Abstract
Purpose
An optimal volume replacement strategy aims to restore systemic hemodynamics with the ultimate goals of improving organ perfusion and microcirculation for sustaining adequate tissue oxygenation. This review presents the (patho)physiological basis of hypovolemia, microcirculation, and tissue oxygenation and presents a literature review on the effects of plasma substitutes on microperfusion and oxygenation in the clinical setting.
Methods
Literature review of the effects of fluid therapy on microcirculation and tissue oxygenation using PubMed search including original papers in English from 1988 to 2009.
Results
We identified a total of 14 articles dealing with the effects of different crystalloids and colloids on organ perfusion, microcirculation, and tissue oxygenation in patients. The results are divergent, but there is a general trend that colloids are superior to crystalloids in improving organ perfusion, microcirculation, and tissue oxygenation. Due to the limited number of studies and different study conditions, a meta-analysis on the effects of the volume replacement strategies on microcirculation is not possible.
Conclusions
Improving the microcirculation by volume replacement appears to be a promising issue when treating the critically ill. The growing insights from animal experiments have to be translated into the clinical setting to identify the optimal fluid regimen for correcting hypovolemia. New techniques for monitoring microcirculation at the bedside might provide such endpoints, although these have to be validated also in the clinical setting. Whether improved microperfusion and tissue oxygenation by fluid therapy will also improve patient outcomes will have to be proven by future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Circulating volume and microcirculatory deficits may occur in the surgical, trauma, burn, and intensive care patient. While bleeding causes absolute volume deficits, vasodilation mediated by vasoactive substances produces relative volume deficits. Hypovolemia may also develop in the absence of obvious fluid loss secondary to generalized impairment of the endothelial barrier caused by inflammation resulting in capillary leakage and fluid shift from the intravascular to the interstitial space. Restoration of intravascular volume can be regarded as a cornerstone of therapy in the critically ill. Diagnosis, choice of fluids, and identification of hemodynamic targets, however, remain controversial. One of the reasons for this uncertainty is that fluid resuscitation has traditionally been targeted to correct macrocirculation, whereas the physiological impact of fluids at the microcirculatory level is still unclear. Uncorrected microcirculatory alterations result in inadequate oxygen transport to achieve sufficient oxidative phosphorylation and, ultimately, cause tissue damage and organ dysfunction [1–4]. The primary aim of optimal fluid resuscitation should be to achieve adequate perfusion without compromising oxygen transport by excessive hemodilution. It still remains unclear whether this can be achieved by correction of hypovolemia itself or whether the kind of volume replacement is also of importance. The ideal volume replacement strategy should correct hypovolemia and restorate systemic hemodynamics, but also improve microcirculatory perfusion and tissue oxygenation [5, 6].
The purpose of this review is to consider the current insights into the effects of fluid therapy on microcirculation and oxygen transport to the parenchymal cells. A review of the literature will be given with regard to the effects of commonly used plasma substitutes on organ perfusion, microcirculation, and tissue oxygenation in the clinical setting.
Pathophysiology of the hypovolemic microcirculation
Hypovolemia leads to inadequate perfusion of the microcirculation resulting in insufficient oxygen availability to meet the needs of mitochondrial oxidative phosphorylation [2, 7]. Weil and Shubin [8] in their keynote paper classified the different types of shock into four main categories: hypovolemic, cardiogenic, obstructive, and distributive shock (Fig. 1, 2). Hypovolemic shock can be described as the condition whereby there is a decrease in circulating volume. Cardiogenic shock occurs where there is a loss of cardiac contractility with elevation of diastolic filling pressure and volume. Obstructive shock can occur as a result of massive pulmonary embolism, tension pneumothorax, or pericardial tamponade where there is a physical obstruction in the circulation resulting in impaired diastolic filling and increased afterload. Distributive shock involves a defect in the (micro)vascular distribution of a normal or even of a supranormal cardiac output resulting in inadequate regional oxygen delivery. Hypovolemia induced by distributive shock is highly heterogeneous and targets the microcirculation. Its detection by measuring systemic hemodynamics is complicated by shunting of the microcirculation resulting in microcirculatory alterations and hypoxia with normal systemic hemodynamics and oxygen-derived variables [9]. Distributive shock especially occurs under conditions of inflammation and infection such as in sepsis and reperfusion injury. Inflammatory mediators and hypoxemia result in abnormal blood flow distributions and shunting leading to a mismatch between oxygen delivery and oxygen need by the parenchymal cells, and thus heterogeneous hypoxemia, and organ dysfunction [9, 10].
