Abstract
Purpose of the Review
The purpose of this review is to describe the outcomes following refractory cardiogenic shock (CS) requiring mechanical circulatory support and factors associated with successful and unsuccessful weaning from VA-ECMO. Based on the presented data, we will propose a weaning and bridging algorithm with the aim of facilitating this complex process.
Recent Findings
Refractory CS requiring VA-ECMO support is associated with high early mortality. Approximately 1/3 of the patients weaned from ECMO do not survive until hospital discharge. When evaluating the ability to wean from ECMO etiology of CS, hemodynamics, end-organ function, pulmonary blood oxygenation, metabolic status, and echocardiographic assessments must be considered. When cardiopulmonary function is not recoverable, heart replacement therapies (HRT) should be considered early as patients may have better outcomes.
Summary
Durable weaning from VA-ECMO is obtainable in well-selected patients. Patients should be separated from the ECMO circuit in the presence of myocardial recovery, hemodynamic stability, and restored end-organ function. If myocardial recovery is unsatisfactory (severe LV dysfunction), HRT should be considered early in suitable candidates. Future research is needed to identify predictors of sustained myocardial recovery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of venoarterial extracorporeal membrane oxygenation (VA-ECMO) and other short-term circulatory assist devices to support patients in refractory cardiogenic shock (CS) has rapidly increased in the US over the last decade [1, 2]. VA-ECMO can be used as the first line of support in patients with refractory cardiopulmonary failure due to various etiologies [3,4,5,6,7,8,9,10,11] as a bridge to recovery [12,13,14,15,16,17,18,19, 20•] or bridge to advanced heart replacement therapies (HRT), including left ventricular assist devices [21,22,23,24] (LVADs) and orthotopic heart transplantation (OHT) [25,26,27]. VA-ECMO support allows hemodynamic and metabolic stabilization with restoration of end-organ perfusion, and, after a few days of support, the patient can be weaned and separated from the ECMO circuit if there are signs consistent with recovery of cardiopulmonary function. Not always, however, complete, or even partial recovery is obtainable and different strategies (HRT) need to be considered. Indeed, successful weaning from VA-ECMO has been reported anywhere between 30 and 70% [12, 14, 16,17,18,19, 25] depending on the etiology of CS, center’s expertise, and definition of successful weaning [12, 14, 15, 28,29,30, 31•]. Another important consideration is the fact that even when weaning from ECMO support is successful, a positive outcome for the patient does not necessarily follow [20•]. In fact, approximately 1/3 of the patients weaned from ECMO do not survive until hospital discharge [13, 17, 20•, 32]. Limited literature assessing the discrepancy between weaned and discharged patients is available and the appropriate interventions to reduce this gap remain poorly studied and described. When cardiopulmonary function is not recoverable, HRT should be considered; however, timing, choice of therapy/device, and patient selection are still mostly based on physician’s experience without defined guidelines. When assessing the timing and ability to wean from ECMO etiology of CS, hemodynamics, end-organ function, pulmonary blood oxygenation, metabolic status, and echocardiographic assessments must be considered. In our experience [20•], durable and sustained weaning from VA-ECMO with acceptable functional status at mid-term follow-up is obtainable in well-selected patients; however, patients who were bridged to recovery had worse mid-term outcomes than those who were bridged to HRT. This may suggest that patients with inadequate myocardial recovery should be evaluated early for HRT, in the absence of absolute contraindications. This remains, however, a single center experience with other studies presenting different findings [19]. Long-term outcomes of this patient population have been rarely described and even more scarce information are available regarding quality of life, functional status, and echocardiographic data. In this manuscript, we will discuss important considerations during weaning trials, factors associated with successful and unsuccessful weaning from support, and short- and mid-term outcomes of VA-ECMO destinations. Considering the presented data, we will propose an algorithm to facilitate weaning and bridging strategies.
