Abstract
Background
Acute renal failure on the intensive care unit is associated with significant mortality and morbidity.
Objectives
To determine recommendations for the prevention of acute kidney injury (AKI), focusing on the role of potential preventative maneuvers including volume expansion, diuretics, use of inotropes, vasopressors/vasodilators, hormonal interventions, nutrition, and extracorporeal techniques.
Method
A systematic search of the literature was performed for studies using these potential protective agents in adult patients at risk for acute renal failure/kidney injury between 1966 and 2009. The following clinical conditions were considered: major surgery, critical illness, sepsis, shock, and use of potentially nephrotoxic drugs and radiocontrast media. Where possible the following endpoints were extracted: creatinine clearance, glomerular filtration rate, increase in serum creatinine, urine output, and markers of tubular injury. Clinical endpoints included the need for renal replacement therapy, length of stay, and mortality. Studies are graded according to the international Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) group system
Conclusions and recommendations
Several measures are recommended, though none carries grade 1A. We recommend prompt resuscitation of the circulation with special attention to providing adequate hydration whilst avoiding high-molecular-weight hydroxy-ethyl starch (HES) preparations, maintaining adequate blood pressure using vasopressors in vasodilatory shock. We suggest using vasopressors in vasodilatory hypotension, specific vasodilators under strict hemodynamic control, sodium bicarbonate for emergency procedures administering contrast media, and periprocedural hemofiltration in severe chronic renal insufficiency undergoing coronary intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) often complicates the course of critical illness and, although previously considered as a marker rather than a cause of adverse outcomes, it is now known to the extent possible to be independently associated with an increase in both morbidity and mortality [1–11]. The major causes of AKI in the intensive care unit (ICU) include renal hypoperfusion, sepsis/systemic inflammatory response syndrome (SIRS), and direct nephrotoxicity, although in most cases etiology is multifactorial [12–15]. Furthermore, epidemiological studies involving patients undergoing cardiothoracic surgery [16, 17] or contrast administration [18, 19] have determined additional risk factors including age, preexisting hypertension, diabetes mellitus, heart failure, and prolonged and complex surgery. It follows that minimizing renal injury should confer a benefit to patients.
This review is the result of an international collaboration of the Critical Care Nephrology Working Group of the European Society of Intensive Care Medicine (ESICM) with the principal aim of providing a critical evaluation of available evidence and to give recommendations, where possible, for clinical practice. We have focused primarily on the role of volume expansion, diuretics, inotropes, vasopressors/vasodilators, hormonal interventions, nutrition, and extracorporeal techniques, which are the major interventions routinely and easily undertaken in intensive care practice.
Methods
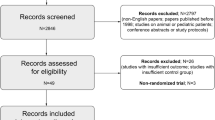
A systematic search of the literature was performed using the following databases: Medline (1966 through 2009, April), Embase (1980 through 2009, week 15), CINAHL (1982 through 2009, April), Web of Science (1955 through 2009), and PubMed/PubMed Central to identify key studies, preferably randomized placebo-controlled trials (RCT) and meta-analyses, on AKI/acute renal failure (ARF) addressing the use of therapeutic strategies to prevent renal dysfunction in adult critically ill patients. The following clinical conditions were considered: major surgery, critical illness, sepsis, shock, and use of potentially nephrotoxic drugs and radiocontrast. Transplantation, primary renal disease (e.g., vasculitis), and the hepatorenal syndrome were not considered.
Search terms and text words are available in electronic supplementary material (ESM), as are the endpoints extracted.
These recommendations are intended to provide guidance for the clinician caring for a patient at risk of AKI. The process of developing these recommendations included a modified Delphi process, three nominal consensus conferences during the annual meetings of the European Society of Intensive Care Medicine 2005 and 2006, followed by electronic-based discussions. The quality of the evidence was judged by predefined Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) criteria, which has also been employed in developing the latest Surviving Sepsis international guidelines [20]. This allows the development and grading of management recommendations through rating the quality of available evidence and grading strength of recommendations in clinical practice, as described in more detail elsewhere [21].
According to the GRADE system the strength of the recommendations is classified as either strong (1) or weak (2) [22]. A strong recommendation (1) indicates that an intervention’s desirable effects clearly outweigh undesirable effects such as risk, cost, and other burdens. Weak recommendations (i.e., suggestions) (2) indicate that the balance between undesirable and desirable effects is less clear.
The degree (i.e., quality) of evidence for the recommendations is classified from high (A) to low (C) according to factors including study design, consistency of the results, and directness of the evidence [22]. We regarded repeated large RCTs including ≥50 patients in each arm and meta-analyses showing low heterogeneity as providing a high degree of evidence (A) and smaller RCTs or larger RCTs whose results could not be reproduced as providing lower-grade evidence (B). For clinical decision-making, the grade of recommendation is considered of greatest clinical importance. It should, however, be emphasized that there may be circumstances in which a recommendation cannot or should not be followed for an individual patient. Furthermore, interventions are generally investigated separately, not in combination, and as such recommendations relate to the primary intervention. Local clinical guidelines will govern the use of either a single intervention or a combination.
Volume expansion
Recommendations
-
1.
We recommend controlled fluid resuscitation in true or suspected volume depletion (grade 1C).
-
2.
There is little evidence-based support for the preferential use of crystalloids, human serum albumin, gelatine-derived colloids or the lowest-molecular-weight hydroxy-ethyl starches (HES) for renal protection as long as derangements of serum electrolytes are prevented.
-
3.
We recommend avoiding 10% HES 250/0.5 (grade 1B) as well as higher-molecular-weight preparations of HES and dextrans in sepsis (grade 2C).
-
4.
We recommend prophylactic volume expansion by isotonic crystalloids in patients at risk of contrast nephropathy (grade 1B). We suggest using isotonic sodium bicarbonate solution, especially for emergency procedures (grade 2B).
-
5.
We suggest prophylactic volume expansion with crystalloids to prevent AKI by certain drugs (specified below) (grade 2C).
Rationale
Both relative and overt hypovolemia are significant risk factors for the development of AKI [23–26]. Consequently, timely fluid administration is a preventive measure which should be effective both through restoration of circulating volume and minimizing drug-induced nephrotoxicity [27]. Where volume replacement is indicated, this should be performed in a controlled fashion directed by hemodynamic monitoring, as injudicious use of fluids carries its own inherent risk [26]. In the ICU patient this may manifest in several ways, including prolonged mechanical ventilation [28] or the development of intra-abdominal hypertension, which itself is a risk factor for AKI [29].
Volume replacement may employ 5% glucose (i.e., free water), crystalloids (isotonic, half-isotonic), colloids, or more commonly in clinical practice, a combination thereof. Glucose solutions substitute free water and are used to correct hyperosmolar states, although the mainstay for correction of extracellular volume depletion remains isotonic crystalloids. However, the increased chloride load may result in hyperchloremic acidosis and associated renal vasoconstriction as well as altered perfusion of other organs such as the gut [30]. Crystalloids expand plasma volume by approximately 25% of the infused volume, whereas colloid infusion results in a greater expansion of plasma volume. The degree of expansion is dependent on concentration, mean molecular weight, and (for HES) the degree of molecular substitution. However, large-volume replacement with colloids in isolation risks hyperoncotic impairment of glomerular filtration [31, 32] as well as osmotic tubular damage, particularly in sepsis [33–35].
Commonly employed colloids include human albumin (HA), gelatins, dextranes, and HES. HA may appear attractive in hypooncotic hypovolemia but is costly [36–38]. A large multicenter RCT comparing 4% albumin with crystalloid failed to demonstrate any difference in outcome parameters including renal function, but proved that albumin itself was safe [39]. Gelatines have an average molecular weight of ca. 30 kDa, and the observed intravascular volume effect is shorter than that observed with HA or HES. Advantages include the lack of deleterious effects on renal function [40, 41] offset by the possibility of prion transmission, histamine release, and coagulation problems, particularly if large volumes are infused [42, 43]. Dextrans are single-chain polysaccharides comparable to albumin in size (40–70 kDa) and with a reasonably high volume effect, although anaphylaxis, coagulation disorders, and indeed AKI may occur at doses higher than 1.5 g/(kg day) [44–47]. HES are highly polymerized sugar molecules characterized by molecular weight, grade of substitution, concentration, and C2/C6 ratio. Their volume effect is greater than that of albumin, especially when larger-sized polymers are employed which degrade through hydrolytic cleavage the products of which undergo renal elimination. These degradation products may be reabsorbed and contribute to osmotic nephrosis and possibly medullary hypoxia [35, 48]. A further problem with HES may be tissue deposition and associated pruritus, which appears to be dose dependent [49–51]. These adverse effects may be less pronounced in the more modern, rapidly degradable HES solutions such as HES 130/0.4 [52–54].
Clinical studies
Unsurprisingly, no studies have specifically addressed the effects of volume expansion in overt hypovolemia compared with no volume resuscitation given the intuitive benefits of volume replacement. A large prospective observational trial of the CRYCO (CRYstalloids or COlloids?) study group found an increased risk of AKI in 1,013 ICU patients with shock receiving either hyperoncotic artificial colloids or albumin as compared with crystalloids [55]. This is in contrast to the results of a large RCT comparing isotonic sodium chloride to human serum albumin in various clinical settings, where no differences in renal function were demonstrated [39] (ESM Table S1). Given the lack of a marked beneficial effect the use of albumin should be limited given its expense.
Small studies performed in the perioperative setting have compared a variety of fluid replacement regimes (mainly between various forms of HES, or HES versus gelatine or albumin) with no definitive conclusions [56–60]. Only one RCT reported better preserved early postoperative renal function with 6% HES 130/0.4 than with 4% gelatine following cardiac surgery [61].
In patients undergoing low-risk surgery with normal renal function, a small RCT comparing lactated Ringers’ solution to three different forms of HES demonstrated no adverse effects of either solution [62].
In severe sepsis the beneficial effects of timely volume replacement on organ failure and mortality are well known and are a cornerstone of the Surviving Sepsis campaign guidelines [20, 63]. A single-center RCT comparing 5% HA with gelatine in ICU patients showed no difference in renal function despite significant differences in serum albumin levels [64]. A study comparing 6% HES 200/0.5 with gelatine in ICU patients with sepsis demonstrated slightly lower serum creatinine levels in the group receiving gelatine, but no effect was observed on endpoints such as the need for renal replacement therapy (RRT) or mortality [41] (ESM Table S1). However, a large German RCT in patients with septic shock (the VISEP trial) showed a higher incidence of AKI, requirement for RRT, and mortality in the group treated with 10% HES 200/0.5 compared with Ringer’s lactate [65]. Adverse effects on renal function may be restricted to higher-molecular-weight HES, as a multivariate analysis in a large European multicenter observational study was unable to detect HES as an independent risk factor, neither in 1,970 ICU patients requiring RRT nor in a subgroup of 822 patients with severe sepsis [66].
Prophylactic volume expansion has been investigated extensively in the setting of contrast-induced nephropathy (CIN) [67–72]. The benefit of this measure has been mainly shown for patients with inherent risk factors of CIN such as reduced glomerular filtration rate (GFR) (<50 ml/min/1.73 m²), heart failure or diabetes characterized by various risk scores (e.g., Thakar score [18] or Mehran score [19]). The first RCT comparing protection in CIN by half normal saline versus half normal saline plus diuretic therapy clearly demonstrated the superiority of volume expansion by half normal saline at a rate of 1 ml/kg 12 h before and after angiography [67]. However, a larger RCT including 1,620 patients undergoing coronary angioplasty showed a lower incidence of CIN when using normal saline compared with half normal saline [68]. Given that normal saline contains a high amount of chloride, the protective effect of isotonic bicarbonate solutions (containing 150–154 mmol/l sodium) in patients with impaired renal function receiving contrast agents has been studied. This showed a significant reduction in the incidence of CIN [69–75], but not in the need of renal replacement therapy or mortality. Most investigations applied a hydration regimen starting at 3(–5) ml/(kg h) 1 h before radiocontrast application, followed by 1 ml/(kg h) for at least 6 h post procedure [69, 71–73, 75]. Although three recent RCTs indicated that hydration with saline or sodium bicarbonate were equally effective [76–78], when included in meta-analyses a benefit of sodium bicarbonate over normal saline could still be detected [74, 79–81] (ESM Table S2). In a recent RCT of cardiac surgical patients at risk for AKI, intravenous sodium bicarbonate was associated with a lower incidence of acute renal dysfunction [82]. Nevertheless, adequately powered RCTs are still needed to determine whether sodium bicarbonate will reduce clinically meaningful outcomes.