The classification of shock according to Weil and Shubin [8]. I Normal conditions. II Cardiogenic shock, related to cardiac pump failure resulting from loss of the pump function of the heart. III Hypovolemic shock as a result of decreased circulating volume from, for example, hemorrhage. IV Obstructive shock as result of an obstruction in the cardiovascular circuit as a result of, for example, massive pulmonary embolism, tension pneumothorax, or pericardial tamponade. V Distributive shock where vascular dysfunction is unable to distribute a normal or even high cardiac output, resulting in underperfused microcirculatory areas being shunted by well perfused areas
Distributive shock provides the biggest challenge with regard to identifying endpoints for assessing an adequate fluid replacement [11]. Currently these endpoints are aimed at correcting changes in systemic hemodynamics. Fluid resuscitation can cause an apparent improvement in systemic circulation while leaving regional and microcirculatory oxygenation and perfusion underresuscitated. In animal investigations it has been shown that fluid resuscitation improved organ blood flow of the gut and kidneys, while leaving other areas hypoxemic [12]. This is important in the light of recent clinical studies using new techniques for monitoring microcirculation, which have shown the persistence of microcirculatory underresuscitation in the presence of normalized systemic hemodynamic variables and association with adverse clinical outcome [5, 13–15].
Adequate microcirculation relies on the function of the different components of the microcirculation. Red and white blood cells, endothelial cells, and smooth muscle cells have to function in close harmony to guarantee adequate microcirculatory blood flow to transport oxygen to the tissues. The function of each of these cellular and subcellular systems is affected by hypovolemia. Deleterious effects on the microcirculation include dysfunction of endothelial signal transductory pathways by inflammatory mediators and reactive oxygen species, deterioration of endothelial barrier function (e.g., glycocalyx), alterations in red blood cell function (deformability, aggregability), and increased leukocyte adhesion and activation [6, 9]. Any one of these alterations either alone or acting together can lead to a loss of functional capillary density resulting in heterogeneous abnormalities in the distribution of blood to the microcirculatory network, enhanced oxygen diffusion distances from the perfused intracapillary lumens to the tissue cell, tissue hypoxemia, and finally organ dysfunction.
Administration of fluids to correct hypovolemia may modulate microcirculatory function by various mechanisms. The most important is increasing flow by enhanced filling of the vasculature, thus generating forcing pressure promoting microcirculatory perfusion. Fluids also modify the hemorheology of blood by decreasing viscosity, which additionally promotes blood flow. There are different effects of fluids on blood viscosity depending on the composition of the fluid; the microcirculation can be either improved or impaired by these effects [16]. Excessive hemodilution can cause shunting of the microcirculation and impair regional tissue oxygenation [14]. This effect can differ among the different organ systems [12].
Acid-base balance has been shown to be influenced by the administration of fluids [17]. Alterations in acid-base status can cause deleterious effects on organ function (e.g., kidney function [18]). Saline solution appears to have the most negative effect on the (micro)circulation [19]. Infusion of large amounts of saline results in increased plasma chloride concentration and causes a reduction in the strong ion difference, which in turn produces an increase in free hydrogen ions [20]. This effect can be avoided when using more plasma-adapted (“balanced”) crystalloids [21].
Release of inflammatory mediators secondary to hypovolemia is another important factor contributing to microcirculatory dysfunction. The volume replacement strategy can modulate inflammatory activation, generation of reactive oxygen, and leukocyte adhesion to the microcirculatory endothelium [22]. Saline solution appears to be the most pro-inflammatory fluid, whereas certain colloids (especially when dissolved in a balanced solution) may be more beneficial in controlling the inflammatory process [23–25]. Studies using intravital microscopy have shown this to be the case by imaging the effects of different fluids on leukocyte adherence on organ microvascular surfaces [26, 27]. In this context colloids showed more beneficial effects on the microcirculation than saline resuscitation in a variety of experimental models using direct observation of leukocyte endothelial interaction [27, 28]. The use of crystalloids as volume replacement has been shown to impair microcirculation and to cause vascular leakage resulting in fluid shift to the interstitial space [29, 30]. The negative effects on oxygen transport pathways occur as a result of increased diffusion path length and poor oxygen solubility in aqueous solutions causing reduced oxygen availability and impaired cellular respiration.
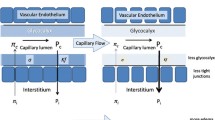
The glycocalyx is a gel-like structure that forms the interface between the intracapillary lumen and the endothelial cells, and its integrity is highly sensitive to oxidative stress [31]. It is likely that endothelial dysfunction and microcirculatory deterioration resulting from hypovolemia and reperfusion injury are caused by a loss of the endothelial glycocalyx [32]. Depletion of this barrier results in leukocyte adhesions and loss of the endothelial barrier function [33, 34]. This in turn results in alterations of the Starling forces promoting tissue edema. The loss of this important supracellular compartment of the microcirculation may form an important rationale for using colloids [32]. Of the colloids, starches have been shown to protect the glycocalyx barrier [35]. It is expected that modifications of the molecular and pharmacological properties of colloids as well as of the solvent may provide additional protection of the glycocalyx and thereby of the microcirculation.
How can the effects of volume replacement be monitored?
