Abstract
Purpose
Detailed extracorporeal membrane oxygenation (ECMO) weaning strategies and specific predictors of ECMO weaning success are lacking. This study evaluated a weaning strategy following support for refractory cardiogenic shock to identify clinical, hemodynamic, and Doppler echocardiography parameters associated with successful ECMO removal.
Methods
Hemodynamically stable patients underwent ECMO flow reduction trials to <1.5 L/min under clinical and Doppler echocardiography monitoring. When a patient had partially or fully recovered from severe cardiac dysfunction, tolerated the weaning trial, and had left ventricular ejection fraction (LVEF) >20–25% and aortic time–velocity integral (VTI) >10 cm under minimal ECMO support, device removal was considered.
Results
Among the 51 patients (34 males, aged 54 ± 14 years) who received ECMO for medical (n = 27), postcardiotomy (n = 11), or posttransplantation (n = 5) cardiogenic shock, 38 tolerated at least one ECMO flow reduction trial and 20 were ultimately weaned. Compared with the 13 patients who tolerated the trial but were not deemed weanable, those successfully weaned had, at each ECMO flow level, higher arterial systolic and pulse pressures, VTI, LVEF, and lateral mitral annulus peak systolic velocity (TDSa). All weaned patients had aortic VTI ≥10 cm, LVEF >20–25%, and TDSa ≥6 cm/s at minimal ECMO flow support. These Doppler echocardiography parameters better separated weaned and nonweaned patients than any other parameters tested.
Conclusions
Patients who tolerated a full ECMO weaning trial and had aortic VTI ≥10 cm, LVEF >20–25%, and TDSa ≥6 cm/s at minimal ECMO flow were all successfully weaned. However, further studies are needed to validate these simple and easy-to-acquire Doppler echocardiography parameters as predictors of subsequent ECMO weaning success in patients recovering from severe cardiogenic shock.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extracorporeal membrane oxygenation (ECMO) should be considered for rescuing patients with refractory cardiogenic shock [1–10]. It has been successfully used as a bridge to myocardial recovery, cardiac transplantation, or implantation of a ventricular assist device (VAD) in patients with overt cardiac failure’s various etiologies, e.g., acute myocardial infarction [7, 11], end-stage dilated cardiomyopathy [9], viral or toxic myocarditis [12–14], complications of cardiac surgery [8, 15, 16], or cardiac arrest [1, 2]. After a few days of mechanical assistance, the device can sometimes be successfully removed, when the patient has partially or fully recovered from the condition that indicated ECMO use. However, to date, detailed weaning strategies following ECMO initiation for refractory cardiogenic shock have never been reported, and only a few studies have evaluated outcome predictors following ECMO institution [1, 2, 4, 7, 9, 15, 17, 18]. Moreover, those studies were not specifically designed to predict which patients can be successfully weaned off ECMO.
Therefore, the objectives of this study are to describe a weaning strategy that tested daily hemodynamic tolerance of ECMO flow reduction trials and to identify clinical, hemodynamic, and Doppler echocardiography parameters associated with successful ECMO removal.
Patients and methods
Patients
This study was conducted between March 2007 and March 2008 at the Cardiology Institute of La Pitié-Salpêtrière Hospital in Paris in accordance with the ethical standards of our hospital’s Committee for the Protection of Human Subjects. Informed consent for demographic, physiological, and hospital outcome data analyses was not obtained, because this observational study did not modify existing diagnostic or therapeutic strategies. Before ECMO onset, the 51 patients included in the study displayed signs of acute refractory cardiogenic shock. Patients receiving venovenous ECMO or those with a mitral prosthesis or severe mitral valvulopathy were not included in the study. Additional details are provided in the Electronic Supplementary Material (ESM).