Hypovolemia may also contribute significantly towards drug-induced renal injury, although the available evidence supporting preventative hydration is observational with no consensus found as to the timing, optimal volume, and type of solution to be employed [12]. Prevention of nephrotoxicity through prophylactic volume expansion has been shown to be of benefit with amphotericin B, antivirals including foscarnet, cidofovir, and adefovir [83–85], as well as drugs causing crystal nephropathy such as indinavir, acyclovir, and sulfadiazine [86].
Diuretics
Recommendations
-
1.
We recommend that loop diuretics are not used to prevent or ameliorate AKI (grade 1B).
Rationale
Oligo-anuria frequently is the first indicator of acute renal dysfunction. A multinational survey has demonstrated that 70% of intensivists used loop diuretics in a wide spectrum of AKI settings [87]. There may indeed be some theoretical attractions in using diuretics to ameliorate AKI, including prevention of tubular obstruction, reduction in medullary oxygen consumption, and increase in renal blood flow [88–90].
Clinical studies
Few prospective randomized trials have addressed the role of diuretics in the prevention of AKI. Most of the available studies have been done in the setting of contrast administration [67, 91, 92] and cardiac surgery [93, 94]. No protection against contrast nephropathy has been observed with diuretics, and in cardiac surgery, higher postoperative serum creatinine levels were found in patients receiving furosemide [93, 94].
To date four randomized controlled trials have examined the role of diuretics in established renal failure in the intensive care setting. No demonstrable improvement in primary outcome parameters, such as recovery of renal function or mortality, has been observed [35, 95–97]. Other studies have compared diuretics with dopamine or against placebo, again with no perceived benefit [33, 98, 99]. Three meta-analyses confirmed that the use of diuretics in established AKI does not alter outcome but carries a significant risk of side-effects such as hearing loss [100–102] (ESM Table S3). Furthermore, in an international cohort study an increase in the risk of death or an increase in established renal failure was observed. These findings, however, could not be confirmed in a second cohort study [103, 104].
Vasopressors and inotropes
Recommendations
-
1.
We recommend that mean arterial pressure (MAP) be maintained ≥60–65 mmHg (grade 1C), however, target pressure should be individualized where possible, especially if knowledge of the premorbid blood pressure is available.
-
2.
In case of vasoplegic hypotension as a result of sepsis or SIRS we recommend either norepinephrine or dopamine (along with fluid resuscitation) as the first-choice vasopressor agent to correct hypotension (grade 1C).
-
3.
We recommend that low-dose dopamine not be used for protection against AKI (grade 1A).
Rationale
Preservation or improvement of renal perfusion can theoretically be achieved by fluid resuscitation, through renal vasodilators, by systemic vasopressors that redirect blood flow to the kidney or by increasing cardiac output through the use of inotropic drugs. Whereas a recent study indicated that any MAP of >60 mmHg may be considered adequate for patients with septic shock [105], additional benefits with regards to renal function were not observed when a target MAP of more than 85 mmHg was compared to a target of 65 mmHg [106]. However, it must be borne in mind that this study may not be widely applicable to the patients admitted to the intensive care unit with preexisting comorbidities. Those at greatest risk of developing AKI include those patients with vascular disease, hypertension, diabetes, increased age, and elevated intra-abdominal pressure, for whom the target mean arterial pressures may have to be individually tailored.
Clinical studies
Low-dose or “renal-dose” dopamine has been advocated in the past to prevent selective renal vasoconstriction in a variety of conditions [107]. Although dopamine infusion may improve renal perfusion in healthy volunteers, no improvement of renal perfusion nor preventive benefit with respect to renal dysfunction has been unequivocally demonstrated in the critically ill, where increased sympathetic activity and local norepinephrine release may contribute to renal vasoconstriction [108–110]. Dopamine also has an inotropic effect, but observed increases in diuresis or even a reduction in serum creatinine during dopamine infusion does not protect against AKI [107, 110, 111]. Several meta-analyses have concluded that “renal-dose” dopamine is of no benefit in either preventing or ameliorating AKI in the critically ill and may even promote AKI [107, 109, 112].
The inotropic agents dobutamine and dopexamine have also been examined, but to date no prospective controlled clinical trial has demonstrated a protective effect on renal function [113]. In contrast, the effect of dobutamine on renal function is variable, even when favorably affecting cardiac output [111].
Norepinephrine is known to be of use in the treatment of vasodilatory shock after adequate fluid loading. In nonrandomized studies, administration of norepinephrine and resulting increases in arterial blood pressure have shown to increase diuresis and creatinine clearance [114]. Whether this is due to increased renal perfusion and thus implies renal protection is unknown. A RCT on 252 adult patients comparing dopamine in septic shock with norepinephrine as the initial vasopressor showed no significant differences between groups with regard to renal function or mortality. Though norepinephrine was associated with significantly less sinus tachycardia and arrhythmias (Patel GP et al., Shock, in press).
Vasopressin is gaining popularity in the treatment of norepinephrine-refractory shock [115] increasing blood pressure and enhancing diuresis in hypotensive oliguric patients but as yet has not been proven to enhance survival nor to prevent or ameliorate AKI in the critically ill [116].
Vasodilators
Recommendations
-
1.
We suggest using vasodilators for renal protection when volume status is corrected and the patient is closely hemodynamically monitored with particular regard to the development of hypotension (grade 2C). When choosing a vasodilator, the clinical condition of the patient, the availability of the drug, and the concomitant interventions should be considered.
-
2.
We suggest the prophylactic use of fenoldopam, if available, in cardiovascular surgery patients at risk of AKI (grade 2B). We recommend that fenoldopam is not used for prophylaxis of contrast nephropathy (grade 1A).
-
3.
We suggest using theophylline to minimize risk of contrast nephropathy, especially in acute interventions (grade 2C) when hydration is not feasible.
-
4.
We suggest that natriuretic peptides are not used as protective agents against AKI in critically ill patients (grade 2B), while its use may be considered during cardiovascular surgery (grade 2B).
Rationale
Reduced tissue perfusion elicits neurohumoral activation leading to increased sympathetic tone, endothelin production, and activation of the renin–angiotensin–aldosterone system which maintains systemic blood pressure often at the cost of both splanchnic and renal vasoconstriction. Glomerular perfusion and filtration pressure are maintained through afferent arteriolar vasodilatation and efferent vasoconstriction, but once these compensatory mechanisms fail, a decline in glomerular filtration ensues. This critical threshold for renal perfusion depends on the ability of compensatory intrarenal vasodilatation. It is increased, for example, with chronic hypertension and high venous pressure states and decreased by endogenous or exogenous vasodilators [117–121]. In circumstances of persistent renal vasoconstriction, vasodilators might have a beneficial effect on kidney function. However, the use of vasodilators may cause hypotension by counteracting compensatory vasoconstriction, thus unmasking occult hypovolemia, and as such the correction of hypovolemia is crucial.
Clinical studies
Fenoldopam is a pure dopamine A-1 receptor agonist. Three RCTs have evaluated the effects of continuous infusion of fenoldopam on renal function in critically ill patients with AKI and observed mixed results [122–124]. Two compared fenoldopam with placebo, and one with dopamine. In one, fenoldopam was found to be beneficial with regard to the primary endpoints of dialysis-free survival at 21 days and need of RRT in the selected subgroups of patients without “diabetes mellitus” or “after cardiac surgery” [124]. In another, fenoldopam caused a significant decrease in mild AKI (defined as a rise in serum creatinine >150 μmol/L) and ICU stay, and a nonsignificant decrease in severe AKI (serum creatinine >300 μmol/L) [123]. In the third, infusion of fenoldopam, but not dopamine, over 4 days significantly reduced serum creatinine [122]. Prophylactic administration of fenoldopam has been studied during surgery and following administration of radiocontrast. A meta-analysis including 1,059 patients undergoing cardiovascular surgery found that fenoldopam consistently and significantly reduced the need for RRT and in-hospital mortality [125]. A second meta-analysis including 1,290 critically ill and surgical patients from 16 randomized studies reported that the use of fenoldopam reduced the incidence of acute renal injury, need for RRT, and hospital mortality [126]. However, none of the larger RCTs showed that using fenoldopam for the prevention of contrast nephropathy confers renal protection [127, 128] (studies summarized in ESM Table S4).
Clonidine is a central and peripheral adrenergic-receptor blocking agent with predominantly α2 blocking effects which also reduces plasma renin levels. Two RCTs, again involving cardiothoracic patients demonstrated some beneficial effects of clonidine on renal function [129, 130]. However, the use of β-blockers as part of current practice is not mentioned in these studies (ESM Table S5).
Anaritide (ANP) is produced by cardiac atria in response to atrial dilatation and induces afferent glomerular dilatation and efferent vasoconstriction as well as increasing urinary sodium excretion, renal blood flow, and GFR in early ischemic AKI with a dose-dependent hypotensive effect [131]. Ularitide and nesiritide (brain natriuretic peptide, BNP) have similar effects. The first large RCT evaluating ANP in AKI found a reduction of RRT in the oliguric subgroup only [132]. Disappointing results were subsequently observed in RCTs of anaritide and ularitide in patients with oliguric AKI [133–135]. In contrast, a RCT in post cardiac surgery patients with heart failure and early renal dysfunction demonstrated that use of continuous low-dose anaritide significantly reduced the need for RRT [136]. The effect of nesiritide was even more pronounced in cardiac surgery patients with reduced ejection fraction (NAPA trial), demonstrating shorter hospital stay and reduced 180-day mortality [137]. The observed differences might be explained by differences in dose and duration of drug administration with a consequent reduction in hypotension observed. Similarly, protective effects on renal function were observed with low-dose nesiritide in elective abdominal aneurysm repair [138] and in cardiac bypass surgery [139]. Of note, a recent meta-analysis comparing non-inotrope-based treatment with nesiritide (h-BNP) therapy indicated that this may be associated with an increased risk of renal failure and death in a select population of patients with acutely decompensated heart failure [140] (ESM Table S6).
Phosphodiesterase (PDE) inhibitors have both vasodilatory and inotropic effects and modulate the inflammatory response, rendering them interesting candidates for the prevention of renal damage. Ten controlled trials evaluating the effect of theophylline on the prevention of contrast nephropathy showed varying efficacy in attenuating increases in serum creatinine after administration of radiocontrast media [141–151]. The results of three successive meta-analyses remained inconclusive [142, 147, 152]. However, a more recent trial has demonstrated a reduction in the incidence of contrast nephropathy by preprocedural administration of 200 mg theophylline in critically ill patients [151]. Anticipated adverse events such as arrhythmias were not observed (ESM Table S7).
Pentoxifylline has been examined in low risk cardiothoracic patients with no benefit [153] but other small studies examined populations at higher risk and demonstrated beneficial effects on both preservation of renal function and clinical outcomes [154, 155].
Enoximone is a selective phosphodiesterase III inhibitor with both vasodilatory and anti-inflammatory properties. Two small studies in cardiac surgery have evaluated the effect of enoximone, with one study combining enoximone with the β-blocker esmolol [156, 157]. Both demonstrated a reduction in post-bypass inflammation and renal protective effects, although the studies were not designed to show effects on clinical outcomes.
A new phosphodiesterase inhibitor with myocardial calcium sensitizer activity, levosimendan, has recently been approved for the treatment of congestive cardiac failure. A small RCT in 80 patients with acute decompensated heart failure demonstrated short-term improvement of renal function as measured by eGFR which could not be achieved by dobutamine alone [158].
The compensatory release of the vasodilator prostaglandin PGE1 contributes to the maintenance of renal perfusion through dilatation of both the afferent arteriole and medullary vessels in renal hypoperfusion. Three clinical studies showed a mild protective effect of vasodilator prostaglandins (PGE1/PGI2) [159–161].
Angiotensin blockade may have renal protective effects in the acute setting; for example, angiotensin blockade augments renal cortical microvascular PO2 [162] and restores endothelial dysfunction [163]. Two studies evaluating the effect of short-term intravenous enalaprilat in a cardiac surgical population with poor cardiac function demonstrated improved cardiac and renal function [164, 165].
Hormonal manipulation and activated protein C
Recommendations
-
1.
We recommend against the routine use of tight glycemic control in the general ICU population (grade 1A). We suggest “normal for age” glycemic control with intravenous (IV) insulin therapy to prevent AKI in surgical ICU patients (grade 2C), on condition that it can be done adequately and safely applying a local protocol which has proven efficacy in minimizing rate of hypoglycemia.