Currently, endpoints of volume replacement are aimed at correcting changes in systemic hemodynamic variables. These include giving volume challenges either by administering a fluid bolus or by autotransfusion by passive leg raising resulting in changes in systemic hemodynamic variables such as stroke volume or venous pressure [11]. Fluid resuscitation can cause an apparent improvement in systemic circulation while leaving regional and microcirculatory oxygenation and perfusion uncorrected [12]. New techniques have been introduced at the bedside aimed at monitoring various aspects of the microcirculation and tissue oxygenation. These have been applied to various areas of the body, and various parameters related to microcirculatory function and tissue oxygenation have been measured. At present, no unifying monitoring technique exists that measures all aspects of microcirculatory function in an integrated fashion, and each technique has its limitations in this respect. It is worth noting that in fact all hemodynamic monitoring modalities suffer from this drawback and that no single technique can be used to measure the integrative function of the cardiovascular system to achieve hemodynamic homeostasis from lung to mitochondria. Thus each microcirculatory monitoring technique should be interpreted for its sensitivity and specificity in identifying a particular physiological component and its ability to predict impending adverse events. It has to be emphasized that these microcirculatory monitoring techniques are at an early stage of development and have to be validated for guiding volume therapy in the clinical setting. Currently used bedside monitoring techniques for assessing the influence of volume therapy on microcirculation are described below.
Laser Doppler flowmetry (LDF) is used for nonquantitative assessment of blood flow and has been applied mainly to the cutaneous microcirculation [36].
Tissue CO2 can be measured using gastric CO2 tonometers or by tissue CO2 electrodes [37]. These measurements have been mostly applied either in the stomach or intestines or sublingually and are assumed to be a measure of the adequacy of microcirculatory perfusion [36–42]. In early studies such CO2 measurements and arterial bicarbonate were entered into the Henderson-Hasselbalch equation to obtain intestinal mucosal pH (pHi). This calculation was later abandoned and instead tissue-to-arterial gradient (pCO2 gap) was used.
A more direct method of evaluating microcirculatory perfusion is by direct imaging of the microcirculation using the recently introduced orthogonal polarizing spectrum (OPS) or sidestream dark field (SDF) imaging [43, 44]. These are optical techniques that are incorporated into hand-held microscopes for direct visual observation of the microcirculation on mucosal organ surfaces. In the perioperative setting these techniques have mainly been applied to study the sublingual microcirculation.
Tissue oxygenation has classically been measured by use of Clark electrodes [45] although more modern versions of such electrodes use solid state or oxygen-dependent fluorescence quenching methods. These electrodes are applied either trans- or subcutaneously, or as needles that can be inserted into muscle tissue.
An alternative method to gain information about microcirculatory oxygen availability is the use of the oxygen-dependent optical properties of microcirculatory Hb, which can be monitored using near infrared spectroscopy (NIRS) [46]. This technique has been applied in the thenar, calf, and forearm, and on the forehead.
A more comprehensive presentation of these techniques will be provided in another review paper to be published in Intensive Care Medicine as part of this current series dedicated to microcirculation.
Clinical studies on the effects of fluid therapy on microcirculation and tissue oxygenation
A PubMed analysis was carried out to investigate the literature on the effects of fluids on the microcirculation and tissue oxygenation in the clinical setting. We included all original studies published in English from 1988 to 2009 and only studies in patients (no volunteer studies) were included (Table 1). Keywords for the search included [microcirculation], [microperfusion] [tissue blood flow] [organ perfusion] [tissue oxygenation] in connection with [hypovolemia], [volume therapy/replacement], [fluid therapy/replacement], [crystalloids], [Ringer’s lactate], [saline solution], [normal saline], [(human) albumin], [gelatin(s)], [dextran(s)], [hydroxyethyl starch, HES], and [hypertonic solution]. Articles were only included when the methods for assessing organ perfusion, microcirculation, and tissue oxygenation were given. Obviously we may have missed several ongoing studies and indeed several are currently under review, but nevertheless we feel this overview gives a comprehensive review of the state of the current art.
Crystalloids
In two prospective, randomized studies in patients undergoing abdominal aortic and gynecologic surgery, the influence of Ringer’s lactate (RL) for intraoperative volume replacement on gastric mucosal perfusion was assessed by measuring gastric intramucosal pH (pHi) by tonometry [47, 48]. In one study, RL did not improve gastric mucosal perfusion [47], while in the other one RL also increased gastric mucosal perfusion, but not nearly as much as HES 200/0.5 did [48]. In patients undergoing major abdominal surgery, volume replacement using saline solution resulted in decreased skeletal muscle tissue pO2 (ptiO2), whereas ptiO2 increased significantly in the patients given HES 130/0.4 in saline [49]. This difference was not identified by monitoring only systemic hemodynamics. Arkilic et al. [50] investigated the effects of “conservative” (2,183 ± 972 mL) versus “aggressive” (3,815 ± 1,853 mL) unspecified crystalloid resuscitation in elective colon resection surgery and measured subcutaneous oxygen tension using oxygen electrodes and capillary blood flow by a thermal diffusion system. They found that supplemental fluid administration increased both tissue oxygen tension and capillary blood flow showing the most increase with the higher doses of fluids. The difference between “conservative” and “aggressive” crystalloid resuscitation seen in the microcirculation was not identified by monitoring systemic hemodynamics.