Clinical, hemodynamic, and echocardiographic parameters
The following data were recorded at time of ECMO onset: age; sex; indication for ECMO support; type of ECMO, peripheral femoral versus central intrathoracic; initiation under cardiopulmonary resuscitation (CPR); Simplified Acute Physiology Score (SAPS) II (range, 0–174) [19]; Sepsis-Related Organ Failure Assessment (SOFA) score [20]; concomitant use of an intra-aortic balloon pump (IABP); patients on mechanical ventilation, on intravenous inotropes, or receiving renal replacement therapy; blood gas analyses; blood lactate, serum creatinine; and prothrombin activity. Hemodynamic status was assessed daily by measuring systolic (SBP), diastolic (DBP) and mean (MBP) arterial blood pressure, pulse pressure (SBP–DBP), and heart rate.
Echocardiography was performed on a daily basis, and the following parameters were recorded: LV ejection fraction (LVEF); aortic time–velocity integral (VTI); transmitral early peak (E) and late diastolic velocities; spectral tissue Doppler lateral mitral annulus peak systolic (TDSa) and early diastolic (Ea) annular velocities [21]. LV filling pressures were estimated with the E/Ea ratio [22, 23]. Additional details are provided in the ESM.
ECMO weaning trials
An ECMO weaning trial was undertaken when the patient was considered hemodynamically stable, i.e., baseline MBP >60 mmHg while receiving no or low-dose vasoactive agents and a pulsatile arterial waveform maintained for at least 24 h, and when pulmonary blood oxygenation was not compromised. The ECMO flow was decreased to 66% for 10–15 min, then to 33% and/or to a minimum of 1–1.5 L/min for another 10–15 min. If MBP dropped significantly and was constantly <60 mmHg during the trial, ECMO flow was returned to 100% of the initial flow and the trial was stopped. Doppler echocardiography was repeated at each ECMO flow level by both intensive care unit (ICU) staff echocardiographists and the study echocardiographist (N.A.). When a patient was not suffering from an end-stage cardiac disease and had partially or fully recovered from the initial cardiac dysfunction, tolerated the full weaning trial, and had LVEF >20–25% and aortic VTI >10 cm under minimal ECMO support, ECMO removal was considered. If the patient remained stable after prolonged (15–20 min) complete-circuit clamping in the operating room, the machine was surgically removed and the mediastinum or femoral access surgically repaired. When ECMO weaning was deemed impossible, bridging to VAD or to transplantation was considered.
Outcome variables
For patients who tolerated maximal ECMO flow reduction, clinical and Doppler echocardiography parameters associated with successful ECMO weaning were evaluated the day before ECMO was successfully removed, or when they either died or were bridged to heart transplantation or VAD. Patients successfully weaned from ECMO were defined as those having ECMO removed and not requiring further mechanical support because of recurring cardiogenic shock over the following 30 days. Patients fulfilling the aforementioned ECMO removal criteria but not disconnected because a deadly complication occurred shortly after the successful weaning trial were excluded from the weaning outcome evaluation.
Other outcome variables included 30-day survival, duration of mechanical ventilation, need for renal replacement therapy, length of ICU stay, and ECMO-associated complications, i.e., cannulation-related injuries such as leg ischemia, femoral hemorrhage due to arterial laceration, deep vein or inferior vena cava thrombosis, cannula insertion-site infection, ischemic or hemorrhagic stroke, pulmonary edema, cardiac tamponade, or other technical problems.
Statistical analyses
Continuous variables were compared with Student’s t test, the Mann–Whitney U test, analysis of variance, or the Kruskal–Wallis statistic, as appropriate. Categorical variables were compared with chi-square tests. Assessment of intra- and interobserver reproducibility was performed on 10 echocardiographic and Tissue Doppler imaging (TDI) recordings selected at random (additional details provided in the ESM). Changes of mean clinical and echocardiographic parameter values from 100% to minimal ECMO flow were compared for weaned and nonweaned patients with repeated-measures analysis of variance. Statistical significance was defined as p < 0.05. Analyses were performed using StatView 5.0 (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics of the study population
Between March 2007 and March 2008, 51 patients (34 males, aged 54 ± 14 years) underwent ECMO for acute cardiogenic shock. Reasons for ECMO support are detailed in Table 1. Femoro-femoral ECMO was used for 26 (51%) patients, 13 (25%) had received an IABP before ECMO implantation, 9 (18%) experienced cardiac arrest during the 12 h preceding ECMO, and 7 (14%) were cannulated under continuous CPR. Mean SAPS II (69 ± 21) and SOFA score (14 ± 5) were high, reflecting disease severity at ICU admission (SAPS II-predicted mortality was >62%). Elevated blood lactate and creatinine, and low prothrombin activity also reflected severe multiorgan failure at ECMO onset.