-
2.
We suggest not to use thyroxine (grade 2C), erythropoietin (grade 2C), activated protein C (grade 2C) or steroids (grade 2C) routinely to prevent AKI.
Rationale
The effects of blood glucose control with insulin on the development or progression of nephropathy in patients with type I and type II diabetes is well documented [166]. In animal models, insulin-like growth factor 1 (IGF-1) has been shown to accelerate recovery from ischemic and cisplatin-induced AKI [167–169], through either renal hemodynamic actions or through a direct metabolic, mitogenic, and antiapoptotic effect on injured tubular cells. Thyroid hormone has also been seen to accelerate renal recovery in various animal models of AKI [170, 171]. There is increasing experimental evidence from ischemia/reperfusion models that erythropoietin (EPO) reduces apoptotic cell death and induces tubular proliferation [172–175]. A retrospective clinical study also showed a slowing of the progression of chronic renal failure in predialysis patients [176]. However, erythropoietin in high doses may induce renal vasoconstriction, systemic hypertension, increase the risk of thrombosis, and increase tumor burden, limiting clinical applicability [177]. New derivatives of EPO that are devoid of hematopoietic activity may be safer [178].
Activated protein C (APC) has pleiotropic actions including anticoagulation, profibrinolysis, anti-inflammation, and antiapoptosis activity, and may therefore be an ideal candidate to prevent ischemia/reperfusion-induced organ failure. Indeed, animal models of ischemia/reperfusion and sepsis have documented beneficial effects on kidney function [179–182].
Clinical studies
A large prospective randomized controlled trial in 1,548 surgical ICU patients compared tight blood glucose control with insulin (blood glucose 80–110 mg/dL) versus standard care (insulin therapy when blood glucose >200 mg/dL resulting in mean blood glucose of 150–160 mg/dL) and showed not only an improved survival rate but also a 41% reduction in AKI requiring RRT. Additionally, tight glucose control also reduced by 27% the number of patients with peak plasma creatinine >2.5 mg/dL [183]. A similar study was undertaken in the medical ICU of the same hospital where tight blood glucose control again improved survival of prolonged ICU stay but had no effect on the need for RRT. However, there was a 34% reduction in newly acquired renal injury, defined as a doubling of serum creatinine compared with the admission level [184]. A combined analysis of the two clinical trials, using modified Rifle criteria, showed a more pronounced renal protection when normoglycemia was achieved. The absence of a reduction in the need for RRT in medical patients could be explained by the higher illness severity with more renal dysfunction on admission, precluding an impact of this mainly protective intervention [185]. Two large sequential cohort studies, one in a medical-surgical ICU [186] and the other in the setting of cardiac surgery [187], also revealed a significant reduction in observed renal dysfunction after the implementation of tight glycemic control. Finally, a recent meta-analysis indicated that survival benefit by applying tight glycemic control, if any, may be restricted only to patients in surgical ICUs [188].
More recent randomized trials in septic [65] and general [189, 190] ICU patients and a meta-analysis [191] do not confirm the renoprotective effect of intensive insulin therapy as evaluated by the need for RRT only.
A major obstacle to the broad implementation of tight glycemic control is the increased risk of hypoglycemia. If the clinician decides to adopt tight glycemic control then steps should be taken to ensure that fluctuations in glucose levels are minimized and standardized and that reliable tools to measure blood glucose are employed [192]. An important concern is the fact that the NICE-Sugar trial found a higher mortality in patients treated with tight glycemic control compared with an intermediate level, which was found independent from hypoglycemia [190]. The clinician should, however, be aware of important differences between the Leuven and the NICE-Sugar trial [192] (ESM Table S8).
The use of IGF-1 has not been exposed to as much scrutiny. A multicenter RCT in 72 patients with AKI failed to show an effect of IGF-1 on renal recovery [193]. A small RCT of IGF-1 administered postoperatively in 54 patients undergoing vascular surgery versus placebo did reveal a lower proportion of patients developing renal dysfunction (fall in GFR compared with baseline), but no difference in need for dialysis or creatinine at discharge was observed [194].
The role of thyroxine has been examined in one RCT in 59 patients with AKI. No effect of thyroxine on any measure of AKI severity compared with placebo was found [195].
With regard to the use of corticosteroids for renal protection one small RCT (n = 20) has been performed on patients undergoing on-pump cardiothoracic surgery. This failed to demonstrate any effect of dexamethasone on the transient renal impairment observed in the postoperative period [196].
Treatment with APC reduces mortality of patients with severe sepsis [197]. A post hoc analysis of secondary endpoints of this trial, however, failed to show an effect on the resolution of renal failure (measured with the SOFA score criteria) in patients with renal dysfunction at baseline. In patients with normal renal function at baseline there was also no effect on the development of renal impairment [198].
No RCT has evaluated the effect of erythropoietin in AKI. A RCT trial on EPO in critically ill patients showed no difference in the incidence of AKI (mentioned as an adverse event, not as an outcome parameter) [199]. A retrospective cohort study in 187 critically ill patients requiring RRT used a propensity-adjusted analysis and found an improvement in renal recovery in patients administered EPO [198].
Metabolic interventions
Recommendations
-
1.
We suggest that all patients at risk of AKI have adequate nutritional support, preferably through the enteral route (grade 2C).
-
2.
We suggest that N-acetylcysteine is not used as prophylaxis against contrast induced nephropathy or other forms AKI in critically ill patients because of conflicting results, possible adverse reactions, and better alternatives (grade 2B).
-
3.
We recommend against the routine use of selenium to protect against renal injury (grade 1B).
Rationale
Metabolic factors are crucial for maintaining cellular integrity and organ function, and nutrition is a basic requirement in the care of any critically ill patients. Starvation accelerates protein breakdown and impairs protein synthesis in the kidney, whereas refeeding might exert the opposite effects and promote renal regeneration. For example, in animal experiments increased protein intake has been shown to reduce tubular injury [200, 201]. Arginine (possibly by producing nitric oxide) helps to preserve renal perfusion and tubular function in both nephrotoxic and ischemic models of AKI. However, amino acids infused before or during ischemia may also enhance tubular damage and accelerate loss of renal function [202]. In part, this “amino acid paradox” is related to the increase in metabolic work for transport processes which may aggravate ischemic injury. However, amino acids per se do not exert intrinsic nephrotoxic effects.
One aspect of nutrition is the adequate supply of nutritional cofactors and antioxidant. The role of reactive oxygen species (ROS) under both normal and pathological conditions has undergone much scrutiny with the NAD(P)H oxidase system believed to be pivotal in their formation and instrumental in the development of certain pathophysiological conditions in the kidney [203–206]. Under certain specific circumstances a role for antioxidant supplementation may be proposed, with potential candidates being the modified form of cysteine, N-acetylcysteine (NAC), the antioxidant vitamins [vitamin E (α-tocopherol) and vitamin C (ascorbic acid)], as well as selenium and other novel antioxidants.
Clinical studies
Protein(s) and amino acids, including glutamine, augment renal perfusion and improve renal function, a fact which was termed “renal reserve capacity” [207]. However, this potential beneficial effect has received little attention in terms of prevention and therapy of AKI to date. Intravenous amino acids appear to improve renal plasma flow and glomerular filtration rate in cirrhotic patients with functional renal failure [208]. One study has reported on a positive effect of enteral versus parenteral nutrition on the resolution of AKI [209].
Most studies on antioxidants have centered on NAC, which besides being an antioxidant, also has a vasodilatory effect [210]. NAC undergoes rapid first-pass metabolism through deacylation and as a result its bioavailability is low even following IV administration. There is no evidence that administration of NAC increases glutathione concentrations in the kidney [211, 212]. NAC has mainly been studied in the setting of contrast nephropathy following the initial publication by Tepel et al. [213] which provided the catalyst for the subsequent proliferation of similar studies [127, 213–222]. This subject continues to generate much research and has led to several meta-analysis [152, 223–228] (ESM Table S9). Most report a risk reduction, although study heterogeneity and positive reporting bias somewhat hinders definitive recommendations [229]. However, studies demonstrating a positive effect have a higher incidence of CIN in the control arms, reflecting differences in risk profile, the route of NAC administration, the dose, the timing (with earlier dosing perhaps being more efficacious), and the total contrast load given. Importantly, some investigators suggest that the reduction in serum creatinine with NAC may be a direct effect of the drug on the creatinine measurement and does not reflect a decrease of GFR, although subsequent work has not replicated these findings when serum creatinine is measured via the Jaffe method [230, 231]. An issue of concern is also the fact that the endpoint used in all the studies is a change in creatinine and not clinically relevant endpoints such as a need for RRT, length of stay or death. This is corroborated by a recent study where contrast-induced AKI was not independently associated with death and had no effect on other clinical events [6, 219]. In patients stratified as high or low risk based on GFR, NAC conferred some benefit when combined with hydration, with multivariate analysis confirming age, volume of contrast, diabetes, and peripheral vascular disease as significant factors for CIN development [232]. However, no benefit was observed in studies using NAC in vascular patients [233, 234] nor in diabetic patients when compared with hydration alone [235]. Several recent studies have employed the use of NAC with other agents such as bicarbonate [236, 237], hydration, and theophylline, with mixed results [70, 151, 237–240].
Several RCTs examining the role of NAC to prevent renal dysfunction in other high-risk groups did not demonstrate a beneficial effect of NAC regarding renal dysfunction or need for renal support [241–245] (ESM Table S9), although one study in 177 patients found a mortality benefit for the patients treated with NAC [246]. It must be considered, though, that especially IV NAC may be harmful, leading to allergic reactions [247] or decreased cardiac output or survival in patients with septic shock [248, 249].
A recent single-center trial showed a protective effect of oral ascorbic acid on the development of contrast nephropathy [250]. Here too, the rate of nephropathy in the control group was high and no patients required renal support. Two further studies investigating protection against contrast nephropathy could not prove protective effects of ascorbic acid [72, 251].
There have been several studies on antioxidant supplementation in the ICU, although few large studies have been conducted. The use of an antioxidant cocktail in patients undergoing elective aortic aneurysm repair resulted in an increased creatinine clearance on the second postoperative day but the incidence of renal failure was very low [252].
A a small RCT in 42 patients showed that selenium supplementation decreased the requirement for RRT from 43% to 14% [253]. These findings, however, could not be reproduced in a larger RCT using selenium in 249 patients with sepsis (SIC study) [254, 255]. More recent studies in the critically ill demonstrated that, although selenium supplementation increased both selenium and glutathione peroxidase levels, there was no effect on the requirement for RRT [256].
Extracorporeal therapies
Recommendations
-
1.
We suggest periprocedural continuous veno-venous hemofiltration (CVVH) in an ICU environment to limit contrast nephropathy after coronary interventions in high-risk patients with advanced chronic renal insufficiency (grade 2C).
Rationale
Extracorporeal therapies may protect the kidney by removal of substance(s) such as contrast, particularly in patients with chronic renal insufficiency, because of the higher contrast load delivered to the remaining nephrons. The relative amount of contrast removal by hemofiltration depends on the pore size of the filter [257], on hemofiltration dose, and on residual renal function. Control of homeostasis or maintenance of fluid balance may be equally important.
Clinical studies
Several studies have employed renal replacement techniques in an attempt to limit contrast nephropathy. Amongst those, three studies utilized periprocedural hemodialysis, with variable benefit [258–261]. Prophylactic CVVH was evaluated in a total of 114 patients at high risk of contrast nephropathy undergoing coronary intervention. The intervention group had a significantly reduced mortality as well as a reduced need for continued renal support, particularly in those with baseline creatinine >300 μmol/l [262]. The same author performed a second RCT in 92 patients, which demonstrated that only combined pre- and postprocedural CVVH, and not postprocedural CVVH alone, had any renoprotective effect [263]. It should be emphasized that the patients receiving CRRT were treated in ICU and those receiving hydration alone were not, which raises the question of whether the hemofiltration procedure itself or the preprocedural optimization in the ICU environment led to a better outcome. Other mechanisms such as pre- and periprocedural hydration during CVVH, correction of acidosis or control of fluid balance might be relevant [264, 265].