Colloids
Human albumin (HA)
In one study in trauma intensive care unit (ICU) patients, volume replacement with HA given over 5 days to guarantee stable hemodynamics assessed with the help of a pulmonary artery catheter (PAC) did not change normal gastric mucosal perfusion measured by tonometry (no changes in pHi). In septic patients, however, pHi was significantly decreased by HA administration despite adequate correction of hypovolemia, suggesting ongoing perfusion abnormalities in this area by volume resuscitation with HA [51].
Dextrans
No study in the clinical setting during the past 20 years was found showing the effects of dextrans on organ perfusion, microcirculation, or tissue oxygenation in patients.
Hydroxethyl starch (HES)
Hydroxyethyl starch (HES) refers to a class of synthetic colloid solutions that are modified natural polysaccharides, similar to glycogen. HES is derived from amylopectin, a highly branched starch that is obtained from waxy maize or potatoes. Polymerized d-glucose units are joined primarily by 1-4 linkages with occasional 1-6 branching linkages. Substituting hydroxyethyl for hydroxyl groups results in highly increased solubility and retards hydrolysis of the compound by amylase, thereby delaying its breakdown and elimination from the blood. The hydroxyethyl groups are introduced mainly at carbon positions C2, C3, and C6 of the anhydroglucose residues. The available HES preparations are characterized by concentration (hypooncotic: 3%; isooncotic: 6%; hyperoncotic: 10%), molar substitution (MS; low: <0.5; medium: 0.5; high: >0.5), the mean molecular weight [Mw, low-molecular weight (LMW)-HES: 70 kD; medium-molecular weight (MMW)-HES: 130–264 kD; high-molecular weight (HMW)-HES: >450 kD], the origin (potato-derived versus maize-derived HES), and the solvent (balanced and unbalanced HES preparations).
Eleven studies using HES in the clinical setting were identified [47, 49, 51–57]. Two studies in septic [54, 55] and one study in cardiac surgery patients [57] showed unchanged organ perfusion by HES. In all of these studies, pHi (or pCO2 gap) was measured by tonometry to assess the influence of volume replacement therapy on gastric mucosal perfusion. Eight studies in patients undergoing cardiac, vessel, trauma, or gynecologic surgery showed that HES improved organ perfusion or tissue oxygenation or that decrease in perfusion was blunted by adminstration of HES [47–49, 51–53, 56, 58]. In six of them, gastric mucosal perfusion (pHi) was measured using tonometry and administration of HES was associated with significantly improved gastric mucosal perfusion [47, 48, 51, 53, 56, 58]. In one study in cardiac surgery patients, LDF was used to measure skin microcirculatory perfusion [52]. Microcirculatory blood flow showed better skin perfusion with HES 200/0.5 than with other HES preparations (HES 70/0.5 and HES 450/0.7). That the type of HES preparation seems to be of importance concerning modulation of the microcirculation has also been shown in a study of patients undergoing abdominal aortic surgery [58]. When using HES 200/0.62, pHi was significantly less decreased compared to HES 130/0.4. In a study in patients undergoing major abdominal surgery, the influence of 6% HES 130/0.4 on tissue pO2 was compared to patients who received saline solution for volume replacement [49]. Skeletal muscle tissue pO2 (ptiO2) was monitored for 24 h after surgery using flexible minimally invasive microsensory pO2 catheters. Although systemic hemodynamics and systemic oxygenation data were similar in both groups, ptiO2 increased significantly in the HES 130/0.4-treated patients (+59%), but decreased in the RL group (−23%).
Gelatins
Three studies were found that used gelatins for improving organ perfusion [54, 56, 58]. Gelatin was given for hemodynamic stabilization in patients suffering from sepsis or undergoing aortic surgery and compared to use of HES. pHi was measured by tonometry to assess gastric mucosal perfusion. In the hypovolemic septic patients [54], use of gelatin very moderately increased pHi (from 7.27 to 7.31) suggesting improved splanchnic perfusion. Of particular importance was also that no difference in systemic hemodynamic variables was found between the two volume replacement strategies, but only on gastric tonometry data. In two other studies from one research group performed in patients undergoing abdominal aortic surgery, either gelatin or HES (HES 200/0.62 or HES 130/0.4) was administered for volume expansion [56, 58]. HES 200/0.62-treated patients showed significantly less reduced splanchnic perfusion than those administered gelatin.
Plasma-adapted versus non-plasma-adapted volume replacement strategy
In a prospective, randomized, blinded trial in elderly surgical patients, either a plasma-adapted (“balanced”) intraoperative fluid regimen consisting of Hartmann’s solution and 6% HES preparation dissolved in a balanced electrolyte solution (Hextend) or a nonplasma-adapted (“unbalanced”) regimen consisting of 0.9% sodium chloride solution and 6% HES dissolved in 0.9% sodium chloride solution was given to guarantee stable hemodynamics using a specific algorithm [59]. Similar amounts of HES (approximately 2,500 mL) and crystalloids (approximately 1,500 mL) were given in both groups. Splanchnic perfusion was assessed by gastric tonometry. Systemic hemodynamics were without group differences, while hyperchloremic acidosis was seen only in the non-plasma-adapted volume group, and better gastric mucosal perfusion was provided by the balanced volume replacement strategy compared with saline-based fluids.