“Successful Weaning”
The definition of successful weaning from VA-ECMO support is arbitrary and varies from center to center. In some cases, weaning is considered successful if the patient is alive 48 h post-decannulation [8, 33, 34] regardless of survival until hospital discharge. Others consider weaning successful if the patient is alive 30 days after decannulation without recurrence of CS and need for mechanical circulatory support (MCS) [12, 30, 31•]. In our experience, we considered weaning successful if the patient is alive until hospital discharge [20•]. These 2 last definitions seem to be the most appropriate as they are clinically relevant. We have, however, reported a 1-year survival (post weaning) around 50% for patients who were weaned from ECMO with a left ventricular ejection fraction (LVEF) < 30% [20•]. Should we consider this successful weaning? Not only the ability to separate the patient from the ECMO circuit but also the durability of recovery is crucial to define successful weaning. It will be important to develop a standardized definition of successful weaning as long-term outcomes and quality of life data will become available over the next 5–10 years. This will allow for a better understanding and improved patient selection for recovery or HRT.
VA-ECMO as a Bridge to Recovery
The first consideration when bridging a patient to recovery is the reversibility of the CS etiology. Patients with an acute on chronic heart failure (HF) decompensation may be less likely to recover baseline function and ECMO support should be carefully considered to avoid futility [35]. By contrast, patients suffering acute myocarditis or primary graft dysfunction post-OHT have generally higher chances of complete recovery [36,37,38]. A few studies have identified factors associated with successful weaning from VA-ECMO and in-hospital mortality after weaning from support [12,13,14,15,16,17,18,19, 20•] (Table 1). All of these are small retrospective observational studies which represent the main limitation when interpreting the results and drawing conclusions. As mentioned above, when assessing the timing and ability to wean from ECMO hemodynamics, end-organ function, pulmonary blood oxygenation, metabolic status, and echocardiographic assessments must be considered.
Hemodynamics
Hemodynamic parameters, monitored with an arterial line and a Swan-Ganz catheter, are useful real-time indicators of critical illness during weaning trials. Patients are considered acceptable candidates for weaning in the presence of a pulsatile arterial waveform for > 24 h, mean arterial pressure (MAP) > 60 mmHg on no or low-dose inotropes (epinephrine < 5 mg/min, dopamine < 6 mg/min, and milrinone < 0.375 mg/min) and vasopressors (vasopressin < 0.02 mg/min and norepinephrine < 5 mg/min), cardiac index > 2.4 L/min/m2, central venous pressure < 17 mmHg, and pulmonary capillary wedge pressure < 18 mmHg [12, 19, 20•, 31•, 39]. Pulse pressure has been reported as an important indicator of successful weaning by two studies [12, 15] even though a predictive threshold was not identified. In both studies, patients successfully weaned had a pulse pressure > 50 mmHg. When assessing hemodynamics during weaning trials, the presence of a normal pulse pressure (> 40 mmHg) should be taken into consideration.
End-Organ Function and Metabolic Status
Before initiating a weaning trial, it is important that appropriate end-organ perfusion and function have been recovered. Satisfactory metabolic status (lactate < 2.5 mmol/L) and hepatic function (transaminases < 100 U/L) are required. Normal renal function is not mandatory as often these patients would be on continuous renal replacement therapy (RRT) because of acute kidney injury (AKI) even though low urine output, AKI, and continuous venovenous hemofiltration (CVVH) have been associated with mortality after weaning from support [13, 15, 25]. Blood lactate is an important indicator of end-organ perfusion and baseline lactate level and lactate clearance after ECMO initiation have been suggested as important predictors of in-hospital mortality in post-cardiotomy shock [16].
