitium alterations during sepsis: relationship to stage, infection, and pathogen. Intensive Care Med 37:1756–1764
Dimopoulou I, Nikitas N, Orfanos SE, Theodorakopoulou M, Vassiliadi D, Ilias I, Ikonomidis I, Boutati E, Maratou I, Tsangaris I, Karkouli G, Tsafou E, Diamantakis A, Kopterides P, Maniatis N, Kotanidou A, Armaganidis A, Ungerstedt U (2011) Kinetics of adipose tissue microdialysis-derived metabolites in critically ill septic patients: associations with sepsis severity and clinical outcome. Shock 35:343–348
Zygun DA, Nortje J, Hutchinson PJ, Timofeev I, Menon DK, Gupta AK (2009) The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Crit Care Med 37:1074–1078
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, SCCM/ESICM/ACCP/ATS/SIS (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 31:1250–1256
Gogos C, Kotsaki A, Pelekanou A, Giannikopoulos G, Vaki I, Maravitsa P, Adamis S, Alexiou Z, Andrianopoulos G, Antonopoulou A, Athanassia S, Baziaka F, Charalambous A, Christodoulou S, Dimopoulou I, Floros I, Giannitsioti E, Gkanas P, Ioakeimidou A, Kanellakopoulou K, Karabela N, Karagianni V, Katsarolis I, Kontopithari G, Kopterides P, Koutelidakis I, Koutoukas P, Kranidioti H, Lignos M, Louis K, Lymberopoulou K, Mainas E, Marioli A, Massouras C, Mavrou I, Mpalla M, Michalia M, Mylona H, Mytas V, Papanikolaou I, Papanikolaou K, Patrani M, Perdios I, Plachouras D, Pistiki A, Protopapas K, Rigaki K, Sakka V, Sartzi M, Skouras V, Souli M, Spyridaki A, Strouvalis I, Tsaganos T, Zografos G, Mandragos K, Klouva-Molyvdas P, Maggina N, Giamarellou H, Armaganidis A, Giamarellos-Bourboulis EJ (2010) Early alterations of the innate and adaptive immune statuses in sepsis according to the type of underlying infection. Crit Care 14:R96
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Marik PE, Sibbald WJ (1993) Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA 269:3024–3029
Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, Denny C, Silliman CC (1999) Age of transfused blood is an independent risk factor for post injury multiple organ failure. Am J Surg 178:570–572
Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Büchele G, Simion D, Chierego ML, Silva TO, Fonseca A, Vincent JL, De Backer D (2010) Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med 36:949–955
De Backer D, Ortiz JA, Salgado D (2010) Coupling microcirculation to systemic hemodynamics. Curr Opin Crit Care 16:250–254
Jhanji S, Lee C, Watson D, Hinds C, Pearse RM (2009) Microvascular flow and tissue oxygenation after major abdominal surgery: association with post-operative complications. Intensive Care Med 35:671–677
Pottecher J, Deruddre S, Teboul JL, Georger JF, Laplace C, Benhamou D, Vicaut E, Duranteau J (2010) Both passive leg raising and intravascular volume expansion improve sublingual microcirculatory perfusion in severe sepsis and septic shock patients. Intensive Care Med 36:1867–1874
Lelubre C, Vincent JL (2011) Red blood cell transfusion in the critically ill patient. Ann Intensive Care 1:43
Doerschug KC, Delsing AS, Schmidt GA, Haynes WG (2007) Impairments in microvascular reactivity are related to organ failure in human sepsis. Am J Physiol Heart Circ Physiol 293:H1065–H1071
Nichol AD, Egi M, Pettila V, Bellomo R, French C, Hart G, Davies A, Stachowski E, Reade MC, Bailey M, Cooper DJ (2010) Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care 14:R25
Pojar M, Mand’ák J, Cibícek N, Lonský V, Dominik J, Palicka V, Kubícek J (2008) Peripheral tissue metabolism during off-pump versus on-pump coronary artery bypass graft surgery: the microdialysis study. Eur J Cardiothorac Surg 33:899–905
Timofeev I, Carpenter KL, Nortje J, Al-Rawi PG, O’Connell MT, Czosnyka M, Smielewski P, Pickard JD, Menon DK, Kirkpatrick PJ, Gupta AK, Hutchinson PJ (2011) Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain 134:484–494
Hollenberg SM (2010) Think locally: evaluation of the microcirculation in sepsis. Intensive Care Med 36:1807–1809
Acknowledgments
We gratefully acknowledge the contribution of the medical and nursing staff of the Second Department of Critical Care Medicine in “Attiko” University Hospital to the completion of this study. We also wish to recognize the technical expertise of Argyrios Diamantakis, RN, who dedicated long hours to helping us in this research project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kopterides, P., Theodorakopoulou, M., Nikitas, N. et al. Red blood cell transfusion affects microdialysis-assessed interstitial lactate/pyruvate ratio in critically ill patients with late sepsis. Intensive Care Med 38, 1843–1850 (2012). https://doi.org/10.1007/s00134-012-2635-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2635-8