Hypertonic solutions
Great enthusiasm has been expressed for hypertonic saline (HS) or hypertonic/colloid solutions (HCS) in the treatment of hypovolemic shock (“small volume resuscitation”). Hypertonic volume replacement has been proposed to correct microcirculatory dysfunction associated with hypovolemia and its subsequent inflammatory effect. HS has been shown to increase perfusion pressure, improve capillary flow distribution, and offer endothelial de-swelling effects [60, 61]. Because hemodynamic effects of HS solutions are reported to be rather transient, HS is often mixed with colloids [hypertonic saline plus hyperoncotic dextran (HSD) or hypertonic saline plus hypertonic HES (HHES)].
In a study in cardiac surgery patients using HHES to double low pulmonary capillary wedge pressure (PCWP), skin microcirculatory blood flow measured by LDF was significantly increased in comparison to HES 200/0.5 [52]. In an observational study in patients with subarachnoid hemorrhage, Al-Rawi et al. [62] administered extremely hypertonic saline solution (23.5%). A significant increase in cerebral perfusion pressure, brain tissue oxygenation, and middle cerebral artery flow velocity was found accompanied by a significant decrease in intracranial pressure. An improvement in cerebral metabolic status in terms of lactate-pyruvate ratio was also seen after infusion of this extreme hypertonic solution.
Discussion
In recent years, microcirculation has gotten increased attention in the treatment of the critically ill patient. This includes the choice for the ideal volume replacement strategy to improve microcirculation. Interestingly, we found only 14 papers on this issue over the past 20 years. This dearth of information on the different volume replacement strategies for organ perfusion, microcirculation, or tissue oxygenation is remarkable as there is an ongoing controversy concerning the ideal plasma substitute to correct hypovolemia. Due to the small number of studies and the divergent study designs, we decided not to perform a meta-analysis or a systematic review. The identified studies differed markedly with regard to the study design: (1) Plasma substitutes were used under different conditions, e.g., in trauma, sepsis, hemorrhage or surgery. (2) The amount of fluids that was administered differed widely among the studies making it difficult to distinguish between what effect is attributed to volume correction/expansion alone and what effect is specific for a certain plasma substitute. (3) Duration of volume administration differed; cases of “single-shot” versus continuous administration over hours were compared. (4) The endpoint of volume administration was not uniformly defined. Mostly fixed amounts of volume were given. A “goal-directed” volume replacement strategy, however, is a better approach than infusing a fixed amount of volume. (5) Although it is clear that measurement of systemic hemodynamic variables has limited sensitivity for identifying hemodynamic alterations associated with shock and resuscitation, most of the techniques used for assessing microcirculation are still at the stage of development and not yet ready for routine clinical use.
Looking at the results of the 14 identified studies, there appears to be a trend that colloids are more effective in beneficially modifying organ perfusion and microcirculation than crystalloids. One important objection to the results of these studies is that most of them used gastric tonometry to assess the effects of volume replacement on perfusion––a surrogate marker of splanchnic perfusion whose validity has often been doubted.
There is an urgent need for more information on the effect of fluids on microcirculation as there is a current trend to keep the patients more on the “dry side” by restricting volume administration. From the pathophysiological point of view, use of vasopressors in an hypovolemic patient to keep up blood pressure may initiate a fatal process on the microperfusion level with detrimental sequel for organ function and even for the patient’s outcome. It has been demonstrated that the use of vasopressors has no effect on prompting microcirculatory flow, emphasizing that a guarantee of adequate microperfusion may not be reached simply by maintaining or even increasing arterial blood pressure.
Whether the effects of a certain volume replacement strategy on microcirculation can be translated into its effects on patient’s outcome (survival) was beyond the scope of this review. Up to now it has not been shown that, by the choice of a certain volume replacement regimen, someone’s life was rescued, although beneficial effects on inflammation, microcirculation, and tissue oxygenation have been shown. This experience is similar to the introduction of new monitoring techniques, antibiotics, feeding strategies, or renal replacement strategies that have been shown to improve certain aspects in the management of the critically ill without improving overall survival. Our actual approach in the treatment of the critically ill aims to improve different parts in the mosaic of pathophysiology of critical illness; improving microcirculation may be one important part in this therapeutic puzzle.
As the commonly used plasma substitutes differ with regard to their physico-chemical characteristics, they also may differ with regard to their effects on organ perfusion, microcirculation, or tissue oxygenation. Based on the insights received from animal studies, questions that have to be addressed in the clinical setting will include how the different fluids affect the determinants of microcirculatory function. Important issues will be the effects on capillary flow and capillary recruitment in terms of functional capillary density, the extent of leukocyte endothelial interaction and glycocalyx determination, release of inflammatory mediators and reactive oxygen species, and finally the effects of the various fluid types on tissue oxygenation. These effects have to be determined for the different types of shock and will have to be related to organ function and clinical outcome.