Pulmonary Blood Oxygenation and Left Ventricular Unloading
Pulmonary blood oxygenation should not be impaired and the PaO2/FiO2 ratio should be > 200 with an FiO2 of 21% on the circuit and < 60% on the ventilator [31•]. In our experience, pulmonary dysfunction at the time of weaning had a strong correlation with poor outcomes suggesting that ECMO weaning should be avoided in the presence of existing pulmonary complications, including pulmonary congestion [20•]. Aissaoui and colleagues suggested that weaning from VA-ECMO should be avoided when the PaO2/FiO2 ratio is less than 100, and patients otherwise presenting with improved cardiac function should be converted to VV-ECMO [30]. A study by Boulate and colleagues [40] suggested that VA-ECMO is associated with a number of risk factors that could lead to acute lung injury in patients who undergo LVAD placement after ECMO support. In their study, 15 of 55 patients developed severe acute lung injury after LVAD implantation with PaO2/FiO2 less than 200, which was associated with mortality in 90% of the patients. The presence of increased pulmonary dysfunction as a risk factor for poor outcomes in patients undergoing VA-ECMO was also suggested by Pappalardo and colleagues [41] in a recent study assessing the efficacy of VA-ECMO in combination with an Impella device (ABIOMED) to unload the LV. Considering the weight of pulmonary dysfunction as a risk factor for mortality, the decision to convert to VV-ECMO should be considered more liberally in the presence of moderate to severe pulmonary dysfunction or only modest radiologic improvement at the time of VA-ECMO weaning. The retrograde arterial flow of peripheral VA-ECMO increases LV afterload, myocardial oxygen demand, and impedes aortic valve opening contributing to pulmonary venous congestion and edema [42]. Therefore, LV unloading strategies may be beneficial in this setting; however, definite evidence is still lacking and undergoing clinical trials will help clarify this controversial topic.
Echocardiography
Transesophageal echocardiography (TEE) is an essential part of current weaning trials and allows for real-time assessment of cardiac function recovery and residual valvular abnormalities [14, 18]. A few studies have reported how ECMO support affects LV and right ventricle (RV) function and how heart and valve function at the time of weaning can affect patients’ outcomes [12, 15, 18, 20•]. Pappalardo and colleagues observed higher mortality in patients with persistent RV failure [15]. Aissaoui and colleagues showed that patients who failed a weaning trial had a significantly lower aortic velocity time integral, LVEF, and lateral mitral annulus peak systolic velocity. They observed a 100% weaning rate in patients with an aortic velocity time integral 10 cm or greater and LVEF greater than 20% to 25%. Huang et al. showed that a right ventricular ejection fraction (RVEF) > 24.6% was associated with higher weaning success and lower in-hospital mortality [18]. In our experience, we observed that any grade of residual mitral regurgitation after weaning from support was associated with higher in-hospital mortality and the presence of severe LV dysfunction (LVEF < 30%) was significantly associated with lower survival after weaning [20•]. A TEE-based weaning protocol is recommended in the presence of certified personnel and equipment.
Proposed Weaning and Bridging Algorithm
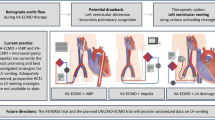
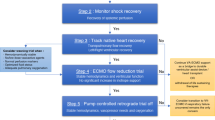
Based on the evidence presented, we propose a VA-ECMO weaning and bridging algorithm (Fig. 1) with the aim of providing guidance during this complex process. A similar approach was presented by Ortuno and colleagues [31•]. Both algorithms highlight the importance of (i) recoverable cardiac function, (ii) hemodynamic stability and restored end-organ function, and (iii) continuous TEE assessment. We also provide insight into bridging strategies to HRT. In the presence of (i) failure of multiple weaning trials, (ii) severe LV dysfunction, (iii) moderate to severe RV dysfunction and valvular abnormalities, and (iv) prolonged ECMO duration (> 14 days), HRT should be considered early, in the absence of contraindications. Prolonged ECMO duration has been identified as an independent factor associated with in-hospital mortality [15, 20•], suggesting that when cardiac function does not show potential for improvement, it is important to consider HRT early rather than extending ECMO support in an attempt for recovery.