Renal replacement techniques have also been used to ameliorate myoglobin-induced tubular damage in rhabdomyolysis or to remove oxalate following ethylene glycol ingestion. Finally, prophylactic use of extracorporeal treatments in oncology patients at high risk of tumor lysis syndrome has been reported. This available literature is limited to case reports and small case series and no recommendation can be drawn. However, it should be emphasized that fomepizole should be used as a first-line treatment before attempting extracorporeal therapy in cases of ethylene glycol intoxication as well as rasburicase following adequate volume expansion in order to prevent tumor lysis syndrome before consideration of extracorporeal therapy. Similarly, the use of extracorporeal techniques to remove myoglobin will require membranes with a high cutoff [266], possibly used in series for a minimal duration of 6 h, which is not readily available [267–273]. Four studies have evaluated leukodepletion during cardiopulmonary bypass [274–277], with three suggesting a beneficial effect on renal function [274–276].
Conclusions and summary
One of the major problems with any review discussing AKI is the fact that definitions used in many publications vary largely. As a consequence comparing studies is fraught with difficulties and calls for uniformly applicable indicators of severity of renal dysfunction, such as duration and degree of oliguria as well as dependence on renal replacement techniques. Fortunately, initiatives in this direction have recently been taken and should aid further study [5, 278].
In order to prevent AKI, prompt resuscitation of the circulation with fluids, inotropes, vasoconstrictors and/or vasodilators is the primary aim and is recommended. Volume expansion is recommended for the prevention of AKI in states of true and suspected hypovolemia, but uncontrolled volume expansion and the use of high-molecular-weight HES preparations and dextrans should be avoided. Following volume resuscitation hypotensive patients should be given a vasoconstrictor which should be titrated individually, with a mean target blood pressure of 60–65 mmHg being adequate in most individuals. Vasodilators are recommended if optimization of cardiac output or reduction in hypertension is required, and some dilators may be considered to prevent deterioration of renal function under strict hemodynamic control. Together with these measures a review of all medications, with the cessation of those known to be nephrotoxic (e.g., amphotericin B, aminoglycosides), is mandatory.
References
Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J (1998) Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 104:343–348
Groeneveld AB, Tran DD, van der MJ, Nauta JJ, Thijs LG (1991) Acute renal failure in the medical intensive care unit: predisposing, complicating factors and outcome. Nephron 59:602–610
Levy EM, Viscoli CM, Horwitz RI (1996) The effect of acute renal failure on mortality. A cohort analysis. JAMA 275:1489–1494
Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, Le G Jr, Druml W (2002) Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med 30:2051–2058
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A (2007) Acute Kidney Injury Network (AKIN): report of an initiative to improve outcomes in acute kidney injury. Crit Care 11:R31
Weisbord SD, Mor MK, Resnick AL, Hartwig KC, Sonel AF, Fine MJ, Palevsky PM (2008) Prevention, incidence, and outcomes of contrast-induced acute kidney injury. Arch Intern Med 168:1325–1332
Joannidis M, Metnitz PG (2005) Epidemiology and natural history of acute renal failure in the ICU. Crit Care Clin 21:239–249
Ricci Z, Cruz D, Ronco C (2008) The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int 73:538–546
Morgera S, Schneider M, Neumayer HH (2008) Long-term outcomes after acute kidney injury. Crit Care Med 36:S193–S197
Cruz DN, Ronco C (2007) Acute kidney injury in the intensive care unit: current trends in incidence and outcome. Crit Care 11:149
Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De BD, Kellum JA (2006) RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 10:R73
Joannidis M (2004) Drug-induced renal failure in the ICU. Int J Artif Organs 27:1034–1042
Kellum JA (2008) Acute kidney injury. Crit Care Med 36:S141–S145
Lameire N, Van BW, Vanholder R (2008) Acute kidney injury. Lancet 372:1863–1865
Bagshaw SM, George C, Gibney RT, Bellomo R (2008) A multi-center evaluation of early acute kidney injury in critically ill trauma patients. Ren Fail 30:581–589
Cruz DN, Bolgan I, Perazella MA, Bonello M, de CM, Corradi V, Polanco N, Ocampo C, Nalesso F, Piccinni P, Ronco C (2007) North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE Criteria. Clin J Am Soc Nephrol 2:418–425
Prescott GJ, Metcalfe W, Baharani J, Khan IH, Simpson K, Smith WC, MacLeod AM (2007) A prospective national study of acute renal failure treated with RRT: incidence, aetiology and outcomes. Nephrol Dial Transplant 22:2513–2519
Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP (2005) A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16:162–168
Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G (2004) A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 44:1393–1399
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med 34:17–60
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW Jr., Zaza S (2004) Grading quality of evidence and strength of recommendations. BMJ 19;328:1490
Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, Phillips B, Raskob G, Lewis SZ, Schunemann H (2006) Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American college of chest physicians task force. Chest 129:174–181
De Mendonca A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, Takala J, Sprung C, Cantraine F (2000) Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med 26:915–921
Guerin C, Girard R, Selli JM, Perdrix JP, Ayzac L (2000) Initial versus delayed acute renal failure in the intensive care unit. A multicenter prospective epidemiological study. Rhone-Alpes Area Study Group on Acute Renal Failure. Am J Respir Crit Care Med 161:872–879
Schwilk B, Wiedeck H, Stein B, Reinelt H, Treiber H, Bothner U (1997) Epidemiology of acute renal failure and outcome of haemodiafiltration in intensive care. Intensive Care Med 23:1204–1211
Himmelfarb J, Joannidis M, Molitoris B, Schietz M, Okusa MD, Warnock D, Laghi F, Goldstein SL, Prielipp R, Parikh CR, Pannu N, Lobo SM, Shah S, D’Intini V, Kellum JA (2008) Evaluation and initial management of acute kidney injury. Clin J Am Soc Nephrol 3:962–967
Badr KF, Ichikawa I (1988) Prerenal failure: a deleterious shift from renal compensation to decompensation. N Engl J Med 319:623–629
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, de Boisblanc B, Connors AF Jr, Hite RD, Harabin AL (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354:2564–2575
Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P, Japiassu A, Kurtop E, De Keulenaer BL, Daelemans R, Del TM, Cosimini P, Ranieri M, Jacquet L, Laterre PF, Gattinoni L (2004) Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med 30:822–829
Wilkes NJ, Woolf R, Mutch M, Mallett SV, Peachey T, Stephens R, Mythen MG (2001) The effects of balanced versus saline-based hetastarch and crystalloid solutions on acid–base and electrolyte status and gastric mucosal perfusion in elderly surgical patients. Anesth Analg 93:811–816
Horgan KJ, Ottaviano YL, Watson AJ (1989) Acute renal failure due to mannitol intoxication. Am J Nephrol 9:106–109
Rozich JD, Paul RV (1989) Acute renal failure precipitated by elevated colloid osmotic pressure. Am J Med 87:359–360
Ahsan N, Palmer BF, Wheeler D, Greenlee RG Jr, Toto RD (1994) Intravenous immunoglobulin-induced osmotic nephrosis. Arch Intern Med 154:1985–1987
Coronel B, Mercatello A, Martin X, Lefrancois N (1997) Hydroxyethylstarch and renal function in kidney transplant recipients. Lancet 349:884
Legendre C, Thervet E, Page B, Percheron A, Noel LH, Kreis H (1993) Hydroxyethylstarch and osmotic-nephrosis-like lesions in kidney transplantation. Lancet 342:248–249
Horstick G, Lauterbach M, Kempf T, Bhakdi S, Heimann A, Horstick M, Meyer J, Kempski O (2002) Early albumin infusion improves global and local hemodynamics and reduces inflammatory response in hemorrhagic shock. Crit Care Med 30:851–855
Inoue M, Okajima K, Itoh K, Ando Y, Watanabe N, Yasaka T, Nagase S, Morino Y (1987) Mechanism of furosemide resistance in analbuminemic rats and hypoalbuminemic patients. Kidney Int 32:198–203
Zhang H, Voglis S, Kim CH, Slutsky AS (2003) Effects of albumin and Ringer’s lactate on production of lung cytokines and hydrogen peroxide after resuscitated hemorrhage and endotoxemia in rats. Crit Care Med 31:1515–1522
Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R (2004) A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 350:2247–2256
Beyer R, Harmening U, Rittmeyer O, Zielmann S, Mielck F, Kazmaier S, Kettler D (1997) Use of modified fluid gelatin and hydroxyethyl starch for colloidal volume replacement in major orthopaedic surgery. Br J Anaesth 78:44–50
Schortgen F, Lacherade JC, Bruneel F, Cattaneo I, Hemery F, Lemaire F, Brochard L (2001) Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet 357:911–916
Mardel SN, Saunders FM, Allen H, Menezes G, Edwards CM, Ollerenshaw L, Baddeley D, Kennedy A, Ibbotson RM (1998) Reduced quality of clot formation with gelatin-based plasma substitutes. Br J Anaesth 80:204–207
Tabuchi N, de HJ, Gallandat Huet RC, Boonstra PW, van OW (1995) Gelatin use impairs platelet adhesion during cardiac surgery. Thromb Haemost 74:1447–1451
Kurnik BR, Singer F, Groh WC (1991) Case report: dextran-induced acute anuric renal failure. Am J Med Sci 302:28–30
Laxenaire MC, Charpentier C, Feldman L (1994) Anaphylactoid reactions to colloid plasma substitutes: incidence, risk factors, mechanisms. A French multicenter prospective study. Ann Fr Anesth Reanim 13:301–310
Mailloux L, Swartz CD, Capizzi R, Kim KE, Onesti G, Ramirez O, Brest AN (1967) Acute renal failure after administration of low-molecular weight dextran. N Engl J Med 277:1113–1118
Messmer KF (1987) The use of plasma substitutes with special attention to their side effects. World J Surg 11:69–74
Bernard C, Alain M, Simone C, Xavier M, Jean-Francois M (1996) Hydroxyethylstarch and osmotic nephrosis-like lesions in kidney transplants. Lancet 348:1595
Bork K (2005) Pruritus precipitated by hydroxyethyl starch: a review. Br J Dermatol 152:3–12
Barron ME, Wilkes MM, Navickis RJ (2004) A systematic review of the comparative safety of colloids. Arch Surg 139:552–563
Wiedermann CJ (2004) Hydroxyethyl starch—can the safety problems be ignored? Wien Klin Wochenschr 116:583–594
Blasco V, Leone M, Antonini F, Geissler A, Albanese J, Martin C (2008) Comparison of the novel hydroxyethylstarch 130/0.4 and hydroxyethylstarch 200/0.6 in brain-dead donor resuscitation on renal function after transplantation. Br J Anaesth 100:504–508
Boldt J, Suttner S, Brosch C, Lehmann A, Rohm K, Mengistu A (2009) The influence of a balanced volume replacement concept on inflammation, endothelial activation, and kidney integrity in elderly cardiac surgery patients. Intensive Care Med 35:462–470
Leuschner J, Opitz J, Winkler A, Scharpf R, Bepperling F (2003) Tissue storage of 14C-labelled hydroxyethyl starch (HES) 130/0.4 and HES 200/0.5 after repeated intravenous administration to rats. Drugs R D 4:331–338