Patients’ outcomes
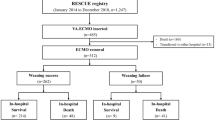
Among the 51 patients who received ECMO, 13 were permanently ECMO dependent and did not undergo or tolerate an ECMO weaning trial (Fig. 1). These patients were older, had more frequently suffered complicated cardiac surgery or cardiac arrest before ECMO initiation, had lower systolic blood pressure, and required more ECMO flow support (Tables 1 and E1, Electronic Supplementary Material). ECMO duration was significantly shorter for these 13 patients (Table 2), of whom 11 rapidly died of refractory multiple organ failure (Fig. 1) and only 2 received a heart transplant (1 survived at D30).
Thirty-eight patients tolerated at least one full ECMO weaning trial (Fig. 1), of whom 20 were ultimately weaned from the device (19 survived at D30, 1 died of radiological contrast-medium-induced anaphylactic shock). Among the 18 patients who were nonweaned, 5 had both LVEF >25% and VTI >10 cm at minimal ECMO flow and developed a fatal complication in the hours/days following the successful trial before being disconnected from the device (4 had severe ischemic or hemorrhagic neurological lesions which subsequently evolved towards brain death; 1 experienced rapidly fatal multiorgan failure due to septic shock). Of the other 13 patients, 4 received a heart transplant (4 survived at D30), 6 were bridged to another assist device (5 survived at D30), and 3 experienced rapidly fatal multiorgan failure due to septic shock (Fig. 1). Overall, successful ECMO weaning rates were lower for patients with ischemic or dilated cardiomyopathies (30% and 0%, respectively). ICU resource utilization was high, as reflected by prolonged ICU stays (18 days on average) and high percentages of patients on mechanical ventilation (46 patients, 90%) and receiving renal replacement therapy (16 patients, 31%).
Analysis of factors associated with successful ECMO weaning
Table 3, Table E2 (Electronic Supplementary Material), and Fig. 2 show hemodynamic and echocardiographic characteristics during the 24 h preceding successful ECMO weaning, death, or bridging to VAD or transplantation for the 33 patients who tolerated a full weaning trial (patients who had both LVEF >25% and VTI >10 cm at minimal ECMO flow and were not disconnected from ECMO because a deadly complication occurred shortly after a successful weaning trial were excluded from this analysis). The inter- and intraobserver reproducibilities of Doppler echocardiography parameters were always good (<10%) (Table E3 and additional details are provided in the ESM). Successfully weaned patients had lower baseline ECMO flows and, at each ECMO flow level, had higher SBP and pulse pressure, and, on Doppler echocardiography, higher aortic VTI, LVEF, and TDSa (Fig. 2). However, indices of LV filling pressure (pulsed Doppler mitral E and TDI Ea velocities and E/Ea) did not differ significantly between the two groups.