Proposed VA-ECMO weaning and bridging algorithm. VA-ECMO, venoarterial extracorporeal membrane oxygenation; LVAD, left ventricular assist device; OHT, orthotopic heart transplant; LVEF, left ventricular assist device; VTI, velocity time integral; TDSa, tissue Doppler lateral mitral annulus peak systolic velocity; MAP, mean arterial pressure; AKI, acute kidney injury; TEE, transesophageal echocardiography; RRT, renal replacement therapy; RVEF, right ventricular ejection fraction; HRT, heart replacement therapy; CVP, central venous pressure; PCWP, pulmonary capillary wedge pressure; VV-ECMO, venovenous extracorporeal membrane oxygenation
VA-ECMO as a Bridge to HRT
When cardiac function does not recover, ECMO support can be used as a bridge to LVAD or OHT safely and effectively in selected patients with acceptable mid-term quality of life [22,23,24, 26, 27]. The few studies, however, that have analyzed this patient population (Table 1) were all observational and included a limited number of patients providing a low level of evidence. Marasco and colleagues showed that bridging INTERMACS I and II patients to LVAD with VA-ECMO is an effective strategy to allow for end-organ function recovery with outcomes comparable to those who underwent LVAD implantation without pre-operative ECMO support [23]. In a similar study, Unai et al. found no difference in outcomes between INTERMACS 1 patients bridged to LVAD with and without ECMO, with comparable qualify at life at 1-year follow-up [24]. Fukuhara et al., in an analysis of the UNOS database, found that patients bridged to OHT with VA-ECMO had significantly worse short- and mid-term outcomes than those bridged with LVAD [26]. By contrast, in a study from Spain, Barge-Caballero and colleagues showed no difference in outcomes in patients bridged to OHT with VA-ECMO compared to those bridged with conventional support [43]. These conflicting results may be due to differences in the organ allocation system between the United States (US) and some European countries. In the US, direct bridging to OHT with VA-ECMO is rare [26] due to excessively long waitlist time for these patients resulting in high waitlist mortality. In Spain, where heart transplant candidates have shorter waitlist time, VA-ECMO as a direct bridge to OHT is much more common [43]. Over the next few years, we may however observe a shift toward the use of VA-ECMO as direct bridge in the US where a new allocation system favoring MCS and high acuity was introduced at the end of 2017. The choice of the most appropriate strategy for each patient remains limited by the low level of evidence currently available and also influenced by national and regional policies. Ongoing randomized clinical trials will help improve patient selection and guide the development of improved allocation policies.
VA-ECMO as a Bridge to Recovery vs VA-ECMO as a Bridge to HRT
To our knowledge, only two studies have compared outcomes of patients bridged to recovery to those bridged to HRT. Garan and colleagues, in a recent retrospective study including only patients with acute MI supported with VA-ECMO, did not find any statistically significant difference in short-term survival between patients weaned and discharged without HRT and patients who failed weaning and who underwent HRT [19]. In our experience, we found a significant difference in mid-term survival between these 2 groups, favoring the use of HRT over nonselective ECMO weaning, which suggests that some patients who are weaned from ECMO may have compromised mid- and long-term outcomes and should be considered for HRT if they are suitable candidates [20•]. In our study, the decrease in patient survival from ECMO weaning to discharge is an important signal that requires further analysis in an attempt to improve patient selection and outcomes.
Short- and Mid-term Outcomes
Refractory CS requiring ECMO support is associated with high early mortality. Around 20 to 50% of the patients placed on VA-ECMO die while on support requiring withdrawal of care (Table 1). As mentioned above, about 1/3 of the patients who are weaned from support die in-hospital and 15 to 50% of those who survive until hospital discharge die within a year from discharge. Despite all the improvements in technologies and patient management over the last decade, it is evident that only a fraction of all the patients placed on VA-ECMO for refractory CS survive beyond 1 year. This highlights large gaps in current knowledge and understanding, especially with regard to strategies to achieve myocardial recovery [44]. Only few studies have reported mid-term outcomes [17, 20•, 24, 26] with a 3-year survival between 40 and 67%. Even fewer studies have reported quality of life data. In our experience, around 50% of the patients were free from HF symptoms (NYHA 1) at mid-term follow-up and 50% had at least one readmission to the hospital due to HF exacerbation [20•]. Longer term outcomes (5–10 years) are still lacking and they will be essential for better understanding and improving patient selection for recovery or HRT. Generally, it may seem that patients who undergo HRT have better short-term outcomes (Table 1). However, no definitive answer can be drawn from the available data considering the low level of evidence of small retrospective single-center cohort studies including heterogenous etiologies of CS. Future studies will be needed to identify factors predictive of durable myocardial recovery and to address the role and timing of HRT in this complex patient population.