Conclusions
Although it is clear from our literature search that the data that are needed to draw definite conclusions concerning the optimal fluid therapy and improvement in microcirculatory perfusion are not yet available, we will draw some careful conclusions from an “evidence level E” perspective. These can be summarized as follows: (1) A robust method or protocol for identifying hypovolemia and guiding fluid resuscitation is yet being sought. (2) Although it is clear that fluid therapy should be effective in correcting systemic hemodynamics, albeit based mainly on experimental studies, it should also be successful in correcting deficits in microperfusion and tissue oxygenation. (3) There is a need for bedside monitoring techniques for guiding fluid therapy integrating systemic, regional, microcirculatory, and oxygenation issues. (4) The choice between crystalloids and colloids is probably dependent on the patient’s condition and the cause of hypovolemia. (5) Compared to colloids, large amounts of crystalloids are needed to achieve similar systemic and microcirculatory endpoints leading to tissue edema and impaired tissue oxygenation. (6) It is expected that an optimal fluid composition for correction of hypovolemia may have to include additional components needed to support organ perfusion, microcirculation, and tissue oxygenation.
We hope that with this paper we have set the scene for important clinical investigations that will have to be carried out to find the optimal fluid composition and identify the optimal (micro)hemodynamic targets for volume resuscitation. We expect that protection of the microcirculatory function will play an important role in the future and will lead to improved treatment of the hypovolemic critically ill patient.
Change history
13 May 2020
The Editor-in-Chief has retracted this article [1] because a number of studies included in this review [2, 3, 4] (originally cited as references 24, 49, 51) have subsequently been retracted. This has rendered the content of the review unreliable.
References
Edouard AR, Degrémont AC, Duranteau J, Pussard E, Berdeaux A, Samii K (1994) Heterogeneous regional vascular responses to simulated transient hypovolemia in man. Intensive Care Med 20:220–414
Ince C (2004) Microcirculation in distress: a new resuscitation end point? Crit Care Med 32:1963–1964
Vollmar B, Menger MD (2004) Volume replacement and microhemodynamic changes in polytrauma. Langenbecks Arch Surg 389:485–491
Perret C, Feihl F (2000) Volume expansion during septic shock. Bull Acad Natl Med 184:1621–1629
Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL (2004) Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 32:1825–1831
Takala J, Jakob SM (2009) Shedding light on microcirculation. Intensive Care Med 35:394–396
Mythen MG, Salmon JB, Webb AR (1993) The rational administration of colloids. Blood Rev 7:223–228
Weil MH, Shubin H (1971) Proposed reclassification of states of shock. Adv Exp Med Biol 23:13–23
Ince C (2005) The microcirculation is the motor of sepsis. Crit Care 9(Suppl 4):S13–S19
De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL (2002) Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 166:98–104
Vincent JL, Weil MH (2006) Fluid challenge revisited. Crit Care Med 34:1333–1337
Van Bommel J, Siegemund M, Henny CP, Ince C (2008) Heart, kidney, and intestine have different tolerances for anemia. Transl Res 151:110–117
Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL (2007) Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med 49:88–98
Van Bommel J, Henny CP, Trouwborst A, Ince C (2001) Microvascular shunting in severe normovolemic hemodilution. Anesthesiology 94:152–160
Wang P, Hauptman JG, Chaudry IH (1990) Hemorrhage produces depression in microvascular blood flow which persists despite fluid resuscitation. Circ Shock 32:307–318
Ehrly AM, Landgraf H (1985) Influence of intravenous infusions of hydroxyethylstarch (HES) (MW 40,000 and 450,000) on the blood flow properties of healthy volunteers. Angiology 36:41–44
Boldt J (2007) The balanced concept of fluid resuscitation. Br J Anaesth 99:312–315
Powell-Tuck J, Gosling P, Lobo DN, Allison SP, Carlson GL, Gore M, Lewington AJ, Pearse RM, Mythen MG (2008) British consensus guidelines on intravenous fluid therapy for adult surgical patients. http://www.bapen.org.uk/pdfs/bapen_pubs/giftasup.pdf
Kellum JA (2002) Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: improved short-term survival and acid-base balance with Hextend compared with saline. Crit Care Med 30:300–305
Scheingraber S, Rehm M, Sehmisch C, Finsterer U (1999) Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology 90:1265–1270
Boldt J (2008) Saline versus balanced hydroxyethyl starch: does it matter? Curr Opin Anaesthesiol 21:679–683