Evolution of clinical and Doppler echocardiography parameters for the 33 hemodynamically stable patients who tolerated maximal ECMO flow reduction at different flow levels for weaned (open triangles) and nonweaned (closed circles) patients. Significant differences existed between weaned and nonweaned patients (repeated-measures analysis of variance) for aortic VTI (p < 0.0001), TDSa (p < 0.001), LVEF (p = 0.04), and pulse pressure (p = 0.007), but not for mitral E wave or E/Ea. VTI time–velocity integral, TDSa spectral tissue Doppler imaging mitral annulus peak systolic velocity, E/Ea, ratio of transmitral early peak (E) diastolic velocity to spectral tissue Doppler lateral mitral annulus peak systolic early diastolic (Ea) annular velocity
Mean values for aortic VTI, TDSa, LVEF, and pulse pressure measured at minimal ECMO flow were higher for the group of weaned patients (Table 3). However, as indicated in Fig. 3, aortic VTI, TDSa, and LVEF at minimal ECMO flow better separated weaned from nonweaned patients than any other parameters tested. Specifically, all weaned patients had aortic VTI ≥10 cm, LVEF >20–25%, and TDSa ≥6 cm/s at minimal ECMO flow support.
Discussion
We observed in our cohort that up to 40% of patients who received ECMO for medical, postcardiotomy, or posttransplantation cardiogenic shock could be successfully weaned from the device. All these patients had partially or fully recovered from cardiogenic shock, had tolerated a full ECMO weaning trial, and had aortic VTI ≥10 cm, LVEF >20–25%, and TDSa ≥6 cm/s at minimal ECMO flow support. These Doppler echocardiography parameters discriminated better between weaned and nonweaned patients than any other parameters tested.
Only a few studies have examined outcome predictors following ECMO implantation, and most of these studies evaluated very early predictors of unsuccessful outcomes. Chen et al. [18] observed that five factors evaluated during the 96 h following ECMO initiation were associated with poorer outcomes: lung dysfunction, peak lactate >3 mmol/L, systemic infection, kidney dysfunction, and peak creatine kinase >10,000 U/L. Smedira et al. [4] reported that successful withdrawal from ECMO was associated with intraaortic balloon pump use and fewer infectious complications and organ failures. Recently, we also reported on a series of 81 patients, who received ECMO support for medical (n = 55), postcardiotomy (n = 16), or posttransplantation (n = 10) cardiogenic shock; independent predictors of ICU death were device insertion under cardiac massage, 24-h urine output <500 mL, prothrombin activity <50%, and female sex, while myocarditis was associated with better outcomes [7]. Still, all those studies were not specifically designed to identify which patients could be successfully weaned.
Indeed, detailed weaning strategies following ECMO initiation for refractory cardiogenic shock have never been reported. In most series described to date, weaning trials were not attempted during the first 24–72 h after ECMO implantation and consisted of gradually decreasing pump flow to 0.5–1.5 L/min for 1–10 min while evaluating heart contractility and cardiac index [1–3, 5, 9, 11, 15, 18]. However, none of those studies analyzed the predictive capability of the criteria they used for deciding ECMO withdrawal. Alternatively, our study described a detailed weaning strategy for a mixed population of medical–surgical ECMO patients who were hemodynamically stable after 24–48 h of mechanical assistance. We evaluated daily ECMO flow reduction tolerance, and clinical and Doppler echocardiography data were monitored before and during the weaning trial. Importantly, Doppler echocardiography parameters were recorded both by the ICU staff physician in charge of the patient and by the study echocardiographist, who was unaware of the medical decision to continue or remove the ECMO machine. Among the parameters monitored, those evaluating LV systolic function (TDSa, LVEF) and LV flow (aortic VTI) had the highest predictive values for successful weaning. LVEF and aortic VTI were higher at 100% ECMO flow for the group of ultimately weaned patients and increased significantly as ECMO flow was lowered, mostly because of increased preload and decreased afterload at the lowest assistance level. This effect was more apparent for weaned patients, who therefore displayed greater contractile reserves when challenged by the weaning trial. In contrast, TDSa was only modestly altered by flow reduction, suggesting its relative preload and afterload independence [24]. Lower ECMO flow before the weaning trial was also associated with successful weaning, reflecting reduced need for mechanical assistance in these patients. Lastly, Doppler parameters reflecting LV filling pressures (mitral E and TDI Ea velocities and E/Ea) did not differ significantly at each ECMO flow level, suggesting that preload conditions were comparable for the two groups and not predictive of weaning outcome. Therefore, markers of systolic (versus diastolic) LV function were better predictors of ECMO weaning success.