Conclusion
Refractory CS requiring short-term mechanical circulatory support remains associated with high early mortality. Weaning trials should be performed under continuous TEE assessment and patients should be separated from the ECMO circuit in the presence of myocardial recovery, hemodynamic stability, and restored end-organ function. If myocardial recovery is incomplete with severe LV dysfunction, HRT should be considered early as patients may have better outcomes. Future studies will need to focus on (i) the identification of factors predictive of durable myocardial recovery, (ii) the development of standardized definition of successful weaning from ECMO support and standardized weaning and bridging algorithms, (iii) and the identification of appropriate timing and patient selection for HRT.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
McCarthy FH, McDermott KM, Kini V, Gutsche JT, Wald JW, Xie D, et al. Trends in U.S. extracorporeal membrane oxygenation use and outcomes: 2002-2012. Semin Thorac Cardiovasc Surg. 2015;27(2):81–8.
Guglin M, Zucker MJ, Bazan VM, Bozkurt B, El Banayosy A, Estep JD, et al. Venoarterial ECMO for adults. J Am Coll Cardiol. 2019;73(6):698–716.
Chen J-S, Ko W-J, Yu H-Y, Lai L-P, Huang S-C, Chi N-H, et al. Analysis of the outcome for patients experiencing myocardial infarction and cardiopulmonary resuscitation refractory to conventional therapies necessitating extracorporeal life support rescue. Crit Care Med. 2006;34(4):950–7.
Schwarz B, Mair P, Margreiter J, Pomaroli A, Hoermann C, Bonatti J, et al. Experience with percutaneous venoarterial cardiopulmonary bypass for emergency circulatory support. Crit Care Med. 2003;31(3):758–64.
Pages ON, Aubert S, Combes A, Luyt CE, Pavie A, Léger P, et al. Paracorporeal pulsatile biventricular assist device versus extracorporal membrane oxygenation-extracorporal life support in adult fulminant myocarditis. J Thorac Cardiovasc Surg. 2009;137(1):194–7.
Rubino A, Costanzo D, Stanszus D, Valchanov K, Jenkins D, Sertic F, et al. Central veno-arterial extracorporeal membrane oxygenation (C-VA-ECMO) after cardiothoracic surgery: a single-center experience. J Cardiothorac Vasc Anesth. 2018;32(3):1169–74.
Chen Y-S, Lin J-W, Yu H-Y, Ko W-J, Jerng J-S, Chang W-T, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372(9638):554–61.
Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg. 2010;139(2):302–311.e1.
Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol. 2014;63(25 Pt A):2769–78.
Bréchot N, Luyt C-E, Schmidt M, Leprince P, Trouillet J-L, Léger P, et al. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit Care Med. 2013 Jul;41(7):1616–26.
Bouabdallaoui N, Demondion P, Leprince P, Lebreton G. Short-term mechanical circulatory support for cardiogenic shock in severe peripartum cardiomyopathy: La Pitié-Salpêtrière experience. Interact Cardiovasc Thorac Surg. 2017;25(1):52–6.
Aissaoui N, Luyt C-E, Leprince P, Trouillet J-L, Léger P, Pavie A, et al. Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med. 2011;37(11):1738–45.
Chang W-W, Tsai F-C, Tsai T-Y, Chang C-H, Jenq C-C, Chang M-Y, et al. Predictors of mortality in patients successfully weaned from extracorporeal membrane oxygenation. Salluh J, editor. PLoS ONE. 2012;7(8):e42687.
Cavarocchi NC, Pitcher HT, Yang Q, Karbowski P, Miessau J, Hastings HM, et al. Weaning of extracorporeal membrane oxygenation using continuous hemodynamic transesophageal echocardiography. J Thorac Cardiovasc Surg. 2013;146(6):1474–9.
Pappalardo F, Pieri M, Arnaez Corada B, Ajello S, Melisurgo G, De Bonis M, et al. Timing and strategy for weaning from venoarterial ECMO are complex issues. J Cardiothorac Vasc Anesth. 2015;29(4):906–11.