Schortgen F, Girou E, Deye N, Brochard L (2008) The risk associated with hyperoncotic colloids in patients with shock. Intensive Care Med 34:2157–2168
Godet G, Lehot JJ, Janvier G, Steib A, De Castro, V, Coriat P (2008) Safety of HES 130/0.4 (Voluven(R)) in patients with preoperative renal dysfunction undergoing abdominal aortic surgery: a prospective, randomized, controlled, parallel-group multicentre trial. Eur J Anaesthesiol 20:1–9
Boldt J, Brosch C, Ducke M, Papsdorf M, Lehmann A (2007) Influence of volume therapy with a modern hydroxyethylstarch preparation on kidney function in cardiac surgery patients with compromised renal function: a comparison with human albumin. Crit Care Med 35:2740–2746
Sedrakyan A, Gondek K, Paltiel D, Elefteriades JA (2003) Volume expansion with albumin decreases mortality after coronary artery bypass graft surgery. Chest 123:1853–1857
Boldt J, Lehmann A, Rompert R, Haisch G, Isgro F (2000) Volume therapy with a new hydroxyethyl starch solution in cardiac surgical patients before cardiopulmonary bypass. J Cardiothorac Vasc Anesth 14:264–268
Boldt J, Brosch C, Rohm K, Lehmann A, Mengistu A, Suttner S (2008) Is albumin administration in hypoalbuminemic elderly cardiac surgery patients of benefit with regard to inflammation, endothelial activation, and long-term kidney function? Anesth Analg 107:1496–1503
Boldt J, Brosch C, Rohm K, Papsdorf M, Mengistu A (2008) Comparison of the effects of gelatin and a modern hydroxyethyl starch solution on renal function and inflammatory response in elderly cardiac surgery patients. Br J Anaesth 100:457–464
Dehne MG, Muhling J, Sablotzki A, Dehne K, Sucke N, Hempelmann G (2001) Hydroxyethyl starch (HES) does not directly affect renal function in patients with no prior renal impairment. J Clin Anesth 13:103–111
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Stockwell MA, Scott A, Day A, Riley B, Soni N (1992) Colloid solutions in the critically ill. A randomised comparison of albumin and polygeline 2. Serum albumin concentration and incidences of pulmonary oedema and acute renal failure. Anaesthesia 47:7–9
Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K (2008) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 358:125–139
Sakr Y, Payen D, Reinhart K, Sipmann FS, Zavala E, Bewley J, Marx G, Vincent JL (2007) Effects of hydroxyethyl starch administration on renal function in critically ill patients. Br J Anaesth 98:216–224
Solomon R, Werner C, Mann D, D’Elia J, Silva P (1994) Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med 331:1416–1420
Mueller C, Buerkle G, Buettner HJ, Petersen J, Perruchoud AP, Eriksson U, Marsch S, Roskamm H (2002) Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med 162:329–336
Merten GJ, Burgess WP, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, Bersin RM, Van MA, Simonton CA III, Rittase RA, Norton HJ, Kennedy TP (2004) Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA 291:2328–2334
Ozcan EE, Guneri S, Akdeniz B, Akyildiz IZ, Senaslan O, Baris N, Aslan O, Badak O (2007) Sodium bicarbonate, N-acetylcysteine, and saline for prevention of radiocontrast-induced nephropathy. A comparison of 3 regimens for protecting contrast-induced nephropathy in patients undergoing coronary procedures. A single-center prospective controlled trial. Am Heart J 154:539–544
Masuda M, Yamada T, Mine T, Morita T, Tamaki S, Tsukamoto Y, Okuda K, Iwasaki Y, Hori M, Fukunami M (2007) Comparison of usefulness of sodium bicarbonate versus sodium chloride to prevent contrast-induced nephropathy in patients undergoing an emergent coronary procedure. Am J Cardiol 100:781–786
Briguori C, Airoldi F, D’Andrea D, Bonizzoni E, Morici N, Focaccio A, Michev I, Montorfano M, Carlino M, Cosgrave J, Ricciardelli B, Colombo A (2007) Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation 115:1211–1217
Recio-Mayoral A, Chaparro M, Prado B, Cozar R, Mendez I, Banerjee D, Kaski JC, Cubero J, Cruz JM (2007) The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the RENO Study. J Am Coll Cardiol 49:1283–1288
Hogan SE, L’Allier P, Chetcuti S, Grossman PM, Nallamothu BK, Duvernoy C, Bates E, Moscucci M, Gurm HS (2008) Current role of sodium bicarbonate-based preprocedural hydration for the prevention of contrast-induced acute kidney injury: a meta-analysis. Am Heart J 156:414–421
Pakfetrat M, Nikoo MH, Malekmakan L, Tabandeh M, Roozbeh J, Nasab MH, Ostovan MA, Salari S, Kafi M, Vaziri NM, Adl F, Hosseini M, Khajehdehi P (2009) A comparison of sodium bicarbonate infusion versus normal saline infusion and its combination with oral acetazolamide for prevention of contrast-induced nephropathy: a randomized, double-blind trial. Int Urol Nephrol 41:629–634
Brar SS, Shen AY, Jorgensen MB, Kotlewski A, Aharonian VJ, Desai N, Ree M, Shah AI, Burchette RJ (2008) Sodium bicarbonate vs sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. JAMA 300:1038–1046
Maioli M, Toso A, Leoncini M, Gallopin M, Tedeschi D, Micheletti C, Bellandi F (2008) Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol 52:599–604
Adolph E, Holdt-Lehmann B, Chatterjee T, Paschka S, Prott A, Schneider H, Koerber T, Ince H, Steiner M, Schuff-Werner P, Nienaber CA (2008) Renal Insufficiency Following Radiocontrast Exposure Trial (REINFORCE): a randomized comparison of sodium bicarbonate versus sodium chloride hydration for the prevention of contrast-induced nephropathy. Coron Artery Dis 19:413–419
Joannidis M, Schmid M, Wiedermann CJ (2008) Prevention of contrast media-induced nephropathy by isotonic sodium bicarbonate: a meta-analysis. Wien Klin Wochenschr 120:742–748
Navaneethan SD, Singh S, Appasamy S, Wing RE, Sehgal AR (2009) Sodium bicarbonate therapy for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Am J Kidney Dis 53:617–627
Kanbay M, Covic A, Coca SG, Turgut F, Akcay A, Parikh CR (2009) Sodium bicarbonate for the prevention of contrast-induced nephropathy: a meta-analysis of 17 randomized trials. Int Urol Nephrol 41:617–627
Haase M, Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Reade MC, Bagshaw SM, Seevanayagam N, Seevanayagam S, Doolan L, Buxton B, Dragun D (2009) Sodium bicarbonate to prevent increases in serum creatinine after cardiac surgery: a pilot double-blind, randomized controlled trial. Crit Care Med 37:39–47
Branch RA (1988) Prevention of amphotericin B-induced renal impairment. A review on the use of sodium supplementation. Arch Intern Med 148:2389–2394
Cheung TW, Jayaweera DT, Pearce D, Benson P, Nahass R, Olson C, Wool GM (2000) Safety of oral versus intravenous hydration during induction therapy with intravenous foscarnet in AIDS patients with cytomegalovirus infections. Int J STD AIDS 11:640–647
Safrin S, Cherrington J, Jaffe HS (1999) Cidofovir. Review of current and potential clinical uses. Adv Exp Med Biol 458:111-20: 111-120
Perazella MA (1999) Crystal-induced acute renal failure. Am J Med 106:459–465
Bagshaw SM, Delaney A, Jones D, Ronco C, Bellomo R (2007) Diuretics in the management of acute kidney injury: a multinational survey. Contrib Nephrol 156:236–49: 236–249
Bayati A, Nygren K, Kallskog O, Wolgast M (1990) The effect of loop diuretics on the long-term outcome of post-ischaemic acute renal failure in the rat. Acta Physiol Scand 139:271–279
Heyman SN, Rosen S, Epstein FH, Spokes K, Brezis ML (1994) Loop diuretics reduce hypoxic damage to proximal tubules of the isolated perfused rat kidney. Kidney Int 45:981–985
Kramer HJ, Schuurmann J, Wassermann C, Dusing R (1980) Prostaglandin-independent protection by furosemide from oliguric ischemic renal failure in conscious rats. Kidney Int 17:455–464
Stevens MA, McCullough PA, Tobin KJ, Speck JP, Westveer DC, Guido-Allen DA, Timmis GC, O’Neill WW (1999) A prospective randomized trial of prevention measures in patients at high risk for contrast nephropathy: results of the P.R.I.N.C.E. Study. Prevention of Radiocontrast Induced Nephropathy Clinical Evaluation. J Am Coll Cardiol 33:403–411
Weinstein JM, Heyman S, Brezis M (1992) Potential deleterious effect of furosemide in radiocontrast nephropathy. Nephron 62:413–415
Brown CB, Ogg CS, Cameron JS (1981) High dose frusemide in acute renal failure: a controlled trial. Clin Nephrol 15:90–96
Lassnigg A, Donner E, Grubhofer G, Presterl E, Druml W, Hiesmayr M (2000) Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Am Soc Nephrol 11:97–104
Cotter G, Weissgarten J, Metzkor E, Moshkovitz Y, Litinski I, Tavori U, Perry C, Zaidenstein R, Golik A (1997) Increased toxicity of high-dose furosemide versus low-dose dopamine in the treatment of refractory congestive heart failure. Clin Pharmacol Ther 62:187–193
Hager B, Betschart M, Krapf R (1996) Effect of postoperative intravenous loop diuretic on renal function after major surgery. Schweiz Med Wochenschr 126:666–673
van der Voort PHJ, Boerma EC, Koopmans M, Zandberg M, de RJ, Gerritsen RT, Egbers PH, Kingma WP, Kuiper MA (2009) Furosemide does not improve renal recovery after hemofiltration for acute renal failure in critically ill patients: a double blind randomized controlled trial. Crit Care Med 37:533–538
Shilliday IR, Quinn KJ, Allison ME (1997) Loop diuretics in the management of acute renal failure: a prospective, double-blind, placebo-controlled, randomized study. Nephrol Dial Transplant 12:2592–2596
Sirivella S, Gielchinsky I, Parsonnet V (2000) Mannitol, furosemide, and dopamine infusion in postoperative renal failure complicating cardiac surgery. Ann Thorac Surg 69:501–506
Ho KM, Sheridan DJ (2006) Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ 333:420
Bagshaw SM, Delaney A, Haase M, Ghali WA, Bellomo R (2007) Loop diuretics in the management of acute renal failure: a systematic review and meta-analysis. Crit Care Resusc 9:60–68
Sampath S, Moran JL, Graham PL, Rockliff S, Bersten AD, Abrams KR (2007) The efficacy of loop diuretics in acute renal failure: assessment using Bayesian evidence synthesis techniques. Crit Care Med 35:2516–2524
Mehta RL, Pascual MT, Soroko S, Chertow GM (2002) Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA 288:2547–2553
Uchino S, Doig GS, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Nacedo E, Gibney N, Tolwani A, Ronco C, Kellum JA (2004) Diuretics and mortality in acute renal failure. Crit Care Med 32:1669–1677
Dünser MW, Takala J, Ulmer H, Mayr VD, Luckner G, Jochberger S, Daudel F, Lepper P, Hasibeder WR, Jakob SM (2009) Arterial blood pressure during early sepsis and outcome. Intensive Care Med 35:1225–1233
Bourgoin A, Leone M, Delmas A, Garnier F, Albanese J, Martin C (2005) Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med 33:780–786
Holmes CL, Walley KR (2003) Bad medicine: low-dose dopamine in the ICU. Chest 123:1266–1275
Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J (2000) Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet 356:2139–2143
Friedrich JO, Adhikari N, Herridge MS, Beyene J (2005) Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med 142:510–524
Girbes AR, Patten MT, McCloskey BV, Groeneveld AB, Hoogenberg K (2000) The renal and neurohumoral effects of the addition of low-dose dopamine in septic critically ill patients. Intensive Care Med 26:1685–1689
Ichai C, Soubielle J, Carles M, Giunti C, Grimaud D (2000) Comparison of the renal effects of low to high doses of dopamine and dobutamine in critically ill patients: a single-blind randomized study. Crit Care Med 28:921–928
Karthik S, Lisbon A (2006) Low-dose dopamine in the intensive care unit. Semin Dial 19:465–471