Boldt J (2006) Do plasma substitutes have additional properties beyond correcting volume deficits? Shock 25:103–116
Kellum JA, Song M, Almasri E (2006) Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest 130:962–967
Boldt J, Suttner S, Brosch C, Lehmann A, Röhm K, Mengistu A (2009) The influence of a balanced volume replacement concept on inflammation, endothelial activation, and kidney integrity in elderly cardiac surgery patients. Intensive Care Med 35:462–470
Matharu NM, Butler LM, Rainger GE, Gosling P, Vohra RK, Nash GB (2008) Mechanisms of the anti-inflammatory effects of hydroxyethyl starch demonstrated in a flow-based model of neutrophil recruitment by endothelial cells. Crit Care Med 36:1536–1542
Kaplan SS, Park TS, Gonzales ER, Gidday JM (2000) Hydroxyethyl starch reduces leukocyte adherence and vascular injury in the newborn pig cerebral circulation after asphyxia. Stroke 31:2218–2223
Kupper S, Torge Mees S, Gassmann P, Brodde M, Kehrel B, Haier J (2007) Hydroxyethyl starch normalizes platelet and leukocyte adhesion within pulmonary microcirculation during LPS-induced endotoxemia. Shock 28:300–308
Inan N, Iltar S, Surer H, Yilmaz G, Alemdaroglu KB, Yazar MA, Basar H (2009) Effect of hydroxyethyl starch 130/0.4 on ischaemia/reperfusion in rabbit skeletal muscle. Eur J Anaesthesiol 26:160–165
Funk W, Baldinger V (1995) Microcirculatory perfusion during volume therapy. A comparative study using crystalloid or colloid in awake animals. Anesthesiology 82:975–982
Hoffmann JN, Vollmar B, Laschke MW, Inthorn D, Schildberg FW, Menger MD (2002) Hydroxyethyl starch (130 kD), but not crystalloid volume support, improves microcirculation during normotensive endotoxemia. Anesthesiology 97:460–470
Rubio-Gayosso I, Platts SH, Duling BR (2006) Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 290:H2247–H2256
Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M (2008) A rational approach to perioperative fluid management. Anesthesiology 109:723–740
Constantinescu AA, Vink H, Spaan JA (2003) Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol 23:1541–1547
Mulivor AW, Lipowsky HH (2002) Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart 283:H1282–H1291
Rehm M, Zahler S, Lötsch M, Welsch U, Conzen P, Jacob M, Becker B (2004) Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology 100:1211–1223
Leslie SJ, Affolter J, Denvir MA, Webb DJ (2003) Validation of laser Doppler flowmetry coupled with intra-dermal injection for investigating effects of vasoactive agents on the skin microcirculation in man. Eur J Clin Pharmacol 59:99–102
Dubin A, Edul VSK, Ince C (2009) Determinants of tissue pCO2 in shock and sepsis: relationship to the microcirculation. In: Vincent JL (ed) Yearbook of intensive care and emergency medicine. Springer, Heidelberg, pp 195–204
Russell JA (1997) Gastric tonometry: does it work? Intensive Care Med 23:3–6
Vallet B, Lund N, Curtis S, Kelly D, Cain S (1994) Gut and muscle tissue PO2 in endotoxemic dogs during shock and resuscitation. J Appl Physiol 76:793–800
Dubin A, Edul VS, Pozo MO, Murias G, Canullan CM, Martins EF, Ferrara G, Canales HS, Laporte M, Estenssoro E, Ince C (2008) Persistent villi hypoperfusion explains intramucosal acidosis in sheep endotoxemia. Crit Care Med 36:535–542
Creteur J, De Backer D, Sakr Y, Koch M, Vincent JL (2004) Sublingual capnometry tracks microcirculatory changes in septic patients. Crit Care Med 32:516–523
Weil MH, Nakagawa Y, Tang W, Sato Y, Ercoli F, Finegan R (1999) Sublingual capnometry: a new noninvasive measurement for diagnosis and quantification of severity of circulatory shock. Crit Care Med 27:1225–1229
Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C (2007) Sidestream dark field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express 15:15101–15114
Groner W, Winkelman JW, Harris AG, Ince C, Bouma GJ, Messmer K, Nadeau RG (1999) Orthogonal polarization spectral imaging: a new method for study of the microcirculation. Nat Med 5:1209–1212
Clark LC (1956) Monitor and control of blood and tissue oxygen tension. Trans Am Soc Artif Intern Org 2:41–46
Jobsis FF (1977) Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198:1264–1267
Marik PE, Iglesias J, Maini B (1997) Gastric intramucosal pH changes after volume replacement with hydroxyethyl starch or crystalloid in patients undergoing elective abdominal aortic aneurysm repair. J Crit Care 12:51–55
Guo X, Xu Z, Ren H, Luo A, Huang Y, Ye T (2003) Effect of volume replacement with hydroxyethyl starch solution on splanchnic oxygenation in patients undergoing cytoreductive surgery for ovarian cancer. Chin Med J 116:996–1000
Lang K, Boldt J, Suttner S, Haisch G (2001) Colloids versus crystalloids and tissue oxygen tension in patients undergoing major abdominal surgery. Anesth Analg 93:405–409