Li C-L, Wang H, Jia M, Ma N, Meng X, Hou X-T. The early dynamic behavior of lactate is linked to mortality in postcardiotomy patients with extracorporeal membrane oxygenation support: a retrospective observational study. J Thorac Cardiovasc Surg. 2015;149(5):1445–50.
García-Gigorro R, Renes-Carreño E, Pérez-Vela JL, Marín-Mateos H, Gutiérrez J, Corrés-Peiretti MA, et al. Mechanical support with venoarterial extracorporeal membrane oxygenation (ECMO-VA): short-term and long-term prognosis after a successful weaning. Med Int. 2017;41(9):513–22.
Huang K-C, Lin L-Y, Chen Y-S, Lai C-H, Hwang J-J, Lin L-C. Three-dimensional echocardiography-derived right ventricular ejection fraction correlates with success of decannulation and prognosis in patients stabilized by venoarterial extracorporeal life support. J Am Soc Echocardiogr. 2018;31(2):169–79.
Garan AR, Eckhardt C, Takeda K, Topkara VK, Clerkin K, Fried J, et al. Predictors of survival and ability to wean from short-term mechanical circulatory support device following acute myocardial infarction complicated by cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2018;7(8):755–65.
• Sertic F, Chavez L, Diagne D, Richards T, Wald J, Acker M, et al. Predictors of in-hospital mortality and midterm outcomes of patients successfully weaned from venoarterial extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2019. https://doi.org/10.1016/j.jtcvs.2019.11.106. This study provides essential information regarding factors associated with mortality after weaning from VA-ECMO and useful insight in guiding patient selection for heart replacement options.
Pagani FD, Aaronson KD, Dyke DB, Wright S, Swaniker F, Bartlett RH. Assessment of an extracorporeal life support to LVAD bridge to heart transplant strategy. Ann Thorac Surg. 2000;70(6):1977–84 discussion 1984-1985.
Scherer M, Moritz A, Martens S. The use of extracorporeal membrane oxygenation in patients with therapy refractory cardiogenic shock as a bridge to implantable left ventricular assist device and perioperative right heart support. J Artif Organs. 2009;12(3):160–5.
Marasco SF, Lo C, Murphy D, Summerhayes R, Quayle M, Zimmet A, et al. Extracorporeal life support bridge to ventricular assist device: the double bridge strategy. Artif Organs. 2016;40(1):100–6.
Unai S, Yamane K, Tanaka D, Cook G, Hirose H, Cavarocchi NC, et al. Quality of life and mid-term survival of patients bridged with extracorporeal membrane oxygenation to left ventricular assist device. ASAIO J. 2017;63(3):273–8.
Rousse N, Juthier F, Pinçon C, Hysi I, Banfi C, Robin E, et al. ECMO as a bridge to decision: recovery, VAD, or heart transplantation? Int J Cardiol. 2015;187:620–7.
Fukuhara S, Takeda K, Kurlansky PA, Naka Y, Takayama H. Extracorporeal membrane oxygenation as a direct bridge to heart transplantation in adults. J Thorac Cardiovasc Surg. 2018;155(4):1607–1618.e6.
Barge-Caballero G, Castel-Lavilla MA, Almenar-Bonet L, Garrido-Bravo IP, Delgado JF, Rangel-Sousa D, et al. Venoarterial extracorporeal membrane oxygenation with or without simultaneous intra-aortic balloon pump support as a direct bridge to heart transplantation: results from a nationwide Spanish registry. Interact Cardiovasc Thorac Surg. 2019;29(5):670–7.
Platts DG, Sedgwick JF, Burstow DJ, Mullany DV, Fraser JF. The role of echocardiography in the management of patients supported by extracorporeal membrane oxygenation. J Am Soc Echocardiogr. 2012;25(2):131–41.
Santise G, Panarello G, Ruperto C, Turrisi M, Pilato G, Giunta A, et al. Extracorporeal membrane oxygenation for graft failure after heart transplantation: a multidisciplinary approach to maximize weaning rate. Int J Artif Organs. 2014;37(9):706–14.