Renton MC, Snowden CP (2005) Dopexamine and its role in the protection of hepatosplanchnic and renal perfusion in high-risk surgical and critically ill patients. Br J Anaesth 94:459–467
Albanese J, Leone M, Garnier F, Bourgoin A, Antonini F, Martin C (2004) Renal effects of norepinephrine in septic and nonseptic patients. Chest 126:534–539
Delmas A, Leone M, Rousseau S, Albanese J, Martin C (2005) Clinical review: Vasopressin and terlipressin in septic shock patients. Crit Care 9:212–222
Russell JA, Walley KR, Singer J, Gordon AC, Hebert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ, Ayers D (2008) Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 358:877–887
Brezis M, Rosen S (1995) Hypoxia of the renal medulla—its implications for disease. N Engl J Med 332:647–655
Cohen RI, Hassell AM, Marzouk K, Marini C, Liu SF, Scharf SM (2001) Renal effects of nitric oxide in endotoxemia. Am J Respir Crit Care Med 164:1890–1895
Lameire NH, Matthys E, Kesteloot D, Waterloos MA (1990) Effect of a serotonin blocking agent on renal hemodynamics in the normal rat. Kidney Int 38:823–829
Linas S, Whittenburg D, Repine JE (1997) Nitric oxide prevents neutrophil-mediated acute renal failure. Am J Physiol 272:F48–F54
Zhang H, Rogiers P, Smail N, Cabral A, Preiser JC, Peny MO, Vincent JL (1997) Effects of nitric oxide on blood flow distribution and O2 extraction capabilities during endotoxic shock. J Appl Physiol 83:1164–1173
Brienza N, Malcangi V, Dalfino L, Trerotoli P, Guagliardi C, Bortone D, Faconda G, Ribezzi M, Ancona G, Bruno F, Fiore T (2006) A comparison between fenoldopam and low-dose dopamine in early renal dysfunction of critically ill patients. Crit Care Med 34:707–714
Morelli A, Ricci Z, Bellomo R, Ronco C, Rocco M, Conti G, De GA, Picchini U, Orecchioni A, Portieri M, Coluzzi F, Porzi P, Serio P, Bruno A, Pietropaoli P (2005) Prophylactic fenoldopam for renal protection in sepsis: a randomized, double-blind, placebo-controlled pilot trial. Crit Care Med 33:2451–2456
Tumlin JA, Finkel KW, Murray PT, Samuels J, Cotsonis G, Shaw AD (2005) Fenoldopam mesylate in early acute tubular necrosis: a randomized, double-blind, placebo-controlled clinical trial. Am J Kidney Dis 46:26–34
Landoni G, Biondi-Zoccai GG, Marino G, Bove T, Fochi O, Maj G, Calabro MG, Sheiban I, Tumlin JA, Ranucci M, Zangrillo A (2008) Fenoldopam reduces the need for renal replacement therapy and in-hospital death in cardiovascular surgery: a meta-analysis. J Cardiothorac Vasc Anesth 22:27–33
Landoni G, Biondi-Zoccai GG, Tumlin JA, Bove T, De LM, Calabro MG, Ranucci M, Zangrillo A (2007) Beneficial impact of fenoldopam in critically ill patients with or at risk for acute renal failure: a meta-analysis of randomized clinical trials. Am J Kidney Dis 49:56–68
Allaqaband S, Tumuluri R, Malik AM, Gupta A, Volkert P, Shalev Y, Bajwa TK (2002) Prospective randomized study of N-acetylcysteine, fenoldopam, and saline for prevention of radiocontrast-induced nephropathy. Catheter Cardiovasc Interv 57:279–283
Stone GW, McCullough PA, Tumlin JA, Lepor NE, Madyoon H, Murray P, Wang A, Chu AA, Schaer GL, Stevens M, Wilensky RL, O’Neill WW (2003) Fenoldopam mesylate for the prevention of contrast-induced nephropathy: a randomized controlled trial. JAMA 290:2284–2291
Kulka PJ, Tryba M, Zenz M (1996) Preoperative alpha2-adrenergic receptor agonists prevent the deterioration of renal function after cardiac surgery: results of a randomized, controlled trial. Crit Care Med 24:947–952
Myles PS, Hunt JO, Holdgaard HO, McRae R, Buckland MR, Moloney J, Hall J, Bujor MA, Esmore DS, Davis BB, Morgan DJ (1999) Clonidine and cardiac surgery: haemodynamic and metabolic effects, myocardial ischaemia and recovery. Anaesth Intensive Care 27:137–147
Sward K, Valsson F, Sellgren J, Ricksten SE (2005) Differential effects of human atrial natriuretic peptide and furosemide on glomerular filtration rate and renal oxygen consumption in humans. Intensive Care Med 31:79–85
Allgren RL, Marbury TC, Rahman SN, Weisberg LS, Fenves AZ, Lafayette RA, Sweet RM, Genter FC, Kurnik BR, Conger JD, Sayegh MH (1997) Anaritide in acute tubular necrosis. Auriculin Anaritide Acute Renal Failure Study Group. N Engl J Med 336:828–834
Lewis J, Salem MM, Chertow GM, Weisberg LS, McGrew F, Marbury TC, Allgren RL (2000) Atrial natriuretic factor in oliguric acute renal failure. Anaritide Acute Renal Failure Study Group. Am J Kidney Dis 36:767–774
Rahman SN, Kim GE, Mathew AS, Goldberg CA, Allgren R, Schrier RW, Conger JD (1994) Effects of atrial natriuretic peptide in clinical acute renal failure. Kidney Int 45:1731–1738
Meyer M, Pfarr E, Schirmer G, Uberbacher HJ, Schope K, Bohm E, Fluge T, Mentz P, Scigalla P, Forssmann WG (1999) Therapeutic use of the natriuretic peptide ularitide in acute renal failure. Ren Fail 21:85–100
Sward K, Valsson F, Odencrants P, Samuelsson O, Ricksten SE (2004) Recombinant human atrial natriuretic peptide in ischemic acute renal failure: a randomized placebo-controlled trial. Crit Care Med 32:1310–1315
Mentzer RM Jr, Oz MC, Sladen RN, Graeve AH, Hebeler RF Jr, Luber JM Jr, Smedira NG (2007) Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: the NAPA trial. J Am Coll Cardiol 49:716–726
Mitaka C, Kudo T, Jibiki M, Sugano N, Inoue Y, Makita K, Imai T (2008) Effects of human atrial natriuretic peptide on renal function in patients undergoing abdominal aortic aneurysm repair. Crit Care Med 36:745–751
Chen HH, Sundt TM, Cook DJ, Heublein DM, Burnett JC Jr (2007) Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: a double-blind placebo-controlled pilot study. Circulation 116:I134–I138
Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K (2005) Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA 293:1900–1905
Abizaid AS, Clark CE, Mintz GS, Dosa S, Popma JJ, Pichard AD, Satler LF, Harvey M, Kent KM, Leon MB (1999) Effects of dopamine and aminophylline on contrast-induced acute renal failure after coronary angioplasty in patients with preexisting renal insufficiency. Am J Cardiol 83:260–263 A5
Bagshaw SM, Ghali WA (2005) Theophylline for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Arch Intern Med 165:1087–1093
Erley CM, Duda SH, Schlepckow S, Koehler J, Huppert PE, Strohmaier WL, Bohle A, Risler T, Osswald H (1994) Adenosine antagonist theophylline prevents the reduction of glomerular filtration rate after contrast media application. Kidney Int 45:1425–1431
Erley CM, Duda SH, Rehfuss D, Scholtes B, Bock J, Muller C, Osswald H, Risler T (1999) Prevention of radiocontrast-media-induced nephropathy in patients with pre-existing renal insufficiency by hydration in combination with the adenosine antagonist theophylline. Nephrol Dial Transplant 14:1146–1149
Huber W, Ilgmann K, Page M, Hennig M, Schweigart U, Jeschke B, Lutilsky L, Weiss W, Salmhofer H, Classen M (2002) Effect of theophylline on contrast material-nephropathy in patients with chronic renal insufficiency: controlled, randomized, double-blinded study. Radiology 223:772–779
Huber W, Schipek C, Ilgmann K, Page M, Hennig M, Wacker A, Schweigart U, Lutilsky L, Valina C, Seyfarth M, Schomig A, Classen M (2003) Effectiveness of theophylline prophylaxis of renal impairment after coronary angiography in patients with chronic renal insufficiency. Am J Cardiol 91:1157–1162
Ix JH, McCulloch CE, Chertow GM (2004) Theophylline for the prevention of radiocontrast nephropathy: a meta-analysis. Nephrol Dial Transplant 19:2747–2753
Kapoor A, Kumar S, Gulati S, Gambhir S, Sethi RS, Sinha N (2002) The role of theophylline in contrast-induced nephropathy: a case–control study. Nephrol Dial Transplant 17:1936–1941
Katholi RE, Taylor GJ, McCann WP, Woods WT Jr, Womack KA, McCoy CD, Katholi CR, Moses HW, Mishkel GJ, Lucore CL (1995) Nephrotoxicity from contrast media: attenuation with theophylline. Radiology 195:17–22
Kolonko A, Wiecek A, Kokot F (1998) The nonselective adenosine antagonist theophylline does prevent renal dysfunction induced by radiographic contrast agents. J Nephrol 11:151–156
Huber W, Eckel F, Hennig M, Rosenbrock H, Wacker A, Saur D, Sennefelder A, Hennico R, Schenk C, Meining A, Schmelz R, Fritsch R, Weiss W, Hamar P, Heemann U, Schmid RM (2006) Prophylaxis of contrast material-induced nephropathy in patients in intensive care: acetylcysteine, theophylline, or both? A randomized study. Radiology 239:793–804
Kelly AM, Dwamena B, Cronin P, Bernstein SJ, Carlos RC (2008) Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med 148:284–294
Kleinschmidt S, Bauer M, Grundmann U, Schneider A, Wagner B, Graeter T (1997) Effect of gamma-hydroxybutyric acid and pentoxifylline on kidney function parameters in coronary surgery interventions. Anaesthesiol Reanim 22:102–107
Boldt J, Brosch C, Piper SN, Suttner S, Lehmann A, Werling C (2001) Influence of prophylactic use of pentoxifylline on postoperative organ function in elderly cardiac surgery patients. Crit Care Med 29:952–958
Hoffmann H, Markewitz A, Kreuzer E, Reichert K, Jochum M, Faist E (1998) Pentoxifylline decreases the incidence of multiple organ failure in patients after major cardio-thoracic surgery. Shock 9:235–240
Boldt J, Brosch C, Suttner S, Piper SN, Lehmann A, Werling C (2002) Prophylactic use of the phospodiesterase III inhibitor enoximone in elderly cardiac surgery patients: effect on hemodynamics, inflammation, and markers of organ function. Intensive Care Med 28:1462–1469
Boldt J, Brosch C, Lehmann A, Suttner S, Isgro F (2004) The prophylactic use of the beta-blocker esmolol in combination with phosphodiesterase III inhibitor enoximone in elderly cardiac surgery patients. Anesth Analg 99:1009–1017 (table)
Yilmaz MB, Yalta K, Yontar C, Karadas F, Erdem A, Turgut OO, Yilmaz A, Tandogan I (2007) Levosimendan improves renal function in patients with acute decompensated heart failure: comparison with dobutamine. Cardiovasc Drugs Ther 21:431–435
Sketch MH Jr, Whelton A, Schollmayer E, Koch JA, Bernink PJ, Woltering F, Brinker J (2001) Prevention of contrast media-induced renal dysfunction with prostaglandin E1: a randomized, double-blind, placebo-controlled study. Am J Ther 8:155–162
Koch JA, Plum J, Grabensee B, Modder U (2000) Prostaglandin E1: a new agent for the prevention of renal dysfunction in high risk patients caused by radiocontrast media? PGE1 Study Group. Nephrol Dial Transplant 15:43–49
Spargias K, Adreanides E, Giamouzis G, Karagiannis S, Gouziouta A, Manginas A, Voudris V, Pavlides G, Cokkinos DV (2006) Iloprost for prevention of contrast-mediated nephropathy in high-risk patients undergoing a coronary procedure. Results of a randomized pilot study. Eur J Clin Pharmacol 62:589–595
Norman JT, Stidwill R, Singer M, Fine LG (2003) Angiotensin II blockade augments renal cortical microvascular pO2 indicating a novel, potentially renoprotective action. Nephron Physiol 94:39–46
Tiefenbacher CP, Friedrich S, Bleeke T, Vahl C, Chen X, Niroomand F (2004) ACE inhibitors and statins acutely improve endothelial dysfunction of human coronary arterioles. Am J Physiol Heart Circ Physiol 286:H1425–H1432
Ryckwaert F, Colson P, Ribstein J, Boccara G, Guillon G (2001) Haemodynamic and renal effects of intravenous enalaprilat during coronary artery bypass graft surgery in patients with ischaemic heart dysfunction. Br J Anaesth 86:169–175
Wagner F, Yeter R, Bisson S, Siniawski H, Hetzer R (2003) Beneficial hemodynamic and renal effects of intravenous enalaprilat following coronary artery bypass surgery complicated by left ventricular dysfunction. Crit Care Med 31:1421–1428