Several limitations should be mentioned when interpreting the results described herein. First, our data reflect the experience of a single center caring for patients with acute refractory cardiogenic shock. The ECMO weaning strategy and criteria we defined should be prospectively validated on other groups of patients from other institutions. Second, because of missing data, it was not possible to analyze day-by-day evolution weaning trials. However, we were able to analyze in detail data obtained during the last complete ECMO flow reduction trial, which preceded successful device removal, bridge to VAD or transplantation, or death. Indeed, this is a critical moment, when physicians must decide whether or not ECMO support can be safely removed. Third, we evaluated a mixed population of patients, who had received peripheral and/or central ECMO support, following medical, postcardiotomy, or posttransplantation cardiogenic shock. Detailed evaluation of each of these specific populations should be the focus of future studies. Fourth, since weaning was not attempted in all 38 patients who tolerated maximal ECMO flow lowering, we cannot exclude that some of the five nonweaned patients who had aortic VTI ≥10 cm and LVEF >20–25% would in fact have tolerated weaning if they had been offered the option. Lastly, the power of our analyses would have been higher and other factors might also have been associated with successful weaning if more patients had been included in this study. However, this is one of the largest population of patients tested to date, using a comprehensive clinical and echocardiographic strategy to evaluate the predictors of successful ECMO weaning.
In conclusion, our data indicate that weaning from ECMO in patients who received the device for medical, postcardiotomy, or posttransplantation refractory cardiogenic shock was achievable in up to 40% of cases. All weaned patients had partially or fully recovered from severe cardiac dysfunction, had tolerated a full ECMO weaning trial, and had aortic VTI ≥10 cm, LVEF >20–25%, and TDSa ≥6 cm/s at minimal ECMO flow support. These Doppler echocardiography parameters better separated weaned and nonweaned patients than any other parameters tested. However, further studies are needed to validate these simple and easy-to-acquire Doppler echocardiography parameters as predictors of subsequent ECMO-weaning success in patients recovering from severe cardiogenic shock.
References
Chen YS, Chao A, Yu HY, Ko WJ, Wu IH, Chen RJ, Huang SC, Lin FY, Wang SS (2003) Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol 41:197–203
Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, Chen WJ, Huang SC, Chi NH, Wang CH, Chen LC, Tsai PR, Wang SS, Hwang JJ, Lin FY (2008) Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 372:554–561
Bakhtiary F, Keller H, Dogan S, Dzemali O, Oezaslan F, Meininger D, Ackermann H, Zwissler B, Kleine P, Moritz A (2008) Venoarterial extracorporeal membrane oxygenation for treatment of cardiogenic shock: clinical experiences in 45 adult patients. J Thorac Cardiovasc Surg 135:382–388
Smedira NG, Moazami N, Golding CM, McCarthy PM, Apperson-Hansen C, Blackstone EH, Cosgrove DM 3rd (2001) Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at five years. J Thorac Cardiovasc Surg 122:92–102
Magovern GJ Jr, Simpson KA (1999) Extracorporeal membrane oxygenation for adult cardiac support: the Allegheny experience. Ann Thorac Surg 68:655–661
Pagani FD, Lynch W, Swaniker F, Dyke DB, Bartlett R, Koelling T, Moscucci M, Deeb GM, Bolling S, Monaghan H, Aaronson KD (1999) Extracorporeal life support to left ventricular assist device bridge to heart transplant: a strategy to optimize survival and resource utilization. Circulation 100(19 Suppl):206–210