Aissaoui N, El-Banayosy A, Combes A. How to wean a patient from veno-arterial extracorporeal membrane oxygenation. Intensive Care Med. 2015;41(5):902–5.
• Ortuno S, Delmas C, Diehl J-L, Bailleul C, Lancelot A, Naili M, et al. Weaning from veno-arterial extra-corporeal membrane oxygenation: which strategy to use? Ann Cardiothorac Surg. 2019;8(1):E1–8 This study provides useful guidance and highlights important considerations on how to wean patients from VA-ECMO.
ECMO. Extra Corporal Life Support Registry Report. In Ann Arbor, Michigan; 2018 [cited 2018 Dec 14]. Available from: https://www.elso.org/Portals/0/Files/Reports/2018/International%20Summary%20January%202018%20First%20Page.pdf. Accessed 22 Nov 2019.
Luo X, Wang W, Hu S, Sun H, Gao H, Long C, et al. Extracorporeal membrane oxygenation for treatment of cardiac failure in adult patients. Interact Cardiovasc Thorac Surg. 2009;9(2):296–300.
Mebazaa A, Combes A, van Diepen S, Hollinger A, Katz JN, Landoni G, et al. Management of cardiogenic shock complicating myocardial infarction. Intensive Care Med. 2018;44(6):760–73.
Bermudez CA, Rocha RV, Toyoda Y, Zaldonis D, Sappington PL, Mulukutla S, et al. Extracorporeal membrane oxygenation for advanced refractory shock in acute and chronic cardiomyopathy. Ann Thorac Surg. 2011;92(6):2125–31.
Lüsebrink E, Stremmel C, Stark K, Joskowiak D, Czermak T, Born F, et al. Update on weaning from veno-arterial extracorporeal membrane oxygenation. J Clin Med. 2020;9(4). https://doi.org/10.3390/jcm9040992.
Marasco SF, Vale M, Pellegrino V, Preovolos A, Leet A, Kras A, et al. Extracorporeal membrane oxygenation in primary graft failure after heart transplantation. Ann Thorac Surg. 2010;90(5):1541–6.
Asaumi Y, Yasuda S, Morii I, Kakuchi H, Otsuka Y, Kawamura A, et al. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J. 2005;26(20):2185–92.
Aziz TA, Singh G, Popjes E, Stephenson E, Mulvey S, Pae W, et al. Initial experience with CentriMag extracorporal membrane oxygenation for support of critically ill patients with refractory cardiogenic shock. J Heart Lung Transplant. 2010;29(1):66–71.
Boulate D, Luyt C-E, Pozzi M, Niculescu M, Combes A, Leprince P, et al. Acute lung injury after mechanical circulatory support implantation in patients on extracorporeal life support: an unrecognized problem. Eur J Cardiothorac Surg. 2013;44(3):544–9 discussion 549-550.
Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G, et al. Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail. 2017;19(3):404–12.
Roumy A, Liaudet L, Rusca M, Marcucci C, Kirsch M. Pulmonary complications associated with veno-arterial extra-corporeal membrane oxygenation: a comprehensive review. Crit Care. 2020;24(1):212.
Barge-Caballero E, Almenar-Bonet L, Villa-Arranz A, Pérez-Villa F, Segovia-Cubero J, Delgado-Jiménez J, et al. Impact of short-term mechanical circulatory support with extracorporeal devices on postoperative outcomes after emergency heart transplantation: data from a multi-institutional Spanish cohort. Int J Cardiol. 2014;176(1):86–93.
Carrier M. Commentary: survival following extracorporeal membrane oxygenation support, a sobering view. J Thorac Cardiovasc Surg. 2019;14. https://doi.org/10.1016/j.jtcvs.2019.11.115.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiogenic Shock: Progress in Mechanical Circulatory Support
Rights and permissions
About this article
Cite this article
Sertic, F., Bermudez, C. & Rame, J.E. Venoarterial Extracorporeal Membrane Oxygenation as a Bridge to Recovery or Bridge to Heart Replacement Therapy in Refractory Cardiogenic Shock. Curr Heart Fail Rep 17, 341–349 (2020). https://doi.org/10.1007/s11897-020-00495-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-020-00495-7