Fioretto P, Bruseghin M, Berto I, Gallina P, Manzato E, Mussap M (2006) Renal protection in diabetes: role of glycemic control. J Am Soc Nephrol 17:S86–S89
Ding H, Kopple JD, Cohen A, Hirschberg R (1993) Recombinant human insulin-like growth factor-I accelerates recovery and reduces catabolism in rats with ischemic acute renal failure. J Clin Invest 91:2281–2287
Miller SB, Martin DR, Kissane J, Hammerman MR (1992) Insulin-like growth factor I accelerates recovery from ischemic acute tubular necrosis in the rat. Proc Natl Acad Sci USA 89:11876–11880
Yasuda H, Kato A, Miyaji T, Zhou H, Togawa A, Hishida A (2004) Insulin-like growth factor-I increases p21 expression and attenuates cisplatin-induced acute renal injury in rats. Clin Exp Nephrol 8:27–35
Michael UF, Logan JL, Meeks LA (1991) The beneficial effects of thyroxine on nephrotoxic acute renal failure in the rat. J Am Soc Nephrol 1:1236–1240
Sutter PM, Thulin G, Stromski M, Ardito T, Gaudio KM, Kashgarian M, Siegel NJ (1988) Beneficial effect of thyroxin in the treatment of ischemic acute renal failure. Pediatr Nephrol 2:1–7
Chatterjee PK (2005) Pleiotropic renal actions of erythropoietin. Lancet 365:1890–1892
Coleman T, Brines M (2004) Science review: recombinant human erythropoietin in critical illness: a role beyond anemia? Crit Care 8:337–341
Bernhardt WM, Eckardt KU (2008) Physiological basis for the use of erythropoietin in critically ill patients at risk for acute kidney injury. Curr Opin Crit Care 14:621–626
Bahlmann FH, Fliser D (2009) Erythropoietin and renoprotection. Curr Opin Nephrol Hypertens 18:15–20
Jungers P, Choukroun G, Oualim Z, Robino C, Nguyen AT, Man NK (2001) Beneficial influence of recombinant human erythropoietin therapy on the rate of progression of chronic renal failure in predialysis patients. Nephrol Dial Transplant 16:307–312
Maiese K, Chong ZZ, Shang YC (2008) Raves and risks for erythropoietin. Cytokine Growth Factor Rev 19:145–155
Yokomaku Y, Sugimoto T, Kume S, Araki S, Isshiki K, Chin-Kanasaki M, Sakaguchi M, Nitta N, Haneda M, Koya D, Uzu T, Kashiwagi A (2008) Asialoerythropoietin prevents contrast-induced nephropathy. J Am Soc Nephrol 19:321–328
Mizutani A, Okajima K, Uchiba M, Noguchi T (2000) Activated protein C reduces ischemia/reperfusion-induced renal injury in rats by inhibiting leukocyte activation. Blood 95:3781–3787
Gupta A, Berg DT, Gerlitz B, Sharma GR, Syed S, Richardson MA, Sandusky G, Heuer JG, Galbreath EJ, Grinnell BW (2007) Role of protein C in renal dysfunction after polymicrobial sepsis. J Am Soc Nephrol 18:860–867
Gupta A, Rhodes GJ, Berg DT, Gerlitz B, Molitoris BA, Grinnell BW (2007) Activated protein C ameliorates LPS-induced acute kidney injury and downregulates renal INOS and angiotensin 2. Am J Physiol Renal Physiol 293:F245–F254
Gupta A, Gerlitz B, Richardson MA, Bull C, Berg DT, Syed S, Galbreath EJ, Swanson BA, Jones BE, Grinnell BW (2009) Distinct functions of activated protein C differentially attenuate acute kidney injury. J Am Soc Nephrol 20:267–277
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R (2001) Intensive insulin therapy in the critically ill patients. N Engl J Med 345:1359–1367
van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van WE, Bobbaers H, Bouillon R (2006) Intensive insulin therapy in the medical ICU. N Engl J Med 354:449–461
Schetz M, Vanhorebeek I, Wouters PJ, Wilmer A, van den BG (2008) Tight blood glucose control is renoprotective in critically ill patients. J Am Soc Nephrol 19:571–578
Krinsley JS (2004) Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc 79:992–1000
Lecomte P, Van VB, Coddens J, Cammu G, Nollet G, Nobels F, Vanermen H, Foubert L (2008) Tight perioperative glucose control is associated with a reduction in renal impairment and renal failure in non-diabetic cardiac surgical patients. Crit Care 12:R154
Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D (2009) Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ 180:821–827
Arabi YM, Dabbagh OC, Tamim HM, Al-Shimemeri AA, Memish ZA, Haddad SH, Syed SJ, Giridhar HR, Rishu AH, Al-Daker MO, Kahoul SH, Britts RJ, Sakkijha MH (2008) Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med 36:3190–3197
Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hebert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ (2009) Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360:1283–1297
Wiener RS, Wiener DC, Larson RJ (2008) Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA 300:933–944
van den Berghe G, Schetz M, Vlasselaers D, Hermans G, Wilmer A, Bouillon R, Mesotten D (2009) Intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target? J Clin Endocrinol Metab 94:3163–3170
Hirschberg R, Kopple J, Lipsett P, Benjamin E, Minei J, Albertson T, Munger M, Metzler M, Zaloga G, Murray M, Lowry S, Conger J, McKeown W, O’shea M, Baughman R, Wood K, Haupt M, Kaiser R, Simms H, Warnock D, Summer W, Hintz R, Myers B, Haenftling K, Capra W (1999) Multicenter clinical trial of recombinant human insulin-like growth factor I in patients with acute renal failure. Kidney Int 55:2423–2432
Franklin SC, Moulton M, Sicard GA, Hammerman MR, Miller SB (1997) Insulin-like growth factor I preserves renal function postoperatively. Am J Physiol 272:F257–F259
Acker CG, Singh AR, Flick RP, Bernardini J, Greenberg A, Johnson JP (2000) A trial of thyroxine in acute renal failure. Kidney Int 57:293–298
Loef BG, Henning RH, Epema AH, Rietman GW, van OW, Navis GJ, Ebels T (2004) Effect of dexamethasone on perioperative renal function impairment during cardiac surgery with cardiopulmonary bypass. Br J Anaesth 93:793–798
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr (2001) Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344:699–709
Vincent JL, Angus DC, Artigas A, Kalil A, Basson BR, Jamal HH, Johnson G III, Bernard GR (2003) Effects of drotrecogin alfa (activated) on organ dysfunction in the PROWESS trial. Crit Care Med 31:834–840
Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Shapiro MJ, Corwin MJ, Colton T (2002) Efficacy of recombinant human erythropoietin in critically ill patients: a randomized controlled trial. JAMA 288:2827–2835
Pons M, Plante I, LeBrun M, Gourde P, Simard M, Grenier L, Thibault L, Labrecque G, Beauchamp D (2003) Protein-rich diet attenuates cyclosporin A-induced renal tubular damage in rats. J Ren Nutr 13:84–92
Roberts PR, Black KW, Zaloga GP (1997) Enteral feeding improves outcome and protects against glycerol-induced acute renal failure in the rat. Am J Respir Crit Care Med 156:1265–1269
Zager RA (1987) Amino acid hyperalimentation in acute renal failure: a potential therapeutic paradox. Kidney Int Suppl 22:S72–S75
Evans RG, Fitzgerald SM (2005) Nitric oxide and superoxide in the renal medulla: a delicate balancing act. Curr Opin Nephrol Hypertens 14:9–15
Haque MZ, Majid DS (2004) Assessment of renal functional phenotype in mice lacking gp91PHOX subunit of NAD(P)H oxidase. Hypertension 43:335–340
Haugen E, Nath KA (1999) The involvement of oxidative stress in the progression of renal injury. Blood Purif 17:58–65
Vaziri ND (2004) Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens 13:93–99
Abel RM, Beck CH Jr, Abbott WM, Ryan JA Jr, Barnett GO, Fischer JE (1973) Improved survival from acute renal failure after treatment with intravenous essential L-amino acids and glucose. Results of a prospective, double-blind study. N Engl J Med 288:695–699
Badalamenti S, Gines P, Arroyo V, Llach J, Piera C, Rimola A, Jimenez W, Gaya J, Casamitjana R, Rivera F (1990) Effects of intravenous amino acid infusion and dietary proteins on kidney function in cirrhosis. Hepatology 11:379–386
Mouser JF, Hak EB, Kuhl DA, Dickerson RN, Gaber LW, Hak LJ (1997) Recovery from ischemic acute renal failure is improved with enteral compared with parenteral nutrition. Crit Care Med 25:1748–1754
Heyman SN, Goldfarb M, Shina A, Karmeli F, Rosen S (2003) N-Acetylcysteine ameliorates renal microcirculation: studies in rats. Kidney Int 63:634–641
Borgstrom L, Kagedal B, Paulsen O (1986) Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol 31:217–222
Olsson B, Johansson M, Gabrielsson J, Bolme P (1988) Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol 34:77–82
Tepel M, van der GM, Schwarzfeld C, Laufer U, Liermann D, Zidek W (2000) Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med 343:180–184
Baker CS, Wragg A, Kumar S, De PR, Baker LR, Knight CJ (2003) A rapid protocol for the prevention of contrast-induced renal dysfunction: the RAPPID study. J Am Coll Cardiol 41:2114–2118
Briguori C, Manganelli F, Scarpato P, Elia PP, Golia B, Riviezzo G, Lepore S, Librera M, Villari B, Colombo A, Ricciardelli B (2002) Acetylcysteine and contrast agent-associated nephrotoxicity. J Am Coll Cardiol 40:298–303
Durham JD, Caputo C, Dokko J, Zaharakis T, Pahlavan M, Keltz J, Dutka P, Marzo K, Maesaka JK, Fishbane S (2002) A randomized controlled trial of N-acetylcysteine to prevent contrast nephropathy in cardiac angiography. Kidney Int 62:2202–2207
Kay J, Chow WH, Chan TM, Lo SK, Kwok OH, Yip A, Fan K, Lee CH, Lam WF (2003) Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention: a randomized controlled trial. JAMA 289:553–558
MacNeill BD, Harding SA, Bazari H, Patton KK, Colon-Hernadez P, DeJoseph D, Jang IK (2003) Prophylaxis of contrast-induced nephropathy in patients undergoing coronary angiography. Catheter Cardiovasc Interv 60:458–461
Miner SE, Dzavik V, Nguyen-Ho P, Richardson R, Mitchell J, Atchison D, Seidelin P, Daly P, Ross J, McLaughlin PR, Ing D, Lewycky P, Barolet A, Schwartz L (2004) N-acetylcysteine reduces contrast-associated nephropathy but not clinical events during long-term follow-up. Am Heart J 148:690–695
Oldemeyer JB, Biddle WP, Wurdeman RL, Mooss AN, Cichowski E, Hilleman DE (2003) Acetylcysteine in the prevention of contrast-induced nephropathy after coronary angiography. Am Heart J 146:E23
Shyu KG, Cheng JJ, Kuan P (2002) Acetylcysteine protects against acute renal damage in patients with abnormal renal function undergoing a coronary procedure. J Am Coll Cardiol 40:1383–1388
Vallero A, Cesano G, Pozzato M, Garbo R, Minelli M, Quarello F, Formica M (2002) Contrast nephropathy in cardiac procedures: no advantages with prophylactic use of N-acetylcysteine (NAC). G Ital Nefrol 19:529–533
Gonzales DA, Norsworthy KJ, Kern SJ, Banks S, Sieving PC, Star RA, Natanson C, Danner RL (2007) A meta-analysis of N-acetylcysteine in contrast-induced nephrotoxicity: unsupervised clustering to resolve heterogeneity. BMC Med 5:32
Gawenda M, Moller A, Wassmer G, Brunkwall J (2007) Prophylaxis of contrast-induced nephropathy with N-acetylcysteine. Zentralbl Chir 132:227–231
Zagler A, Azadpour M, Mercado C, Hennekens CH (2006) N-acetylcysteine and contrast-induced nephropathy: a meta-analysis of 13 randomized trials. Am Heart J 151:140–145
Duong MH, MacKenzie TA, Malenka DJ (2005) N-acetylcysteine prophylaxis significantly reduces the risk of radiocontrast-induced nephropathy: comprehensive meta-analysis. Catheter Cardiovasc Interv 64:471–479
Pannu N, Manns B, Lee H, Tonelli M (2004) Systematic review of the impact of N-acetylcysteine on contrast nephropathy. Kidney Int 65:1366–1374
Alonso A, Lau J, Jaber BL, Weintraub A, Sarnak MJ (2004) Prevention of radiocontrast nephropathy with N-acetylcysteine in patients with chronic kidney disease: a meta-analysis of randomized, controlled trials. Am J Kidney Dis 43:1–9
Hoffmann U, Fischereder M, Kruger B, Drobnik W, Kramer BK (2004) The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol 15:407–410