Combes A, Leprince P, Luyt CE, Bonnet N, Trouillet JL, Leger P, Pavie A, Chastre J (2008) Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med 36:1404–1411
Doll N, Kiaii B, Borger M, Bucerius J, Kramer K, Schmitt DV, Walther T, Mohr FW (2004) Five-year results of 219 consecutive patients treated with extracorporeal membrane oxygenation for refractory postoperative cardiogenic shock. Ann Thorac Surg 77:151–157 discussion 157
Schwarz B, Mair P, Margreiter J, Pomaroli A, Hoermann C, Bonatti J, Lindner KH (2003) Experience with percutaneous venoarterial cardiopulmonary bypass for emergency circulatory support. Crit Care Med 31:758–764
Megarbane B, Leprince P, Deye N, Resiere D, Guerrier G, Rettab S, Theodore J, Karyo S, Gandjbakhch I, Baud FJ (2007) Emergency feasibility in medical intensive care unit of extracorporeal life support for refractory cardiac arrest. Intensive Care Med 33:758–764
Chen JS, Ko WJ, Yu HY, Lai LP, Huang SC, Chi NH, Tsai CH, Wang SS, Lin FY, Chen YS (2006) Analysis of the outcome for patients experiencing myocardial infarction and cardiopulmonary resuscitation refractory to conventional therapies necessitating extracorporeal life support rescue. Crit Care Med 34:950–957
Pages ON, Aubert S, Combes A, Luyt CE, Pavie A, Leger P, Gandjbakhch I, Leprince P (2009) Paracorporeal pulsatile biventricular assist device versus extracorporal membrane oxygenation-extracorporal life support in adult fulminant myocarditis. J Thorac Cardiovasc Surg 137:194–197
Asaumi Y, Yasuda S, Morii I, Kakuchi H, Otsuka Y, Kawamura A, Sasako Y, Nakatani T, Nonogi H, Miyazaki S (2005) Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J 26:2185–2192
Jan SL, Lin SJ, Fu YC, Chi CS, Wang CC, Wei HJ, Chang Y, Hwang B, Chen PY, Huang FL, Lin MC (2010) Extracorporeal life support for treatment of children with enterovirus 71 infection-related cardiopulmonary failure. Intensive Care Med 36:520–527
Muehrcke DD, McCarthy PM, Stewart RW, Foster RC, Ogella DA, Borsh JA, Cosgrove DM 3rd (1996) Extracorporeal membrane oxygenation for postcardiotomy cardiogenic shock. Ann Thorac Surg 61:684–691
Minev PA, El-Banayosy A, Minami K, Kortke H, Kizner L, Korfer R (2001) Differential indication for mechanical circulatory support following heart transplantation. Intensive Care Med 27:1321–1327
Fiser SM, Tribble CG, Kaza AK, Long SM, Zacour RK, Kern JA, Kron IL (2001) When to discontinue extracorporeal membrane oxygenation for postcardiotomy support. Ann Thorac Surg 71:210–214
Chen YS, Ko WJ, Chi NH, Wu IH, Huang SC, Chen RJ, Chou NK, Hsu RB, Lin FY, Wang SS, Chu SH, Yu HY (2004) Risk factor screening scale to optimize treatment for potential heart transplant candidates under extracorporeal membrane oxygenation. Am J Transplant 4:1818–1825
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Vieillard-Baron A, Slama M, Cholley B, Janvier G, Vignon P (2008) Echocardiography in the intensive care unit: from evolution to revolution? Intensive Care Med 34:243–249
Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA (1997) Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 30:1527–1533
Combes A, Arnoult F, Trouillet JL (2004) Tissue Doppler imaging estimation of pulmonary artery occlusion pressure in ICU patients. Intensive Care Med 30:75–81
Park YS, Park JH, Ahn KT, Jang WI, Park HS, Kim JH, Lee JH, Choi SW, Jeong JO, Seong IW (2010) Usefulness of mitral annular systolic velocity in the detection of left ventricular systolic dysfunction: comparison with three dimensional echocardiographic data. J Cardiovasc Ultrasound 18:1–5
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aissaoui, N., Luyt, CE., Leprince, P. et al. Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med 37, 1738–1745 (2011). https://doi.org/10.1007/s00134-011-2358-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2358-2