Arkiliç C, Taguchi A, Sharma N, Ratnaraj J (2003) Supplemental perioperative fluid administration increases tissue oxygen pressure. Surgery 133:49–55
Boldt J, Heesen M, Muller M, Pabsdorf M, Hempelmann G (1996) The effects of albumin versus hydroxyethyl starch solution on cardiorespiratory and circulatory variables in critically ill patients. Anesth Analg 83:254–261
Boldt J, Zickmann B, Herold C, Ballesteros M, Dapper F, Hempelmann G (1991) Influence of hypertonic volume replacement on the microcirculation in cardiac surgery. Br J Anaesth 67:595–602
Mythen MG, Webb AR (1995) Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Ann Surg 130:423–429
Asfar P, Kerkeni N, Labadie F, Gouello JP, Brenet O, Alquier P (2000) Assessment of hemodynamic and gastric mucosal acidosis with modified fluid gelatin versus hydroxyethyl starch: a prospective, randomized study. Intensive Care Med 26:1282–1287
Forrest DM, Baigorri F, Chittock DR, Spinelli JJ, Rusel JA (2000) Volume expansion using pentastarch does not change gastric-arterial PCO2 gradient or gastric intramucosal pHi in patients who have sepsis syndrome. Crit Care Med 28:2254–2258
Rittoo D, Gosling P, Bonnici C, Burnley S, Millns P, Simms MH, Smith SR, Vohra RK (2002) Splanchnic oxygenation in patients undergoing abdominal aortic aneurysm repair and volume expansion with eloHAES. Cardiovasc Surg 10:128–133
Hofmann D, Thuemer O, Schelenz C, van Hoot N, Sakka SG (2005) Increasing cardiac output by fluid loading: effects on indocyanine green plasma disappearance rate and splanchnic microcirculation. Acta Anaesthesiol Scand 49:1280–1286
Mahmood A, Gosling P, Barclay R, Kilvington F, Vohra R (2009) Splanchnic microcirculation protection by hydroxyethyl starches during abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg 37:319–325
Wilkes NJ, Woolf R, Mutch M, Mallett SV, Peachey T, Stephens R, Mythen MG (2001) The effects of balanced versus saline-based hetastarch and crystalloid solutions on acid-base and electrolyte status and gastric mucosal perfusion in elderly surgical patients. Anesth Analg 93:811–816
Kreimeier U, Bruckner UB, Niemczyk S, Messmer K (1990) Hyperosmotic saline dextran for resuscitation from traumatic-hemorrhagic hypotension: effect on regional blood flow. Circ Shock 32:83–99
Behrman SW, Fabian TC, Kudsk KA, Proctor KG (1991) Microcirculatory flow changes after initial resuscitation of hemorrhagic shock with 7.5% hypertonic saline/6% dextran 70. J Trauma 31:589–598
Al-Rawi PG, Zygun D, Tseng MY, Hutchinson PJ, Matta BF, Kirkpatrick PJ (2005) Cerebral blood flow augmentation in patients with severe subarachnoid haemorrhage. Acta Neurochir (Suppl) 95:123–127
Acknowledgments
The authors gratefully acknowledge the talents of Darryl Milstein who produced Figs. 1 and 2 in this paper. This study was supported only by an institutional grant.
Conflict of interest statement
Boldt and his institution have received funding from B. Braun (Germany); Fresenius-Kabi (Germany); Serumwerke Bernburg (Germany); Baxter (Europe). Ince holds a patent on SDF imaging, has stock in Microvision Medical, and has received educational grants from Hutchinison Technology, Baxter, Novartis, and Eli Lilly.
Author information
Authors and Affiliations
Corresponding author
Additional information
The Editor-in-Chief has retracted this article [1] because a number of studies included in this review [2, 3, 4] (originally cited as references 24, 49, 51) have subsequently been retracted. This has rendered the content of the review as unreliable.
Author Joachim Boldt has not responded to any correspondence from the publisher about this retraction. Author Can Ince agrees to the retraction.
[1] Boldt, J., Ince, C. The impact of fluid therapy on microcirculation and tissue oxygenation in hypovolemic patients: a review. Intensive Care Med 36, 1299–1308 (2010). https://doi.org/10.1007/s00134-010-1912-7
[2] Boldt J, Suttner S, Brosch C, Lehmann A, Röhm K, Mengistu A (2009) The influence of a balanced volume replacement concept on inflammation, endothelial activation, and kidney integrity in elderly cardiac surgery patients. Intensive Care Med 35:462–470
[3] Lang K, Boldt J, Suttner S, Haisch G (2001) Colloids versus crystalloids and tissue oxygen tension in patients undergoing major abdominal surgery. Anesth Analg 93:405–409
[4] Boldt J, Heesen M, Muller M, Pabsdorf M, Hempelmann G (1996) The effects of albumin versus hydroxyethyl starch solution on cardiorespiratory and circulatory variables in critically ill patients. Anesth Analg 83:254–261
About this article
Cite this article
Boldt, J., Ince, C. RETRACTED ARTICLE: The impact of fluid therapy on microcirculation and tissue oxygenation in hypovolemic patients: a review. Intensive Care Med 36, 1299–1308 (2010). https://doi.org/10.1007/s00134-010-1912-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1912-7