Rank N, Michel C, Haertel C, Lenhart A, Welte M, Meier-Hellmann A, Spies C (2000) N-Acetylcysteine increases liver blood flow and improves liver function in septic shock patients: results of a prospective, randomized, double-blind study. Crit Care Med 28:3799–3807
Poletti PA, Saudan P, Platon A, Mermillod B, Sautter AM, Vermeulen B, Sarasin FP, Becker CD, Martin PY (2007) I.v. N-acetylcysteine and emergency CT: use of serum creatinine and cystatin C as markers of radiocontrast nephrotoxicity. AJR Am J Roentgenol 189:687–692
Chen SL, Zhang J, Yei F, Zhu Z, Liu Z, Lin S, Chu J, Yan J, Zhang R, Kwan TW (2008) Clinical outcomes of contrast-induced nephropathy in patients undergoing percutaneous coronary intervention: a prospective, multicenter, randomized study to analyze the effect of hydration and acetylcysteine. Int J Cardiol 126:407–413
Rashid ST, Salman M, Myint F, Baker DM, Agarwal S, Sweny P, Hamilton G (2004) Prevention of contrast-induced nephropathy in vascular patients undergoing angiography: a randomized controlled trial of intravenous N-acetylcysteine. J Vasc Surg 40:1136–1141
Lawlor DK, Moist L, DeRose G, Harris KA, Lovell MB, Kribs SW, Elliot J, Forbes TL (2007) Prevention of contrast-induced nephropathy in vascular surgery patients. Ann Vasc Surg 21:593–597
Coyle LC, Rodriguez A, Jeschke RE, Simon-Lee A, Abbott KC, Taylor AJ (2006) Acetylcysteine In Diabetes (AID): a randomized study of acetylcysteine for the prevention of contrast nephropathy in diabetics. Am Heart J 151:12–1032
Heng AE, Cellarier E, Aublet-Cuvelier B, Decalf V, Motreff P, Marcaggi X, Deteix P, Souweine B (2008) Is treatment with N-acetylcysteine to prevent contrast-induced nephropathy when using bicarbonate hydration out of date? Clin Nephrol 70:475–484
Staniloae CS, Doucet S, Sharma SK, Katholi RE, Mody KR, Coppola JT, Solomon R (2009) N-Acetylcysteine added to volume expansion with sodium bicarbonate does not further prevent contrast-induced nephropathy: results from the cardiac angiography in renally impaired patients study. J Interv Cardiol 22:261–265
Fung JW, Szeto CC, Chan WW, Kum LC, Chan AK, Wong JT, Wu EB, Yip GW, Chan JY, Yu CM, Woo KS, Sanderson JE (2004) Effect of N-acetylcysteine for prevention of contrast nephropathy in patients with moderate to severe renal insufficiency: a randomized trial. Am J Kidney Dis 43:801–808
Gomes VO, Poli de Figueredo CE, Caramori P, Lasevitch R, Bodanese LC, Araujo A, Roedel AP, Caramori AP, Brito FS Jr, Bezerra HG, Nery P, Brizolara A (2005) N-Acetylcysteine does not prevent contrast induced nephropathy after cardiac catheterisation with an ionic low osmolality contrast medium: a multicentre clinical trial. Heart 91:774–778
Ristikankare A, Kuitunen T, Kuitunen A, Uotila L, Vento A, Suojaranta-Ylinen R, Salmenpera M, Poyhia R (2006) Lack of renoprotective effect of i.v. N-acetylcysteine in patients with chronic renal failure undergoing cardiac surgery. Br J Anaesth 97:611–616
Burns KE, Chu MW, Novick RJ, Fox SA, Gallo K, Martin CM, Stitt LW, Heidenheim AP, Myers ML, Moist L (2005) Perioperative N-acetylcysteine to prevent renal dysfunction in high-risk patients undergoing CABG surgery: a randomized controlled trial. JAMA 294:342–350
Macedo E, Abdulkader R, Castro I, Sobrinho AC, Yu L, Vieira JM Jr (2006) Lack of protection of N-acetylcysteine (NAC) in acute renal failure related to elective aortic aneurysm repair-a randomized controlled trial. Nephrol Dial Transplant 21:1863–1869
Komisarof JA, Gilkey GM, Peters DM, Koudelka CW, Meyer MM, Smith SM (2007) N-Acetylcysteine for patients with prolonged hypotension as prophylaxis for acute renal failure (NEPHRON). Crit Care Med 35:435–441
Sisillo E, Ceriani R, Bortone F, Juliano G, Salvi L, Veglia F, Fiorentini C, Marenzi G (2008) N-Acetylcysteine for prevention of acute renal failure in patients with chronic renal insufficiency undergoing cardiac surgery: a prospective, randomized, clinical trial. Crit Care Med 36:81–86
Adabag AS, Ishani A, Koneswaran S, Johnson DJ, Kelly RF, Ward HB, McFalls EO, Bloomfield HE, Chandrashekhar Y (2008) Utility of N-acetylcysteine to prevent acute kidney injury after cardiac surgery: a randomized controlled trial. Am Heart J 155:1143–1149
Wijeysundera DN, Beattie WS, Rao V, Granton JT, Chan CT (2007) N-Acetylcysteine for preventing acute kidney injury in cardiac surgery patients with pre-existing moderate renal insufficiency. Can J Anaesth 54:872–881
Sandilands EA, Bateman DN (2009) Adverse reactions associated with acetylcysteine. Clin Toxicol (Phila) 47:81–88
Molnar Z, Shearer E, Lowe D (1999) N-Acetylcysteine treatment to prevent the progression of multisystem organ failure: a prospective, randomized, placebo-controlled study. Crit Care Med 27:1100–1104
Peake SL, Moran JL, Leppard PI (1996) N-Acetyl-l-cysteine depresses cardiac performance in patients with septic shock. Crit Care Med 24:1302–1310
Spargias K, Alexopoulos E, Kyrzopoulos S, Iokovis P, Greenwood DC, Manginas A, Voudris V, Pavlides G, Buller CE, Kremastinos D, Cokkinos DV (2004) Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation 110:2837–2842
Boscheri A, Weinbrenner C, Botzek B, Reynen K, Kuhlisch E, Strasser RH (2007) Failure of ascorbic acid to prevent contrast-media induced nephropathy in patients with renal dysfunction. Clin Nephrol 68:279–286
Wijnen MH, Vader HL, Van Den Wall Bake AW, Roumen RM (2002) Can renal dysfunction after infra-renal aortic aneurysm repair be modified by multi-antioxidant supplementation? J Cardiovasc Surg (Torino) 43:483–488
Angstwurm MW, Schottdorf J, Schopohl J, Gaertner R (1999) Selenium replacement in patients with severe systemic inflammatory response syndrome improves clinical outcome. Crit Care Med 27:1807–1813
Angstwurm MW, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, Strauss R, Meier-Hellmann A, Insel R, Radke J, Schuttler J, Gartner R (2007) Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med 35:118–126
Reade MC, Fink MP (2005) Bench-to-bedside review: amelioration of acute renal impairment using ethyl pyruvate. Crit Care 9:556–560
Mishra V, Baines M, Perry SE, McLaughlin PJ, Carson J, Wenstone R, Shenkin A (2007) Effect of selenium supplementation on biochemical markers and outcome in critically ill patients. Clin Nutr 26:41–50
Schindler R, Stahl C, Venz S, Ludat K, Krause W, Frei U (2001) Removal of contrast media by different extracorporeal treatments. Nephrol Dial Transplant 16:1471–1474
Lee PT, Chou KJ, Liu CP, Mar GY, Chen CL, Hsu CY, Fang HC, Chung HM (2007) Renal protection for coronary angiography in advanced renal failure patients by prophylactic hemodialysis. A randomized controlled trial. J Am Coll Cardiol 50:1015–1020
Frank H, Werner D, Lorusso V, Klinghammer L, Daniel WG, Kunzendorf U, Ludwig J (2003) Simultaneous hemodialysis during coronary angiography fails to prevent radiocontrast-induced nephropathy in chronic renal failure. Clin Nephrol 60:176–182
Gabutti L, Marone C, Monti M, Malfanti M, Zwahlen U, Pasotti E, Colucci G, Schonholzer C (2003) Does continuous venovenous hemodiafiltration concomitant with radiological procedures provide a significant and safe removal of the iodinated contrast ioversol? Blood Purif 21:152–157
Huber W, Jeschke B, Kreymann B, Hennig M, Page M, Salmhofer H, Eckel F, Schmidt U, Umgelter A, Schweigart U, Classen M (2002) Haemodialysis for the prevention of contrast-induced nephropathy: outcome of 31 patients with severely impaired renal function, comparison with patients at similar risk and review. Invest Radiol 37:471–481
Marenzi G, Marana I, Lauri G, Assanelli E, Grazi M, Campodonico J, Trabattoni D, Fabbiocchi F, Montorsi P, Bartorelli AL (2003) The prevention of radiocontrast-agent-induced nephropathy by hemofiltration. N Engl J Med 349:1333–1340
Marenzi G, Lauri G, Campodonico J, Marana I, Assanelli E, De MM, Grazi M, Veglia F, Fabbiocchi F, Montorsi P, Bartorelli AL (2006) Comparison of two hemofiltration protocols for prevention of contrast-induced nephropathy in high-risk patients. Am J Med 119:155–162
Reinecke H, Fobker M, Wellmann J, Becke B, Fleiter J, Heitmeyer C, Breithardt G, Hense HW, Schaefer RM (2007) A randomized controlled trial comparing hydration therapy to additional hemodialysis or N-acetylcysteine for the prevention of contrast medium-induced nephropathy: the Dialysis-versus-Diuresis (DVD) Trial. Clin Res Cardiol 96:130–139
Cruz DN, Perazella MA, Bellomo R, Corradi V, de CM, Kuang D, Ocampo C, Nalesso F, Ronco C (2006) Extracorporeal blood purification therapies for prevention of radiocontrast-induced nephropathy: a systematic review. Am J Kidney Dis 48:361–371
Naka T, Jones D, Baldwin I, Fealy N, Bates S, Goehl H, Morgera S, Neumayer HH, Bellomo R (2005) Myoglobin clearance by super high-flux hemofiltration in a case of severe rhabdomyolysis: a case report. Crit Care 9:R90–R95
Amyot SL, Leblanc M, Thibeault Y, Geadah D, Cardinal J (1999) Myoglobin clearance and removal during continuous venovenous hemofiltration. Intensive Care Med 25:1169–1172
Schenk MR, Beck DH, Nolte M, Kox WJ (2001) Continuous veno-venous hemofiltration for the immediate management of massive rhabdomyolysis after fulminant malignant hyperthermia in a bodybuilder. Anesthesiology 94:1139–1141
Shigemoto T, Rinka H, Matsuo Y, Kaji A, Tsukioka K, Ukai T, Shimaoka H (1997) Blood purification for crush syndrome. Ren Fail 19:711–719
Heney D, Essex-Cater A, Brocklebank JT, Bailey CC, Lewis IJ (1990) Continuous arteriovenous haemofiltration in the treatment of tumour lysis syndrome. Pediatr Nephrol 4:245–247
Meier M, Nitschke M, Perras B, Steinhoff J (2005) Ethylene glycol intoxication and xylitol infusion—metabolic steps of oxalate-induced acute renal failure. Clin Nephrol 63:225–228
Moreau CL, Kerns W, Tomaszewski CA, McMartin KE, Rose SR, Ford MD, Brent J (1998) Glycolate kinetics and hemodialysis clearance in ethylene glycol poisoning. META Study Group. J Toxicol Clin Toxicol 36:659–666
Saccente SL, Kohaut EC, Berkow RL (1995) Prevention of tumor lysis syndrome using continuous veno-venous hemofiltration. Pediatr Nephrol 9:569–573
Bolcal C, Akay HT, Bingol H, Doganci S, Yildirim V, Yenicesu M, Demirkilic U, Tatar H (2007) Leukodepletion improves renal function in patients with renal dysfunction undergoing on-pump coronary bypass surgery: a prospective randomized study. Thorac Cardiovasc Surg 55:89–93
Tang AT, Alexiou C, Hsu J, Sheppard SV, Haw MP, Ohri SK (2002) Leukodepletion reduces renal injury in coronary revascularization: a prospective randomized study. Ann Thorac Surg 74:372–377
Treacher DF, Sabbato M, Brown KA, Gant V (2001) The effects of leucodepletion in patients who develop the systemic inflammatory response syndrome following cardiopulmonary bypass. Perfusion 16(Suppl):67–73
Salamonsen RF, Anderson J, Anderson M, Bailey M, Magrin G, Rosenfeldt F (2005) Total leukocyte control for elective coronary bypass surgery does not improve short-term outcome. Ann Thorac Surg 79:2032–2038
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004) Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: doi:10.1007/s00134-009-1683-1.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00134-010-1780-1
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Joannidis, M., Druml, W., Forni, L.G. et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit. Intensive Care Med 36, 392–411 (2010). https://doi.org/10.1007/s00134-009-1678-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1678-y